Abstract
Craniopharyngioma has benign histological character. However, because of proximity to optic pathways, pituitary gland, and hypothalamus, it may cause severe and permanent damage to such critical structures and can even be life threatening. Total surgical resection is often difficult. This study aims to evaluate treatment results of Novalis stereotactic radiotherapy (SRT) for craniopharyngioma adjacent to optic pathways. Ten patients (six men, four women) with craniopharyngioma and median age of 56.5 years (range 10–74 years) were treated by SRT using Novalis from July 2006 through March 2009. Median volume of tumor was 7.9 ml (range 1.1–21 ml). Three-dimensional noncoplanar five- or seven-beam SRT or coplanar five-beam SRT with intensity modulation was performed. Total dose of 30–39 Gy in 10–15 fractions (median 33 Gy) was delivered to the target. Ten patients were followed up for 9–36 months (median 25.5 months). Response rate was 80% (8/10), and control rate was 100%. Improvement of neurological symptoms was observed in five patients. No serious complications due to SRT were found. SRT for craniopharyngioma may be a safe and effective treatment. Longer follow-up is necessary to determine long-term tumor control or late complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical resection has been the first-line treatment for craniopharyngioma. However, total removal is often difficult because of proximity to critical structures such as optic pathways, pituitary gland, and hypothalamus. Recurrence may often occur [1, 2]. In such conditions, fractionated external-beam radiotherapy (EBRT) has been a treatment choice but sometimes causes side-effects on those important structures [3–5]. Successful results of single-session gamma knife radiosurgery (GKS) have been reported with less side-effects, if the tumor is not large. However, if the tumor is large or involves optic pathways, it is not easy to deliver high dose in a single session while sparing those important structures [6–8]. Hypofractionated stereotactic radiotherapy (SRT) has the radiobiologic advantage of conventional fractionation and is expected to be a promising alternative treatment method to deliver high dose precisely while avoiding such complications. This study aims to evaluate treatment results of Novalis SRT for craniopharyngioma adjacent to optic pathways.
Materials and methods
We treated ten cases (one child, nine adults) of craniopharyngioma by SRT using Novalis from July 2006 through March 2009 (Table 1). Median age of patients was 56.5 years (range 10–74 years). Male-to-female ratio was 6:4. All patients harbored craniopharyngioma based on histological diagnosis, though histological confirmation was done after Novalis SRT in one of ten patients. Seven were of adamantinomatous type and three of squamous papillary type. All patients initially presented with symptoms attributable to the tumor. Nine patients had had surgical resection as initial treatment 2–136 months (median 6 months) before Novalis SRT. Surgical resection was done prior to SRT once in five patients and twice in four patients, and GKS in two patients. Table 1 summarizes symptoms and signs of patients after previous surgery and those just before SRT. Neurological or endocrinological status was deteriorating because of tumor progression after prior resection until SRT in five among nine patients. At Novalis SRT, nine had visual field defect, four had decreased visual acuity, two had blindness in one eye, nine had diabetes insipidus (DI), and eight had hypopituitarism.
Stereotactic radiotherapy was done as initial treatment in one patient (case 1) and for macroresidual tumors or regrowing tumors located in supraseller region adjacent to optic pathways and pituitary gland in the other nine patients. Median tumor volume of tumors was 7.9 ml (range 1.1–21 ml), and median diameter was 2.6 cm (1.3–3.5 cm) (Table 2). We classified pre-SRT radiological characteristic into three categories: cystic, mixed, and solid. Cystic tumor was observed in two patients and mixed tumor in eight, with no solid tumors.
Stereotactic radiotherapy technique
The Novalis system consists of several stereotactic components. Infrared passive reflectors attached noninvasively to the patient’s body surface, immobilized by a head–neck–shoulder shell, were used for positioning of the target close to the isocenter of a linear accelerator (LINAC). Radiographic image guidance was used for fine positioning adjustments based on internal anatomy, such as the skull-base bony structures [9]. Infrared guidance was also used to monitor external patient motion during treatment.
The infrared positioning system consists of a pair of cameras in the radiotherapy room that emit and detect infrared radiation reflected from markers placed on the patient’s body surface. Data are gathered to yield real-time positional information. Translation and rotation in all three major axes (six dimensions) are displayed on multiple monitors. The treatment couch is driven automatically to position the patient near the isocenter, based on information derived from the infrared positioning to determine the position of the head relative to the isocenter. Radiographs are digitally transferred to a computer, where they are compared with digitally reconstructed radiography (DRR) generated from pretreatment computerized tomography (CT). DRR represents the position of a perfectly aligned patient. Radiographs and DRR are fused automatically to determine whether any patient positioning adjustments are necessary, and the treatment couch is moved to reposition the patient appropriately. After shifts have been applied, a pair of radiographs are exposed to confirm patient positioning. The infrared positioning system is used to monitor external patient motion throughout the treatment process. A thermoplastic shell restraining system minimizes patient movement during treatment. Treatment was delivered by a dedicated 6-MV LINAC with 3-mm multileaf collimator. All patients were irradiated with a single isocenter.
Dose planning
Dose planning for SRT was based on T2- and T1-weighted enhanced three-dimensional magnetic resonance imaging (MRI) and contrast-enhanced thin-slice CT using a BrainScan computer workstation (BrainLAB, Tokyo). The clinical tumor volume (CTV) was delineated on thin-slice CT and MRI, including the cystic portion, if present. The planned target volume (PTV) was determined with margin of 2–3 mm. In one patient, 3.0-T MRI was taken for reference to delineate visual pathways. Three-dimensional noncoplanar five- or seven-beam SRT or coplanar five-beam SRT with intensity modulation was planned. The lesions were covered with 95% or higher isodose level. Tumor volume was 1.1–21 ml (median 7.9 ml). Total dose of 30–39 Gy in 10–15 fractions (median 33 Gy) over 2–3 weeks was delivered to the target [BED (biologically effective dose) = 48.8–68.3 GyE in α/β = 4 Gy]. Total dose and schedule of fractionation were determined considering tumor volume, history of irradiation, and optic nerve involvement. Six patients with intermediate risk (without previous radiation therapy) were treated with 60–63 GyE in α/β = 4 Gy, three patients with high risk (with previous radiation therapy or involvement of bilateral optic pathways by the tumor) were treated with 49–52 GyE, and one patient with low risk (small tumor of about 1 ml) was treated with 68 GyE. The maximum dose delivered to optic pathways was kept low at less than 90 GyE in α/β = 2 Gy.
Methods of evaluation
Clinical status and imaging examination were evaluated every 3–6 months after treatment. Tumor volume, including cystic portion if present, was calculated for evaluation. A four-grade system was devised for evaluation of tumor response: complete response (CR, tumor disappearance), partial response (PR, ≥50% tumor volume reduction), stable disease (SD, <50% reduction), and progression (PG, tumor or cyst enlargement >25%). From these values, response rate was calculated as (CR + PR)/total and control rate as (CR + PR + SD)/total. Clinical evaluation included changes in neurological status, such as visual function and endocrinological function.
Results
Imaging and clinical follow-up were obtained in all ten patients for 9–36 months after SRT (median 25.5 months) (Table 3).
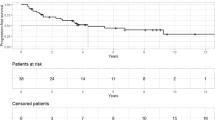
The treated tumor decreased in size in eight of ten patients (80%). CR was obtained in one (10%), PR in seven (70%), and SD in two (20%). No progression was observed. Tumor response rate was 80% (8/10), and control rate was 100% (10/10).
Neurological symptoms due to optic nerve involvement were improved completely or partially in five of nine patients who had had visual disturbance since before treatment (55%). In three patients, visual field defects deteriorated. In one patient (case 1), subtle visual field defect developed due to partial cyst enlargement 19 months after SRT, while the rest of the tumor shrunk. In this patient, open biopsy and Ommaya setting into the cystic part of tumor were performed for the purpose of not only decompression of the right optic nerve but also histological confirmation of craniopharyngioma. In another patient (case 10), before SRT, temporary cyst enlargement was developing with progressive decrease of visual acuity. Though deterioration of visual impairment continued until 3 months after SRT, since 9 months after SRT the treated tumor began to shrink and the patient regained better visual function than that before SRT. In the other patient (case 7), lateral partial hemianopsia in the left eye deteriorated to complete hemianopsia, though the tumor remained shrunk, which was thought to be due to radiation-induced neuropathy. In this patient, GKS had already been performed twice previously. In one patient (case 3), DI had been improving after surgery and continued to improve after SRT. In all other patients, endocrinological status was unchanged during the follow-up period or until death compared with before SRT. One patient (case 2), a 74-year-old male, died 2 years 3 months after SRT from cerebral cortex infarction, which was thought to be unrelated to SRT.
Illustrative cases
Case 5: 25-year-old female (Fig. 1)
The patient developed hydrocephalus due to suprasellar tumor 11 years before Novalis SRT. The tumor was resected gross-totally, and ventriculo-peritoneal shunt was placed. Histological diagnosis was craniopharyngioma of adamantinomatous type. After surgical resection she had suffered from hypopituitarism and DI. Four years later, GKS was performed for recurrent suprasellar mass. Margin dose of 11.1 Gy was delivered for 19 ml of tumor. Another year later, second surgical resection was done for regrowth of the suprasellar mass with cyst formation. The tumor was subtotally resected except for a small part on the left hypothalamus. As the tumor recurred again in the suprasellar region mainly on the left side, the patient was referred to our institute. Visual function of both eyes was gradually deteriorating. Novalis SRT was performed for 5.2 ml of tumor with total dose of 30 Gy (at 100% isodose = normalization point) in ten fractions by noncoplanar seven-beam method. The tumor was covered by the 95% isodose area. The tumor decreased in size remarkably within 6 months and disappeared within 27 months. Neurological and endocrinological status including visual function has been stable, though hypopituitarism and DI continue.
Case 5. a Dose planning for craniopharyngioma. b Coronal magnetic resonance image (MRI) with gadolinium (Gd) enhancement at Novalis stereotactic radiotherapy (SRT). Total dose of 30 Gy in ten fractions was delivered by noncoplanar seven-beam stereotactic radiotherapy. c Coronal MRI with Gd enhancement 17 months after Novalis SRT. Complete response was obtained
Case 7: 45-year-old male (Fig. 2)
The patient developed hydrocephalus due to suprasellar tumor 4 years before Novalis SRT. The tumor was resected partially, and ventriculo-peritoneal shunt was placed. Histological diagnosis was craniopharyngioma of adamantinomatous type. After surgical resection, he had suffered from short-term memory disturbance. Two months later, the suprasellar tumor recurred with cyst formation. GKS was performed for the solid part located in the anterior lower left portion, and Ommaya reservoir was placed in the cystic part. Margin dose of 10 Gy was delivered. Two months later, second GKS was performed for growing upper part of the tumor, including the cystic part extending into the third ventricle. After that, the tumor decreased in size and the cyst shrunk. Three and a half years later, the suprasellar tumor relapsed remarkably. He had memory disturbance, hypopituitarism, and DI. Incomplete bitemporal hemianopsia was observed. He complained of blurred vision in the right eye. Novalis SRT was performed for 21 ml of tumor with total dose of 30 Gy (at 100% isodose) in 12 fractions by coplanar five-beam method with intensity modulation. The tumor was covered by the 95% isodose area. The tumor decreased in size remarkably within 4 months and remained shrunk until 24 months after SRT. Neurological and endocrinological status was stable. Hemianopsia in the left eye became complete without tumor progression, but blurred vision in the right eye was improved. His performance status is stable and he continued with daily life with some support.
Case 7. a Dose planning for craniopharyngioma. b Coronal MRI with Gd enhancement at Novalis SRT. Total dose of 30 Gy in 12 fractions was delivered by coplanar five-beam stereotactic radiotherapy with intensity modulation. c Coronal MRI with Gd enhancement 20 months after Novalis SRT. The tumor remarkably decreased in size within 4 months after Novalis SRT. Visual field before SRT (d) and 14 months later (e). Hemianopsia in the left eye became complete after SRT, but visual field in the right eye was much improved
Discussion
Craniopharyngioma is a benign tumor thought to be derived from a residual cell nest of stomodeum [10]. It may be solid, cystic, or mixed in nature and is commonly calcified. Despite its benign histological features, craniopharyngioma may be life threatening because of proximity to critical structures. It may cause severe and permanent damage to optic pathways, pituitary gland, and hypothalamus, and disrupt visual, hypothalamic, endocrinological, and neurocognitive functions. Total removal by microsurgery is ideal, but complete removal without deterioration of neurological functions is always difficult. Hoffman et al. [1] accomplished total removal in 90% of their cases but reported that the recurrence rate was not low (29%) even after “total” removal. Yasargil et al. [2] did aggressive surgery, but it was frequently associated with significant morbidity (16%) and mortality (16.7%), although with relatively low recurrence rate (7%). Recently, Effenterre et al. [11] reported recurrence rate of 13% and mortality rate of 2.5%. On the other hand, subtotal resection or only biopsy is not adequate treatment for craniopharyngioma because of high recurrence rate with further morbidity and mortality resulted from tumor regrowth and repeat surgery [12].
Combined treatment with partial removal and subsequent conventional EBRT to a relatively broad area including the tumor bed is another treatment strategy. Cure or control rate has been relatively good, up to 91% of 10-year tumor control rate, as shown in recent reports [5, 13–17], but late radiation injury to surrounding organs can produce major side-effects (Table 4). The rate of complications including hypothalamic-pituitary dysfunction, visual disturbance, DI, and epilepsy after surgery is increased further by EBRT, due to not only tumor progression but also radiation toxicity. In addition, especially in pediatric patients, generation of secondary disorders such as glioblastoma and moyamoya disease is sometimes problematic in long-term follow-up [5, 14, 16, 18]. Rajan et al. [15] reported that extent of surgical removal did not influence outcome when surgery was combined with EBRT, and that less invasive surgery followed by EBRT was associated with low morbidity. When EBRT was used as salvage treatment for tumor relapse after aggressive surgery, quality of life was also found to be decreased more as compared with intentional limited resection followed by planned EBRT [13, 19]. Recently, surgery tends to be performed to alleviate symptoms by tumor volume reduction, to provide a specimen for histological diagnosis, and to reduce the volume of irradiation, though sometimes even the chance of cure by total resection exists. The main aim of surgery should be decompression of optic pathways when visual disturbance due to tumor involvement is progressing. It is important to achieve clear space between the residual tumor and the optic pathways as much as possible, not only to improve impaired visual function but also to enable subsequent effective treatment, such as GKS, or SRT as we describe below.
In terms of development of radiation therapy, recently GKS has been employed to deliver more localized irradiation with steeper dose gradient between tumor and surrounding normal structures in order to minimize postradiation long-term complications while maintaining high efficacy [20–25]. Chung et al. [21] reported 87% tumor control rate by GKS. They reported better control results in smaller tumors (<4.2 ml volume, <2 cm diameter) than in larger ones. Kobayashi et al. [6–8, 22] reported treatment results of GKS for relatively small craniopharyngioma (median 3.5 ml volume). Survival rate was 91% at 10 years, and tumor control rate was 54% at 10 years. They reduced the dose to avoid radiation injury of optic pathways if they were close to the tumor. Optic apparatus is vulnerable to radiation, and possible radiation injury is thought to be dose dependent, with 0–2% incidence of optic neuropathy when less than 10 Gy was delivered, and 27% incidence for 10–15 Gy [26]. Kobayashi et al. [22] considered the optimal marginal dose for tumor control to be 12.1 Gy or more, according to their analysis of 100 cases, because mean marginal dose in CR cases was 12.1 Gy. If there is sufficient distance between the tumor and optic apparatus, greater marginal dose can be given in order to control tumor. Otherwise, GKS will not be effective. Kobayashi et al. also reported that it was more difficult to control cystic tumors than solid tumors. They thought this was because GKS was apt not to achieve the optimal dose to tumor wall located at the marginal area due to the steep dose gradient.
Fractionated SRT has merits not only in terms of the mechanical precision of stereotactic technique but also the radiobiologic advantage of conventional fractionation. Stereotactic technique reduces adverse effects by reducing the dose delivered to surrounding normal structures, not only optic pathways but also pituitary grand and hypothalamus. In addition, fractionation allows further sparing of those normal adjacent tissues, by giving them an interval to recover from radiation between each fractions. It seems that SRT is suitable for tumor of large volume, especially with large cystic part and proximal to optic pathways. In this study we report successful results of Novalis SRT for craniopharyngioma of relatively large volume (median 7.9 ml), adjacent to optic pathways. There have been some reports on treatment results of SRT using various modalities [27–29], using total dose of 50–52.2 Gy with small fraction size of 1.5–1.8 Gy like in conventional EBRT. Combs et al. [27] reported good results of 100% local control at 10 years with less complication (10%) and no serious complications. Response rate was 72.5%, and visual acuity improved after SRT in 13% of all patients. Selch et al. [28] and Minniti et al. [29] reported relatively low response rates of 50% and 41%, respectively. Visual acuity improved after SRT in 25% and 17%, respectively, among those who had visual disturbance prior to SRT. Selch et al. mentioned that initial imaging response following SRT was delayed compared with after single-session stereotactic radiosurgery (SRS) such as GKS. They also mentioned that SRT, in their approach of small fraction size like in EBRT, could not be relied upon to relieve symptoms associated with tumor compression of optic chiasm, hypothalamus, or third ventricle. In our series, we used SRT with less fractionation, with total dose of 30–39 Gy (median 33 Gy) in 10–15 fractions over 2–3 weeks. The control rate was 100%, though the follow-up period was not long. Tumor response rate was better at 80%, and improvement rate of visual function was also better at 55%. In addition, side-effect due to SRT was observed in only one case and was not serious. Lee et al. [30] also reported successful results using CyberKnife. They gave mean marginal dose of 21.6 Gy in 3–10 fractions. High control rate of 91% and response rate of 64% were obtained, albeit with relatively small number of cases and short follow-up time. They also reported that no morbidity or mortality was observed.
External-beam radiotherapy, the field of which is broad, may cause more adverse effects in long-term follow-up. SRT has the merit of reducing the radiation dose to surrounding critical structures. However, as described above, most reports of SRT still employ small fraction size (large number of fractions: 25 or more), which may have caused lower response rate. Though it is important to reduce the possibility of side-effects, one of the aims of SRS or SRT is to recover symptoms of the patient while they are reversible. In our series we could obtain better response rate together with less adverse effects. We think the better results of our cases, and maybe those of the CyberKnife series, were due to benefit of large fraction size, though optimal fraction size and optimal total dose remain to be determined by analysis in larger population of patients with longer follow-up period.
Craniopharyngioma tends to recur repeatedly. It seems better to continue to follow up patients even after irradiation. Timely assessment including repeat surgery, GKS, or SRT is necessary, before the tumor grows to be large. In this series we treated two cases of recurrent tumors after GKS. Both tumors were successfully controlled by Novalis SRT with reduced radiation dose. Caution must be exercised in dose selection when some irradiation has been applied previously. In another case of our series, the size of the whole tumor was stable after SRT, but a small cystic part protruded and compressed the right optic nerve. Aspiration of the cyst recovered the visual disturbance successfully. Cyst enlargement is sometimes observed even after irradiation in craniopharyngioma. It is thought to develop due to not only tumor progression but also tumor degeneration. Continuous formation of microcysts has been reported as part of the natural history of craniopharyngioma or as a result of radiation or surgical trauma. Minniti et al. [29] reported that 28% of patients had enlargement of the cystic portion causing visual impairment and hydrocephalus, and all required cyst aspiration.
Conclusions
Despite the small sample size and short follow-up times, we conclude that Novalis SRT may be a useful and safe treatment option for craniopharyngioma, even if the size of tumor is relatively large and optic pathways are involved. Longer follow-up is necessary to further determine outcomes.
References
Hoffman HJ, DeSilva M, Humphreys RP et al (1992) Aggressive surgical management of craniopharyngiomas in children. J Neurosurg 76:47–52
Yasargil MG, Curcic M, Kis M et al (1990) Total removal of craniopharyngiomas: approaches and long-term results in 144 patients. J Neurosurg 73:3–11
Thomsett MJ, Conte FA, Kaplan SL et al (1980) Endocrine and neurologic outcome in childhood craniopharyngioma: review of effect of treatment in 42 patients. J Pediatr 97:728–735
Glauser TA, Packer RJ (1991) Cognitive deficits in long-term survivors of childhood brain tumors. Childs Nerv Syst 7:2–12
Habrand JL, Ganry O, Couanet D, Rouxel V et al (1999) The role of radiation therapy in the management of craniopharyngioma: a 25-year experience and review of the literature. Int J Radiat Oncol Biol Phys 44:255–263
Kobayashi T (2009) Long-term results of gamma knife radiosurgery for 100 consecutive cases of craniopharyngioma and a treatment strategy. Prog Neurol Surg 22:63–76
Kobayashi T (2007) Treatment strategy and pathological background of radiosurgery for craniopharyngiomas. Prog Neurol Surg 20:180–191
Kobayashi T, Kida Y, Mori Y, Hasegawa T (2005) Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg 103(6 Suppl):482–488
Mori Y, Hashizume C, Shibamoto Y, Kobayashi T et al (2010) Preliminary results of stereotactic radiotherapy for spinal lesions using the Novalis system. Radiosurgery 7:378–383
Luse SA, Kernohan JW (1955) Squamous cell nests of the pituitary gland. Cancer 8:623–638
Effenterre RV, Boch AL (2002) Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 97:3–11
Weiss M, Sutton L, Marcial V, Fowble B et al (1989) The role of radiation therapy in the management of childhood craniopharyngiomas. Int J Radiat Oncol Biol Phys 17:1313–1321
Moon SH, Kim IH, Park SW, Kim I et al (2005) Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas—a study in single institute. Childs Nerv Syst 21:799–807
Regine WF, Mohiuddin M, Kramer S (1993) Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol 27:13–21
Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJ, Marsh H, Brada M (1993) Craniopharyngioma- long-term results following limited surgery and radiotherapy. Radiother Oncol 26:1–10
Varlotto JM, Flickinger JC, Kondziolka D, Lunsford LD, Deutsch M (2002) External beam irradiation of craniopharyngiomas: long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys 54:492–499
Pemberton LS, Dougal M, Magee B, Gattamaneni HR (2005) Experence of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol 77:99–104
Hetelekidis S, Barnes PD, Tao ML, Fischer EG, Schneider L, Scott RM, Tarbell NJ (1993) 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys 27:189–195
Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, Lustig RH, Kun LE (2002) Craniopharyngioma: the St. Jude children’s research hospital experience. Int J Radiat Oncol Biol Phys 53:533–542
Minniti G, Esposito V, Amichetti M, Enrici RM (2009) The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg Rev 32:125–132
Chung WY, Pan DHC, Shiau CY, Guo WY, Wang LW (2000) Gamma knife radiosurgery for craniopharyngiomas. J Neurosurg 93(Suppl):47–56
Kobayashi T, Mori Y, Uchiyama Y, Hayashi N, Kida Y, Hasegawa T (2006) New treatment strategy for craniopharyngioma using gamma knife radiosurgery. Radiosurgery 6:152–163
Chiou SM, Lunsford LD, Niranjan A, Kondziolka D, Flickinger JC (2001) Stereotactic radiosurgery of residual or recurrent craniopharyngioma, after surgery, with or without radiation therapy. Neuro Oncol 3:159–166
Ulfarsson E, Lindquist C, Roberts M, Rahn T, Lindquist M, Thoren M, Lippitz B (2002) Gamma knife radiosurgery for craniopharyngiomas: long-term results in the first Swedish patients. J Neurosurg 97(Suppl 5):613–622
Yomo S, Hayashi M, Chernov M, Tamura N, Izawa M, Okada Y et al (2009) Stereotactic radiosurgery of residual or recurrent craniopharyngioma: new treatment concept using Leksell gamma knife model C with automatic positioning system. Stereotact Funct Neurosurg 87:360–367
Leber KA, Berglofff J, Pendl G (1998) Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg 88:43–50
Combs SE, Thilmann C, Huber PE, Hoess A et al (2007) Achievement of long-term local control in patients with craniopharyngiomas using high precision stereotactic radiotherapy. Am Cancer Soc 109:2308–2314
Selch MT, DeSalles AA, Wade M, Lee SP et al (2002) Initiak clinical results of stereotactic radiotherapy for the treatment of craniopharyngioma. Technol Cancer Res Treat 1:51–59
Minniti G, Saran F, Traish D, Soomal R et al (2007) Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngioma. Radiother Oncol 82:90–95
Lee M, Kalani MY, Cheshier S, Gibbs IC, Adler JR, Chang SD (2008) Radiation therapy and CyberKnife radiosurgery in the management of craniopharyngiomas. Neurosurg Focus 24:1–7
Author information
Authors and Affiliations
Corresponding author
Additional information
Rights and permissions
About this article
Cite this article
Hashizume, C., Mori, Y., Kobayashi, T. et al. Stereotactic radiotherapy using Novalis for craniopharyngioma adjacent to optic pathways. J Neurooncol 98, 239–247 (2010). https://doi.org/10.1007/s11060-010-0180-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0180-2