Abstract
Purpose
Stereotactic radiosurgery (SRS) is an important management strategy for residual and recurrent craniopharyngiomas. The current study evaluated the factors which affected tumor control and complications in craniopharyngioma SRS.
Methods
This study includes 53 consecutive patients who underwent single-session SRS for recurrent or residual craniopharyngiomas. The median age was 41 years with 28 male and 25 females. The median tumor volume was 0.63 cm3 and median margin dose was 12 Gy (range 9–25 Gy).
Results
The overall 3-, 5-, and 10-year survival rates were 97.8%, 92.7% and 88.5%. The overall 3-, 5-, and 10-year tumor control rates were 81.0%, 72.1%, and 53.4%. In univariate analysis, ≥ 3 mm distance from optic structures (p = 0.002), only solid or cystic tumor type (p = 0.037), and ≥ 12 Gy to ≥ 85% of the tumor (p < 0.001) were significantly associated with improved tumor control. In multivariate analysis, only solid or cystic tumor type, (p = 0.034), and ≥ 85% of the tumor receiving ≥ 12 Gy (p = 0.004) were significantly associated with better tumor control. When ≥ 85% of the tumor received ≥ 12 Gy the tumor control rates at 3-, 5-, and 10-year were 100%, 93.3%, and 93.3%. Higher conformity index was not associated with better tumor control.
Conclusions
The tumor control rates after recurrent or residual craniopharyngiomas SRS were improved by ensuring that at least 85% of the tumor received ≥ 12 Gy even when the distance between the tumor and the optic system is < 3 mm. This concept refutes the conformity theory that a high conformity index is a critical feature of effective SRS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas are generally considered slowly progressive, relatively rare (1.2–4.6% of all intracranial tumors) growths arising from embryological remnants of the craniopharyngeal duct or Rathke`s cleft [1]. Although located in close proximity to the optic apparatus, pituitary stalk and critical vascular structures, surgical resection coupled with endocrine and visual preservation remains the optimal goal. Because some craniopharyngiomas are adherent to these surrounding structures residual and recurrent tumors are frequent [2]. Gamma knife stereotactic radiosurgery (SRS) had been used as a minimally invasive alternative for both newly diagnosed as well as residual or recurrent craniopharyngiomas. Published literature suggests that radiosurgery is associated with 5-years tumor control rates of 62.2–73.6% [3,4,5,6,7,8]. The present study assesses the outcomes of SRS and evaluates whether optimizing the dose delivered while minimizing radiation in adjacent critical structures improves tumor control.
Methods
Patient population
From 1988 to 2019, a total of 91 craniopharyngioma patients were treated with Gamma Knife stereotactic radiosurgery at the University of Pittsburgh Medical Center. We excluded patients without prior histological confirmation and those who had undergone prior radiation therapy or intracavitary radiation using instillation of the Beta emitting isotope Phosphorus-32. We identified 53 patients eligible for this retrospective study. A patient flow diagram is shown in Fig. 1. There were 28 males and the median patient age was 41 years (range 4–87 years). We included 17 children (≤ 18 years) and 36 adults. Seven patients had normal endocrine function, 46 had anterior lobe hypopituitarism, and 36 had diabetes insipidus (DI) at the time of SRS. Twenty-six patients had normal visual fields, and 27 had abnormal visual fields and optic neuropathy at the time of SRS. Two patients had tumor biopsy only before SRS, 47 patients had undergone surgical resection, one patient had biopsy and cyst aspiration, and one patient had biopsy, cyst aspiration, and surgical resection. We included two additional patients whose craniopharyngioma was verified at the time of delayed progression after SRS. These 53 patients underwent a total of 73 gamma knife procedures, which included repeat procedures performed after detection of tumor progression between 0.9 and 25.6 years after the initial SRS procedure. At the first SRS, three patients had two tumors in different anatomic locations, and one patient had three tumors. During follow-up, ten patients developed new tumors distinct from the initial tumor volume. A total of 68 tumors underwent SRS. At the time of SRS, 40 tumors were solid, four were cystic, and 24 were mixed tumors having both solid and cystic components. The patient characteristics are shown in Table 1. This retrospective study was approved by the University of Pittsburgh Institutional Review Board.
Radiosurgery technique
SRS was performed in single procedure that began with stereotactic head frame application under conscious sedation and local anesthesia. General anesthesia was used for children < 13 years. Patients then underwent thin slice MRI after frame application. During these three decades, SRS was performed using various models of the Leksell Gamma Knife [Model U, B, C, 4C, Perfexion and Icon (Elekta AB)]. Dose planning was performed using various versions of the Leksell dose planning software (KULA or Leksell GammaPlan ®, AB Elekta). Our dose planning strategy was to encompass the 3D tumor volume at the 50% isodose line. In selected cases we purposefully reduced dose to portions of the tumor adjacent to the optic apparatus. Thus, majority of the tumor volume received a higher dose while only a small component near the optic structures received a lower dose. The median tumor volume was 0.63 cm3 (range 0.03–7.61 cm3). The median margin dose was 12.0 Gy (range 9.0–25.0 Gy), the median maximum dose was 24.0 Gy (range 14.3–50.0 Gy), and the median number of isocenters was 5 (1–19). The median maximum dose of optic nerve was 9.7 Gy (range 3.6–13 Gy) in 33 procedures measured the dose of optic nerve. All patients received an intravenous injection of 20–40 mg methylprednisolone or 100 mg of hydrocortisone after SRS.
Follow-up
Our follow up protocol recommended clinical and image evaluations at 3–6 months intervals for the first 2 years after SRS. The follow up interval was increased to every year for patients without tumor progression or new symptoms at 2 years after SRS. MRI was recommended when new visual or endocrinological symptoms were reported. The median follow-up was 86 months (range 6–390 months).
Statistical analysis
Most craniopharyngiomas are adjacent to the optic nerve or chiasm. In order to reduce the risk of radiation related optic neuropathy, in selected patients the dose delivered to tumor adjacent to the optic nerve was reduced. In such cases the prescribed target volume is strategically less than the gross tumor volume. The tumor margin was defined with thin-slice MRI (Gadolinium enhanced T1 and/or T2 high signal volume) by the responsible neurosurgeon and radiation oncologist. The percent tumor volume that received at least 12 Gy was calculated using Leksell GammaPlan® software. This data was available for 40 of 68 tumors. The median 12 Gy coverage was 94.3% (range 41–100%). The 12 Gy coverage was used because it was the median margin dose in this study. Kaplan–Meier plots for overall survival rate were constructed based on the time of SRS and the date of death or last contact. Kaplan–Meir plots for tumor control rate were constructed based on the time of SRS and the date of tumor enlargement or last image evaluation if the tumor was controlled. Tumor volume enlargement was defined as increase of > 25% from baseline. The follow-up volume was determined by multiplying measurements for the maximal X, Y, and Z tumor dimensions multiplied by 0.5 cm3. Univariate analysis was performed on the Kaplan–Meier curve using a log-rank test. Multivariate analysis was performed with Cox proportional hazard models. The factors studies for overall survival variable included age at first SRS (child vs. adult), sex, number of prior surgical resections (≤ 1 vs. 2 ≤), presence of visual deficits, presence of diabetes insipidus (DI), and presence of anterior hypopituitarism. The factors studied for tumor control included patient age at SRS, sex, tumor type (mixed tumor vs. cystic tumor or solid tumor), margin dose, tumor volume, distance between tumor and optic nerve, ≥ 12 Gy tumor coverage, and Paddick conformity index (the coverage ratio at margin dose multiplied by selectivity ratio) [9, 10]. We investigated the factors related to higher 12 Gy coverage rate using the Mann–Whitney U test. The suggestive cutoff value for variables (tumor volume, margin dose, distance to optic nerve, 12 Gy tumor coverage, and conformity index) were determined by Youden index based on receiver operating characteristic curve analysis [11]. The data was analyzed using SPSS Statistics, version 25.0 (IBM, New York, USA).
Results
Overall survival
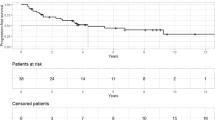
At a median of 118 months (range 23–183), 45 patients were alive and eight had died. The overall survival rate after SRS was 97.8% at 3 years, 92.7% at 5 years, and 88.5% at 10 years (Fig. 2A). At the time of death of eight patients, four had no known tumor recurrence and had undergone no additional treatment for craniopharyngioma; three patients had tumor recurrence leading to death; one patient died related to status epilepticus. None of following factors were associated with overall survival: age at first SRS (p = 0.060), sex (p = 0.064), number of prior surgical resection (p = 0.225), presence of visual deficit (p = 0.658), presence of DI (p = 0.160), and presence of anterior hypopituitarism (p = 0.511).
A Kaplan–Meier plot demonstrating overall survival rate. B Kaplan–Meier plot demonstrating progression free survival rate. C Kaplan–Meier plot comparing progression free survival rates in patients with mixed type tumors and other type tumor (only solid and only cystic tumor). Mixed tumor type was significantly associated with a poor progression free survival rate (p = 0.037). D Kaplan–Meier plot comparing progression free survival rate for craniopharyngioma close to the optic nerve and away from the optic nerve. Tumors away from the optic nerve were significantly associated with improved progression free survival rate (≥ 3 mm, p = 0.002). E Kaplan–Meier plot comparing progression free survival rate for craniopharyngioma treated with higher 12 Gy coverage and lower 12 Gy coverage. Tumors treated with higher 12 Gy coverage were significantly associated with improved progression free survival rate (≥ 85%, p < 0.001). F Box plot illustrating the relationship between distance to the optic nerve and 12 Gy coverage. Tumors away from the optic nerve were significantly associated with higher 12 Gy coverage (≥ 3 mm, p < 0.001). G In tumors near optic apparatus, Kaplan–Meier plot comparing progression free survival rate for craniopharyngioma treated with higher 12 Gy coverage and lower 12 Gy coverage. Craniopharyngiomas which undergo SRS and ≥ 85% of the tumor volume receives 12 Gy or higher had improved progression free survival rates (p = 0.027)
Tumor growth control
The overall tumor control rate was 81.0% at 3 years, 72.1% at 5 years, and 53.4% at 10 years (Fig. 2B). Twenty-four patients (one patient had two tumors) underwent additional treatment during follow-up after SRS. The median interval between the date of SRS and additional intervention was 48.2 months (range 4–297 months). Of 25 tumors, ten underwent surgical resection, three had additional SRS, two had radiation therapy, two cysts were treated with P32 implantation, four patients had surgical resection and additional SRS, one had resection and radiotherapy, one was managed with resection and bleomycin injection, one was treated with resection and P32 implantation, and one underwent cyst aspiration and P32 implantation. In univariate analysis, none of the following factors were associated with tumor control: age, sex, margin dose, maximum dose, tumor volume, and conformity index. Mixed solid-cystic tumor was significantly associated with lower tumor control rate (p = 0.037, Fig. 2C). Greater distance between tumor and the optic nerve (≥ 3 mm, p = 0.002, Fig. 2D) and ≥ 85% volume of the tumor receiving at least 12 Gy (p < 0.001, Fig. 2E) were significantly associated with improved tumor control rates (Table 2).
Multivariate analysis confirmed that mixed tumors had worse tumor control (p = 0.034, HR 4.927, 95% CI 1.13–21.51) and lower coverage with 12 Gy (≤ 85%) was associated with worse tumor control (p = 0.004, HR 22.633, 95% CI 2.63–194.44) (Table 2). The tumor control rate of mixed solid-cystic craniopharyngioma was 65.2% at 3 years, 59.3% at 5 years, and 44.5% at 10 years. In contrast the tumor control rate of patients with only solid or only cystic tumors was 90.1% at 3 years, 79.9% at 5 years, and 61.0% at 10 years (Fig. 2C). The tumor control rate of craniopharyngioma patients whose tumor was treated so that ≥ 85% received at least 12 Gy was 100% at 3 years and 93.3% at 5 and 10 years. In contrast, the tumor control rate of tumors with 12 Gy coverage < 85% was 68.1% at 3 years, 56.7% at 5 years, and 21.3% at 10 years. The cutoff value for tumor control that maximized the Youden index was 85% for the 12 Gy coverage. This analysis suggests that tumor control can be optimized by strategic planning so that at least 85% of the tumor volume receives at least 12 Gy, even if the dose at the tumor margin near the optic nerve, chiasm or pituitary stalk received a reduced dose.
Distance between tumor and optic nerve, and 12 Gy coverage rate
Radiosurgery dose plans for 61 tumors were available for retrospective review. This distance ranged from 0 to 35.1 mm, and 47 tumors were classified as close to optic apparatus (< 3 mm). Fourteen tumors with greater distance between optic nerve and tumor margin received a higher margin dose (median of 14.5 Gy, range 12–20 Gy) compared to tumors near the optic nerve (median margin dose of 12 Gy, range 9–20 Gy) (p < 0.001). The only patient with no tumor-nerve gap and whose margin dose was 20 Gy was completely blind prior to SRS. Thirteen of 14 tumors with > 3 mm distance from the optic nerve were stable during follow up. Tumor type and tumor volume were not associated with higher percent volume 12 Gy tumor coverage rate. Higher margin dose (≥ 12 Gy, p < 0.001) and enough distance to optic nerve (≥ 3 mm, p < 0.001, Fig. 2F) were significantly associated with higher 12 Gy coverage rate (Table 3).
In 24 tumors close to the optic nerve (distance to optic nerve < 3 mm), ≥ 85% percent volume coverage of 12 Gy was significantly associated with better tumor control p = 0.027). The tumor control rate can be estimated to be 80% at 10 years by increasing the 85 percent volume of the tumor receiving at least 12 Gy (Fig. 2G).
Adverse radiation effect
Five patients had worsening of visual field defects at the last follow up. Four of five patients had tumor progression, but one had worse vision without tumor progression at 1 month after SRS. One patient with visual field deficit at SRS had an improved visual field deficit.
One patient had reduced anterior hypopituitarism, but one patient additional anterior endocrine function without tumor progression 24 months after SRS. Two patients developed delayed diabetes insipidus after additional resection for tumor progression.
Discussion
Craniopharyngiomas develop and grow in a narrow anatomic space wherein lies structures that critically affect quality of life—the pituitary stalk and gland, the optic nerve and chiasm, and critical vascular structures that affect the hypothalamus and basal ganglia [12]. Gross total resection as the first line management option confirms the pathological diagnosis and facilitates visual recovery in patients with symptomatic optic neuropathy related to tumor compression. The rate of gross total resection varies between 59 and 90% [2, 13,14,15,16]. Many patients require either additional resection, radiosurgery, intracavitary radiation for cyst progression, or in rare cases fractionated radiation therapy to achieve long tumor control [2, 17].
Overall survival
Previous studies reported overall survival for craniopharyngioma ranging from 91.5 to 97.1% at 5 years and from 82.0 to 91.0% at 10 years after SRS [4,5,6, 18]. Most long survival patients had multimodality management. Such strategies should continue to focus on methods to improve quality of life, including visual, hormonal, and neurocognitive preservation. The use of SRS is an important option that can improve tumor control with reduced risks compared to additional surgical resection [15, 19].
Tumor control
In previous reports of craniopharyngioma patients who underwent SRS, tumor control rates varied from 73.1 to 84.8% at 3 years, 60.8 to 73.6% at 5 years, and 42.6 to 60.2% at 10 years [4,5,6,7,8, 18]. Results of the current study are similar to prior publications. In previous reports, an adequate distance between the tumor and the optic nerve, smaller tumor volume, higher margin dose, and normal vision were reported as predictors for better tumor control [4, 6,7,8, 20]. In our univariate analysis, a greater percent of the tumor receiving at least 12 Gy, greater distance between the optic nerve and the tumor, and purely solid or cystic tumors were factors associated with better tumor control. In the multivariate analysis, greater percent receiving 12 Gy coverage and pure solid or cystic tumors again were significant. An increased distance between the tumor and the optic nerve was not significant in the multivariate analysis because a small number of patients who were blind at the time of SRS were treated with complete coverage and long-term tumor control. Of note a single patient whose tumor margin dose was 18 Gy and the 100% of the tumor received ≥ 12 Gy had a late progression at 164 months after SRS. This single example emphasizes the importance of long-term vigilance of such tumors.
Enhanced 12 Gy coverage improved tumor control
In order to prevent tumor growth while reducing the risk of optic neuropathy, we have advocated a prescribed margin dose of 12 Gy for better tumor control. Because of the anatomic location of craniopharyngiomas, including those that are in contact with the optic system, dose reduction near such critical structures are needed to reduce the risk of further optic neuropathy. This is the first study to suggest that strict conformity (100% of the tumor receives at least 12 Gy) may not be necessary. Instead we found that when ≥ 85% of the tumor volume receives 12 Gy or greater, tumor control can be maximized while reducing the risk of optic nerve injury. Risk in radiosurgery does not come from what the target gets but rather from what adjacent brain, cranial nerves, or pituitary gland receive. The cutoff value for tumor control that maximized the Youden index was 0.85 for the 12 Gy coverage. Higher margin dose (≥ 12 Gy, p < 0.001) and greater distance from optic system (≥ 3 mm, p < 0.001) also were significant. For the tumors near the optic nerve, it is necessary to reduce the tumor margin dose so that the optic nerve receives a tolerance dose [21]. Although this study had only 24 tumors that were near the optic nerve, higher 12 Gy coverage rate was significantly associated with improved tumor control (≥ 85%, p = 0.027). Even if the craniopharyngioma is close to the optic nerve, the tumor control was improved by ensuring that ≥ 85% received at least 12 Gy. Dose fall off (selectivity) using the Gamma knife also is improved by using small isocenters with selective beam blockage.
SRS conformity and selectivity concepts
Since the advent of advanced dose planning strategies, various authors have emphasized two critical aspects of SRS. First the tumor must be precisely defined (contouring) after which using either prospective or inverse dose planning, the tumor must have 100% conformity of the dose delivery [9]. Secondly, the dose outside the tumor must fall off as rapidly as possible to reduce injury to nearby critical structures (selectivity). Paddick et al. have developed conformity indices that are helpful guides to dose planning especially in less experienced planners [9]. As yet no data shows that both tumor response and risk reduction are improved by conformity concepts. It is important to understand that risk comes from what adjacent critical structures outside the target receive, not from what the target itself receives. The conformity index does not take into account the location of critical structures in relation to the tumor. In a study of brain metastases SRS, Aiyama et al. had reported that tumor control actually was reduced as the conformity index was increased [22].
The present study found that for tumors such as craniopharyngioma the conformity index had no relationship to tumor control or to adverse radiation effect. Instead when deliberately reducing the tumor dose near the optic system while at the same time ensuring that at least 85% of the tumor received ≥ 12 Gy, tumor control was improved and risk was low (Fig. 3). This finding parallels the report of Kano et al. who demonstrated that AVM obliteration results can be improved significantly when at least 63% of the AVM receives a dose ≥ 20 Gy; this also confirmed that margin dose critical predictor of successful obliteration [23]. Using the Gamma knife, we can increase the percent volume receiving higher dose by adding low weighted isocenters within the target volume while not changing the prescription margin dose or conformity [23].
Axial MRI (A) with coronal (B) and sagittal (C) reformatted images showing GammaPlan for a craniopharyngioma showing 91% tumor coverage with 12 Gy and conformity index of 0.66. Part of the tumor close to right optic nerve and optic chiasm was treated with doses less than 12 Gy. Five-year follow-up MRI (with 12 Gy line projected from dose plan) shows significantly regressed and stable tumor. This figure illustrates the value of ≥ 85% coverage with 12 Gy. The CI is not clinically significant for tumor control
Adverse radiation effect
In a previous large SRS craniopharyngioma series, the rate of visual field deterioration without tumor progression was 0–2.2% [4, 5, 7]. In the present study, a single patient had visual field deterioration unrelated to tumor progression. The patient was treated with a margin dose of 9 Gy and the dose reduction to the optic nerve was performed by selective beam channel blocking of isocenters closer to the optic nerve. The cause of this presumed radiation related optic neuropathy is unclear. If the maximum point dose delivered to the optic nerve is 10 Gy and the average dose is < 8 Gy, the risk of optic neuropathy is < 1% in patients without prior radiation therapy [24, 25]. This is achieved by enhancing selectivity using only small isocenters near the target periphery and by selective beam channel blocking. Margin doses can be reduced to assist, as long as 85% of the tumor receives a dose of 12 Gy. This same concept applies to pituitary gland and stalk radiation dose.
In the current study, a single patient at 24 months developed additional hormone loss in the absence of tumor progression. This patient was treated with a margin dose of 11 Gy.
Study limitations
This is a retrospective study. Since the clinical course of patients with craniopharyngioma is complex and their incidence is rare, it is difficult to have a homogenous cohort. Both selection bias and prior treatment bias may interact. Since no patient in this study also had fractionated radiation therapy, we cannot assess the benefit or the risks of this alternative strategy.
Conclusion
Craniopharyngiomas are complex tumors that often require multimodality management. Tumor control and risk reduction using SRS after initial surgery were improved by ensuring that at least 85% of the tumor received ≥ 12 Gy while the optic apparatus dose is reduced to below 10 Gy. In this study the conformity index had no correlation with tumor control.
Data availability
Research data are not available at this time.
References
Müller HL, Merchant TE, Warmuth-Metz M et al (2019) Craniopharyngioma. Nat Rev Dis Prim 5:1–19
Park HJ, Dho YS, Kim JH et al (2020) Recurrence rate and prognostic factors for the adult craniopharyngiomas in long-term follow-up. World Neurosurg 133:e211–e217
Hasegawa T, Kobayashi T, Kida Y (2010) Tolerance of the optic apparatus in single-fraction irradiation using stereotactic radiosurgery: evaluation in 100 patients with craniopharyngioma. Neurosurgery 66:688–694
Lee CC, Yang HC, Chen CJ et al (2014) Gamma Knife surgery for craniopharyngioma: report on a 20-year experience. J Neurosurg 121:167–178
Niranjan A, Kano HIK, Athieu DAM et al (2010) Radiosurgery for craniopharyngioma. Int J Radiation Oncology Biol Phys 78:64–71
Tsugawa T, Kobayashi T, Hasegawa T et al (2020) Gamma knife surgery for residual or recurrent craniopharyngioma after surgical resection: a multi-institutional retrospective study in Japan. Cureus 12:1–15
Xu Z, Yen CP, Schlesinger D et al (2011) Outcomes of Gamma Knife surgery for craniopharyngiomas. J Neurooncol 104:305–313
Kobayashi T, Mori Y, Tsugawa T et al (2012) Prognostic factors for tumor recurrence after gamma knife radiosurgery of partially resected and recurrent craniopharyngiomas. Nagoya J Med Sci 74:141–147
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. J Neurosurg 93:219–222
Feuvret L, Noël G, Mazeron JJ et al (2006) Conformity index: a review. Int J Radiat Oncol Biol Phys 64:333–342
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Yaşargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P (1990) Total removal of craniopharyngioma. J Neurosurg 73:3–11
Van Effenterre R, Boch AL (2002) Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 97:3–11
Fahlbusch R, Honegger J, Paulus W et al (1999) Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg 90:237–250
Morisako H, Goto T, Goto H et al (2016) Aggressive surgery based on an anatomical subclassification of craniopharyngiomas. Neurosurg Focus 41:1–15
Mortini P, Losa M, Pozzobon G et al (2011) Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series—clinical article. J Neurosurg 114:1350–1359
Kobayashi T, Kida Y, Mori Y et al (2005) Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg 103:482–488
Caldarelli M, Massimi L, Tamburrini G et al (2005) Long-term results of the surgical treatment of craniopharyngioma: the experience at the Policlinico Gemelli, Catholic University, Rome. Child’s Nerv Syst 21:747–757
Dho YS, Kim YH, Kim JW et al (2018) Optimal strategy of gamma knife radiosurgery for craniopharyngiomas. J Neurooncol 140:135–143
Tishler RB, Loeffler JS, Lunsford LD et al (1993) Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys 27:215–221
Aiyama H, Yamamoto M, Kawabe T et al (2018) Clinical significance of conformity index and gradient index in patients undergoing stereotactic radiosurgery for a single metastatic tumor. J Neurosurg 129:103–110
Kano H, Flickinger JC, Nakamura A et al (2019) How to improve obliteration rates during volume-staged stereotactic radiosurgery for large arteriovenous malformations. J Neurosurg 130:1809–1816
Milano MT, Grimm J, Soltys SG et al (2018) Single- and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2018.01.053
Girkin CA, Comey CH, Lunsford LD et al (1997) Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology 104:1634–1643
Funding
None.
Author information
Authors and Affiliations
Contributions
AO: Data collection, statistics, drafting and approval of final version of manuscript. AN: Review of data and statistics, critical review of manuscript and approval of the final version. HK: review of statistics, review and approval of final version of manuscript. JCF: Review and approval of final version of manuscript. LDL: Critical review and approval of final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Lunsford reported being a consultant for the Insightec Data and Safety Monitoring Board and an AB Elekta stockholder.
Ethical approval
This study (PITT_PRO_STUDY20010256) was reviewed and approved by Institutional Review Board of University of Pittsburgh.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ogino, A., Niranjan, A., Kano, H. et al. Optimizing stereotactic radiosurgery in patients with recurrent or residual craniopharyngiomas. J Neurooncol 154, 113–120 (2021). https://doi.org/10.1007/s11060-021-03806-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03806-7