Abstract
Purpose
Craniopharyngiomas account for 5.6–13% of intracranial tumors in children. Despite being histologically benign, these tumors remain a major neurosurgical challenge because of the typical tight adherence to adjacent critical structures. The optimal therapeutic approach for this disease is controversial. Large cystic size and adherence to neurovascular, neuroendocrine, and optic structures without a clear line of cleavage make complete resection problematic and often hazardous. For these reasons, partial resection and adjuvant treatment play an important role. Post-operative radiation therapy (RT) following either complete or incomplete tumor removal is associated with significantly decreased recurrence rates. The aim of this review is to analyze the potential advantage of the most modern technical advancements for RT of craniopharyngiomas.
Methods
This narrative review on the topic of craniopharyngiomas was based on published data available on PUBMED/Medline. All data concerning adjuvant or upfront radiation therapy treatment of craniopharyngioma were reviewed and summarized. A more detailed analysis of fractionated frameless steretactic radiosurgery of these tumors is provided as well.
Results
We reviewed the possible improvement provided by intensity modulated beams, arc therapy, image guidance, proton radiation, and fractionated stereotactic radiosurgery. Many published findings on outcome and toxicity after RT involve the use of relatively outdated RT techniques. Technologic improvements in imaging, radiation planning, and delivery have improved the distribution of radiation doses to desired target volumes and reduced the dose to nearby critical normal tissues. Currently available techniques, providing image guidance and improved radiation doses distribution profile, have shown to maintain the efficacy of conventional techniques while significantly reducing the toxicity.
Conclusions
Image-guided radiosurgery holds the dose distributions and precision of frame-based techniques with the remarkable advantage of multiple-session treatments that are better tolerated by sensitive peritumoral structures, such as the optic pathway and hypothalamus. This, together with the comfort of a frameless technique, candidates frameless image-guided radiosurgery to be the first option for the adjuvant post-operative treatment of craniopharyngiomas in children and young adults when total resection cannot be achieved, in particular those with hypothalamic involvement, and when the residual tumor is mostly solid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas account for 2–5% of all primary intracranial neoplasms and 5.6–13% of intracranial tumors in children. Despite being histologically benign, these tumors remain a major neurosurgical challenge because of the typical tight adherence to adjacent critical structures lying in the parasellar area including vessels, optic pathway, hypothalamus, and pituitary stalk. This often prevents from a definitive surgical cure and even after apparently successful surgical treatment, craniopharyngioma may relapse. For this reason, several reports in the literature refer to childhood-onset craniopharyngioma as a chronic disease [1, 2]. Thus, 10 years after the operation, 48% of patients exhibit major visual field deficits; the probability of hyperphagia and weight gain, manifestations of severe hypothalamic injury, is around 40% [3]. Permanent motor deficits, epilepsy, psychological disorders requiring treatment, and complete dependency for basal daily activities were detected in 11%, 12%, 15% and 9% of the subjects, respectively. Craniopharyngiomas are, not surprisingly, associated with decreased survival expectations [4].
The optimal therapeutic approach is controversial. If complete removal is the primary goal of treatment, large size and adherence to vital neurovascular structures without a clear line of cleavage make complete resection problematic and often hazardous. In particular, gross total resection should be avoided in cases of hypothalamic involvement to prevent further hypothalamic damage, which exacerbates sequelae and finally results in lower probability of long-term survival and lower quality of life [5, 6]. The recurrence is a surgical challenge even worse than the first treatment. Perioperative mortality of recurrent tumor surgery is higher than that observed after primary surgery. Furthermore, the rate of total removal is dramatically limited.
For these reasons, minimal-risk surgery and adjuvant treatment play an important role. Post-operative radiation therapy (RT) following either complete or incomplete tumor removal is associated with significantly decreased recurrence rate. On the other hand, RT may cause relevant toxicity. Many published findings on outcome and toxicity after RT involve the use of relatively outdated RT techniques. Technologic improvements in imaging and in radiation planning and delivery have improved the conformality of radiation doses to target volumes and reduced the doses to nearby normal tissues. The aim of this review is to analyze the potential advantage of the most modern technical advancements in image guidance and conformal delivery of radiation to treat craniopharyngiomas.
Which results can be expected by radiation therapy of craniopharyngiomas?
Radiotherapy has been used for decades now to treat craniopharyngiomas, but available data are rather inconsistent due to the low incidence of craniopharyngioma and the lack of guidelines). In external beam radiotherapy (EBRT), photon beam radiation was delivered using a limited number of fixed fields, usually two to six, on a target defined on the base of two-dimensional (2D) conventional and more recently three-dimensional (3D) conformal radiotherapy planning. Because of this limited number of fields used, the final radiation dose was less conformal to the target. This, together with the lack of image guidance, determines that large portions of normal tissues receive potentially harmful radiation doses. Thus, 25/30 fractions and a daily dose of 1.8/2 Gy (a dose of 50.4 to 54 Gy at daily fractions of 1.8 Gy) were conventionally used to take advantage of the different sensitivity to radiation of the tumor tissue as compared to normal brain and cranial nerves when delivered in this range of daily doses and number of fractions.
To understand the therapeutic potential of RT, it is worth mentioning two of studies. The data of the German multicenter trial “Kraniopharingeom 2000” showed that irradiated patients had an 88% lower risk of recurrence/progression compared with patients treated without irradiation [7].
In a prognostic univariate analysis of 122 patients from the University of California [3], it was demonstrated that there was no significant difference in progression-free survival (PFS) and overall survival (OS) between patients treated with gross total resection (GTR) or subtotal resection (STR) + RT. Also, STR alone resulted in significantly shortened PFS compared to STR + RT or GTR (p < 0.001 for both). Furthermore, STR was associated with significantly shortened OS compared to STR + RT ( = 0.050) and trended to shorter OS compared to GTR (p = 0.066). On the other hand, GTR was associated with significantly greater risk of developing diabetes insipidus (56.3 vs. 13.3% with STR + RT) and panhypopituitarism (54.8 vs. 26.7% with STR + RT) [3].
Is radiation therapy toxic?
As mentioned above, the use of less conformal irradiation techniques carries the risk of a substantial irradiation of the normal structures surrounding the tumor with a definite risk of radiation-induced complications. These include mainly visual deficits, endocrinological dysfunctions, and neurocognitive deficits.
Visual deficits
In studies where a two-dimensional (2D) planning technique was used, the rates of visual deficits ranged 0–24% [8,9,10,11,12,13,14,15]. The risk increased significantly when the total dose overcomes 55 Gy [16]. Thus, in modern three-dimensional conformal radiotherapy (3DCRT), the doses used are 50–54 Gy in 1.8–2 Gy daily fraction and reported incidence of visual impairment ranges 0–2.5% [17,18,19,20,21]. It is worth mentioning that comparative series have been published that describe that permanent visual impairment is twice as common in surgical (13–54%) than in radiotherapy (6–24%) subgroups [3, 8, 15, 22].
Endocrinological dysfunctions
In comparative retrospective series, it has been shown that there is no difference in terms of pituitary morbidity between surgery alone or in combination with RT. [8, 11, 15, 22] As mentioned above, Schoenfeld et al. [3] reported that GTR was associated with significantly higher risk of developing diabetes insipidus compared with STR + RT (56.3 versus 13.3%) and panhypopituitarism (54.8 versus 26.7%). Nonetheless, the role of RT in causing endocrinological dysfunctions must not be underestimated. In particular, RT-induced hypopituitarism is more common in pediatric patients than in adult patients and with nonconformal radiation doses exceeding 61 Gy [16].
In the Royal Marsden series, after limited surgery and 2D planned radiation treatment, 25% required anti-diuretic hormone (7% new patients), 41% growth hormone (GH) (11% new patients), 66% thyroid supplements (56% new patients), 39% gonadal replacements (27% new patients), and 59% steroid supplements (52% new patients) [23].
Hypothalamic dysfunction leading to obesity, severe electrolyte imbalance, temperature dysregulation, and cognitive impairment is the worst consequence of either a craniopharyngioma or its subsequent treatment. The onset of new post-operative hypothalamic deficits has been reported in the range of 27–70% after GTR [8, 15, 24,25,26,27,28,29,30,31,32], but no significant correlation between radiotherapy and hypothalamic morbidity has been reported in series of craniopharyngiomas irradiated with 2D RT [8, 10, 11, 13,14,15,16,17,18, 20,21,22,23, 27, 33, 34] or when analyzing only series using highly conformal techniques [17, 18, 20, 21, 34].
Neurocognitive and neuropsychological dysfunction
Two different studies report data on neurocognitive and neuropsychological impairment of patients with craniopharyngioma who were irradiated in the last two decades [11, 34]. Merchant et al. [11] [37] reported that patients who received GTR had a greater decline in IQ scores (mean of 9.8 point decline) as compared with a group in which safe resection was followed by RT (mean of 1.25 points). In a prospective series, including exclusively pediatric patients treated with 3DCRT, the same group described a greater decline in IQ correlated with radiation volume receiving > 45 Gy, treatment at a younger age (< 7 years), extensive surgery, multiple surgical procedures, cerebrospinal fluid shunting, and Ommaya reservoir location [34].
What is fractionated stereotactic radiotherapy?
Fractionated stereotactic radiotherapy (FSRT) represents an evolution of external beam RT. It is usually delivered using a linear accelerator-based system with a relocatable (not invasively fixed to the patient head, i.e., using a bite) stereotactic frame for patient immobilization [17, 18, 20, 21]. These systems may be combined with high-resolution stereotactic image techniques. Multileaf or micro-multileaf collimators (micro-MLC) have replaced traditional cylindrically shaped collimators. This allows the conformal shaping of the radiation beam while it is delivered with dose distributions that are much more favorable [17, 20]. Modern linacs such as Novalis (BrainLAB AG, Heimstetten, Germany), Versa HD (Elekta AB, Stockholm, Sweden), and TrueBeam STx and EDGE (Varian, Medical Systems, Palo Alto, CA) have image guidance and dosimetric characteristics that make them suitable for FSRT applications. Conventional fractionation (25/30 daily fractions of 1.8/2 Gy) is normally adopted. The gross tumor volume (GTV) contains all solid and cystic parts of the craniopharyngeoma and is expanded to the clinical target volume (CTV) by a 3- to 5-mm margin.
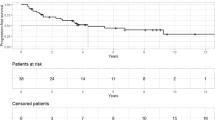
Local control rates are generally high ranging between 62 and 100% at 10 years, whereas reported morbidity is minimal with visual impairment risk, which is close to 0% [17, 20] (Table 1).
Overall reported treatment-induced endocrine morbidity tends to be lower in series using minimal-risk surgery and FSRT: 2.5% new partial hypopituitarism and 2.5% new panhypopituitarism in one series [17]. In the series from Minniti et al., only 3/10 (30%) patients with a normal pituitary function before FSRT developed new endocrine deficits during follow-up [20].
Is proton therapy the gold standard for pediatric craniopharyngiomas?
Protons have an intrinsic inverse dose profile when they cross tissues. While with X-ray the dose absorbed by the tissue has an exponential decay with increasing thickness. For protons and ions, indeed, the dose increases with increasing thickness until a specific point is reached, at the end of the particle range, in which all the energy of the incident beam is delivered (this point is called Bragg peak). The Bragg peak can be modulated in proton beam therapy (PBT) using magnets and also shaped to achieve high conformal irradiation. Protons’ dose distribution results in a significantly reduced integral dose of radiation to the surrounding normal tissues as compared to 3D conformal RT and is therefore considered the best standard for pediatric patients.
In two early clinical series of patients treated with PBT, local control rates were 93% at 5 years [33, 35] Luu et al. [33] [47] treated 16 patients entirely with PBT with a daily dose of 1.8 cobalt Gy equivalent (CGE; 1 proton Gy = 1.1 CGE) for a total CGE of 50.4 to 59.4. Visual and endocrine deteriorations were similar to those obtained with other conformal techniques. Fitzek et al. [35] reported that visual complications occurred in 2/15 patients and 4/15 children required testosterone replacement after irradiation. No treatment-related neurocognitive deficits were recorded within the follow-up period and functional status, IQ, and professional abilities were unaltered after PBT [33]. Luu et al. [33] reported one case of panhypopituitarism and one case of cerebrovascular event among 16 patients treated with PBT [33].
In a recent comparative study, Bishop et al. [36] analyzed data of 52 children treated with PBT (n = 21) or intensity modulated radiotherapy (IMRT) (n = 31) at two institutions. At the 3-year follow-up, neither OS nor disease control differed between treatment groups. During therapy, 40% of patients had cyst growth (20% requiring intervention). Immediately after therapy, 17 out of 52 patients (33%) had cyst growth (transient in 14), more commonly in the IMRT group (42% vs. 19% PBT, p = 0.082), and 27% experienced late cyst growth (32% IMRT, 19% PBT, p = 0.353), with surgical intervention required in 40% of the cases. Toxicity did not differ between groups. Patients given radiation as salvage therapy (for recurrence) rather than adjuvant therapy had higher rates of visual and endocrine (p = 0.017 and 0.024) dysfunction [36].
What is single fraction stereotactic radiosurgery?
In gamma knife radiosurgery (GKS), multiple cobalt-sourced beams (201) are arranged in a hemispherical array and then collimated to create spherical treatment volumes, the isocenter, of variable diameter. Because of the geometry of dose delivery (i.e., the isocenter is generated by 201 radiation beams), the radiation dose fall-off is extremely steep. This is a great advantage for tumors of limited size (i.e., < 3 cm), because a very limited portion of normal brain will receive a potentially harmful dose. To get the maximal conformal dose delivery, multiple spherical isocenters are used to get a 3D volume extremely close to the real volume of the tumor. The GKS requires the patient to be immobilized using a surgically fixed frame to deliver high doses into a stereotactically defined point and therefore typically delivers treatments in a single session.
In the published gamma knife series (Table 1), tumor control rates ranged from 87 to 94%, prescribing a marginal dose of 12–14 Gy in one single fraction [37,38,39,40,41,42,43,44,45]. The reported rates of complications directly attributable to gamma knife radiosurgery include visual deterioration in 0–38%, endocrinological dysfunction in 0–19%, and neurological complications in 0–2% [37,38,39,40,41,42,43,44]. In a recent published series, spanning 20 years of experience and including 137 patients [46], the progression-free survival rates for patients after GKS for a residual or recurrent craniopharyngioma were 70% and 43.8% at the 5- and 10-year follow-up periods, respectively; hypopituitarism was recorded in 8%, adverse radiation effects in 1.5%, visual deterioration in 1.5%, and new-onset cranial nerve palsy in 0.7%. The results of this study suggest that GKS is a relatively safe modality for the treatment of recurrent or residual craniopharyngiomas and is associated with improved tumor control and in-field progression-free survival rates. Acceptable rates of complications were observed [46].
Frameless real-time image-guided robotic radiosurgery
The CyberKnife (Accuray, Sunnyvale, CA) is a frameless system for stereotactic radiosurgery [47,48,49,50,51,52,53,54,55]. It uses noninvasive image-guided localization, a lightweight high-energy radiation source, and a robotic delivery system to deliver stereotactic radiosurgery (SRS) in single or multiple sessions). Frameless technology offers the opportunity to perform multisession radiosurgery or “hypofractionated” treatments (usually two to five fractions). Such strongly hypofractionated treatments allows the delivery of still ablative, radiosurgical doses to the lesion in association with an enhanced protection of the adjacent tissues receiving lower doses and lower dose rates but also being allowed precious time to recover between fractions (Fig. 1).
Patient setup, treatment delivery, and quality assurance
With the CyberKnife, patients are immobilized on the treatment couch utilizing a mask that was custom-fitted at the time of the planning CT. In-room lasers define the center of the imaging system and provide the radiation therapists with an estimate of the patient’s initial alignment. For treatment tracking, the 6D skull tracking mode is used. The treatment location system (TLS) compares orthogonal kV X-ray pairs, i.e., live images, obtained during the patient setup, to the planning system-generated digitally reconstructed radiographs (DRR) obtained from the planning CT scan. The patient is then aligned to within a few millimeters of the final treatment location by the robotic couch. The system recommends shifts and rotations, including pitch, roll, and yaw, which were then confirmed and implemented by the therapists. This process continues iteratively until the residual offsets are within the acceptable values, i.e., < 1 mm in translation and < 0.5° in rotation that are within the limits corrected by the robotic arm during treatment. The TLS calls for an X-ray image and confirmation of the patient’s position at defined time intervals. A frequency of 15 s can be used for patients to minimize any intrafraction inaccuracy. Any residual offset between the patient’s simulation position and that at the time of treatment is accounted for through robotic positional adjustments. At each beam position, the robot adjusts the linac position according to the patient offsets.
Safety in treatment of craniopharyngiomas and other perioptic tumors
As a matter of fact, tumors lying < 3 mm from the optic nerves and chiasm, as typically occurring in many craniopharyngiomas, are not amenable to single fraction radiosurgery because of the high sensitivity to radiations of these structures (i.e., usually a maximum of 8–10 Gy can be delivered to optic nerve and chiasm). Multisession radiosurgery of lesions affecting the optic nerves substantially enhances treatment safety and prevention of dreaded neurological complications [49, 56,57,58]. Furthermore, the CyberKnife is not limited to deliver spherical dose distributions (i.e., treatment planning and delivery can be nonisocentric). Nonisocentric planning provides a straightforward approach for the treatment of irregularly shaped lesions.
The first series reporting of the use of CyberKnife multisession radiosurgery of perioptic tumors was that of the Stanford University, where the CyberKnife radiosurgery system was developed [56]. Adler et al. treated 49 patients with perioptic tumors, including 27 meningiomas, in two to five sessions with a cumulative average marginal dose of 20.3 Gy. At an intermediate-term follow-up of 49 months (range, 6–96 months), vision was unchanged post-radiosurgery in 38 patients improved in eight (16%) and worse in three (6%). In two of these three patients, the deterioration was due to tumor progression. On the other hand, the use of a more aggressive treatment (21.0 Gy delivered in three sessions) was associated with loss of vision in the remaining patient [56]. In contrast, the protocol used by Romanelli et al. in four patients with optic nerve sheath meningiomas and consisting of 20 Gy delivered in four sessions induced fast resolution of visual symptoms and good preservation of vision after a mean follow-up of 37 months [59, 60]. In the largest series on meningiomas treated with the CyberKnife, Colombo et al. [61] treated 29 perioptic meningiomas using up to 5 fractions and up to 25.0 Gy. Authors recorded a visual worsening in two cases (1% of the overall series); again, this was ascribed to the tumor progression. Marchetti et al. [62] treated 21 patients with optic nerve sheath meningiomas with 25 Gy in 5 fractions. No patient had visual deterioration at a mean follow-up of 30 months; visual improvement was recorded in 35% of patients.
Conti et al. [49] treated 64 patients with perioptic meningiomas in 2–15 fractions. Neither radio-induced optic neuropathy, nor tumor progression was recorded at an overall mean follow-up of 32 ± 23 months.
Two CyberKnife series, specifically addressing the treatment of craniopharyngioma, are currently available [63, 64].
Tumor control rates were 91% at 2 years [64] and 85% at 3 years [63]. Lee et al. [64] delivered a mean marginal dose of 21.6 Gy (range 18–38 Gy) to a mean isodose line of 75%, in three to ten fractions to 11 patients (mean age of 34.5 years), with residual craniopharyngiomas within 2 mm of the optic apparatus or pituitary gland, using the CyberKnife SRS system. The mean target volume was 6 cm3 and was most often located in the suprasellar region and in ten cases was found to be against or displacing the optic nerve or chiasm. The mean follow-up time was 15.4 months (range 4–64 months). All ten patients with visual field or acuity problems either improved or remained stable after CyberKnife radiosurgery. In this series, treatment plans were designed to keep the dose experienced by the optic apparatus to < 5 Gy during any single session. The volume of the optic apparatus that received 80% of the prescribed dose was < 0.05 cm3, whereas the volume that received 50% of the dose was < 0.5 cm3. Therefore, the actual volume of the optic segment that received 5 Gy would be small relative to the total volume of the optic apparatus.
Iwata et al. [63] treated three cases in a single fraction to a marginal dose of 13–16 Gy, whereas the other 40 cases were treated in two to five fractions to a marginal dose of 13–25 Gy. The five-fraction schedule was used for young patients (< 20 years old) and for those with tumors that were large (> 15 cm3) or adjacent to optic pathways (< 2 mm). Tumor volumes ranged from 0.09 to 20.8 cm3 (median 2.0 cm3). The median follow-up period was 40 months (range 12–92 months). The 3-year overall survival and local control rates were 100 and 85%, respectively. In-field cyst enlargement was observed in nine patients. These tumors had significantly larger volumes (mean 6.9 cm3; 95% confidence interval, CI, 2.8–10.9 cm3) than the 34 controlled tumors (2.9 cm3; CI 1.5–4.3 cm3) (p = 0.02). Out-field tumor regrowth was observed in four patients. No radiation-induced symptomatic visual disorder or brain necrosis was observed. Hypopituitarism was observed in only one patient.
Conclusions
A large amount of data has rather clearly shown that post-operative RT following either complete or incomplete tumor removal is associated with significantly decreased recurrence rate. On the other hand, RT may result toxic for parasellar normal structures especially in young patients. Currently available techniques, providing image guidance and improved radiation doses distribution profile, have shown to maintain the efficacy of conventional techniques while significantly reducing the toxicity. The best technique for pediatric patients still needs to be determined. Actually, FSRT has shown excellent results, but still requires the use of a conventional fractionation scheme lasting 5 to 6 weeks to be completed. Protons have a clear advantage over photons because of their physical profile. They are generated by cumbersome systems currently available in few centers and the lack of demonstrated superiority currently outweighs the costs for this indication. Furthermore, their modulation is less refined than that of photons which are generated by very light linac mounted over a gantry or a robotic arm and efficiently moved around the patient head delivering hundreds of beams to minimize the effect on the normal tissue. Frame-based radiosurgery is a treatment that can be typically offered to adult patients because of the necessity of an invasively fixed frame and can be used to lesion of moderate size with a distance from the optic structures. Frameless radiosurgery holds the dose distributions and precision of frame-based techniques with the remarkable advantage of multiple-session treatments that are better tolerated by sensitive peritumoral structures, such as the optic pathway and hypothalamus. This, together with the comfort of a frameless technique, candidates frameless image-guided radiosurgery to be the first option for the adjuvant post-operative treatment of craniopharyngiomas in children and young adults when total resection cannot be achieved, in particular those with hypothalamic involvement, and when the residual tumor is mostly solid.
References
Muller H (2018) Craniopharyngioma - a chronic disease. Swiss Med Wkly 148:w14548
Muller HL (2015) Craniopharyngioma: long-term consequences of a chronic disease. Expert Rev Neurother 15:1241–1244
Schoenfeld A, Pekmezci M, Barnes MJ, Tihan T, Gupta N, Lamborn KR, Banerjee A, Mueller S, Chang S, Berger MS, Haas-Kogan D (2012) The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J Neuro-Oncol 108:133–139
Bulow B, Attewell R, Hagmar L, Malmstrom P, Nordstrom CH, Erfurth EM (1998) Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J Clin Endocrinol Metab 83:3897–3904
Daubenbuchel AM, Hoffmann A, Gebhardt U, Warmuth-Metz M, Sterkenburg AS, Muller HL (2015) Hydrocephalus and hypothalamic involvement in pediatric patients with craniopharyngioma or cysts of Rathke’s pouch: impact on long-term prognosis. Eur J Endocrinol 172:561–569
Sterkenburg AS, Hoffmann A, Gebhardt U, Warmuth-Metz M, Daubenbuchel AM, Muller HL (2015) Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: newly reported long-term outcomes. Neuro-Oncology 17:1029–1038
Muller HL, Gebhardt U, Schroder S, Pohl F, Kortmann RD, Faldum A, Zwiener I, Warmuth-Metz M, Pietsch T, Calaminus G, Kolb R, Wiegand C, Sorensen N (2010) Analyses of treatment variables for patients with childhood craniopharyngioma--results of the multicenter prospective trial KRANIOPHARYNGEOM 2000 after three years of follow-up. Horm Res Paediatr 73:175–180
Hetelekidis S, Barnes PD, Tao ML, Fischer EG, Schneider L, Scott RM, Tarbell NJ (1993) 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys 27:189–195
Habrand JL, Saran F, Alapetite C, Noel G, El Boustany R, Grill J (2006) Radiation therapy in the management of craniopharyngioma: current concepts and future developments. J Pediatr Endocrinol Metab 19(Suppl 1):389–394
Varlotto JM, Flickinger JC, Kondziolka D, Lunsford LD, Deutsch M (2002) External beam irradiation of craniopharyngiomas: long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys 54:492–499
Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, Lustig RH, Kun LE (2002) Craniopharyngioma: the St. Jude Children’s Research Hospital experience 1984–2001. Int J Radiat Oncol Biol Phys 53:533–542
Karavitaki N, Wass JA (2008) Craniopharyngiomas. Endocrinol Metab Clin N Am 37:173–193 ix–x
Moon SH, Kim IH, Park SW, Kim I, Hong S, Park CI, Wang KC, Cho BK (2005) Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas--a study in single institute. Childs Nerv Syst 21:799–807
Pemberton LS, Dougal M, Magee B, Gattamaneni HR (2005) Experience of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol 77:99–104
Lin LL, El Naqa I, Leonard JR, Park TS, Hollander AS, Michalski JM, Mansur DB (2008) Long-term outcome in children treated for craniopharyngioma with and without radiotherapy. J Neurosurg Pediatr 1:126–130
Regine WF, Mohiuddin M, Kramer S (1993) Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol 27:13–21
Combs SE, Thilmann C, Huber PE, Hoess A, Debus J, Schulz-Ertner D (2007) Achievement of long-term local control in patients with craniopharyngiomas using high precision stereotactic radiotherapy. Cancer 109:2308–2314
Kalapurakal JA, Goldman S, Hsieh YC, Tomita T, Marymont MH (2000) Clinical outcome in children with recurrent craniopharyngioma after primary surgery. Cancer J 6:388–393
Merchant TE (2006) Craniopharyngioma radiotherapy: endocrine and cognitive effects. J Pediatr Endocrinol Metab 19(Suppl 1):439–446
Minniti G, Saran F, Traish D, Soomal R, Sardell S, Gonsalves A, Ashley S, Warrington J, Burke K, Mosleh-Shirazi A, Brada M (2007) Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngiomas. Radiother Oncol 82:90–95
Selch MT, DeSalles AA, Wade M, Lee SP, Solberg TD, Wallace RE, Ford JM, Rubino G, Cabatan-Awang C, Withers HR (2002) Initial clinical results of stereotactic radiotherapy for the treatment of craniopharyngiomas. Technol Cancer Res Treat 1:51–59
Karavitaki N, Brufani C, Warner JT, Adams CB, Richards P, Ansorge O, Shine B, Turner HE, Wass JA (2005) Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol 62:397–409
Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJ, Marsh H, Brada M (1993) Craniopharyngioma--a long-term result following limited surgery and radiotherapy. Radiother Oncol 26:1–10
Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI (1992) Aggressive surgical management of craniopharyngiomas in children. J Neurosurg 76:47–52
Honegger J, Buchfelder M, Fahlbusch R (1999) Surgical treatment of craniopharyngiomas: endocrinological results. J Neurosurg 90:251–257
Poretti A, Grotzer MA, Ribi K, Schonle E, Boltshauser E (2004) Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol 46:220–229
Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N, Zerah M, Bezerra M, Renier D, Pierre-Kahn A, Sainte-Rose C (2007) Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg 106:3–12
Sands SA, Milner JS, Goldberg J, Mukhi V, Moliterno JA, Maxfield C, Wisoff JH (2005) Quality of life and behavioral follow-up study of pediatric survivors of craniopharyngioma. J Neurosurg 103:302–311
Shi XE, Wu B, Fan T, Zhou ZQ, Zhang YL (2008) Craniopharyngioma: surgical experience of 309 cases in China. Clin Neurol Neurosurg 110:151–159
Tomita T, Bowman RM (2005) Craniopharyngiomas in children: surgical experience at Children’s Memorial Hospital. Childs Nerv Syst 21:729–746
Van Effenterre R, Boch AL (2002) Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 97:3–11
Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P (1990) Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg 73:3–11
Luu QT, Loredo LN, Archambeau JO, Yonemoto LT, Slater JM, Slater JD (2006) Fractionated proton radiation treatment for pediatric craniopharyngioma: preliminary report. Cancer J 12:155–159
Merchant TE, Kiehna EN, Kun LE, Mulhern RK, Li C, Xiong X, Boop FA, Sanford RA (2006) Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg 104:94–102
Fitzek MM, Linggood RM, Adams J, Munzenrider JE (2006) Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at Harvard Cyclotron Laboratory and Massachusetts General Hospital. Int J Radiat Oncol Biol Phys 64:1348–1354
Bishop AJ, Greenfield B, Mahajan A, Paulino AC, Okcu MF, Allen PK, Chintagumpala M, Kahalley LS, McAleer MF, McGovern SL, Whitehead WE, Grosshans DR (2014) Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys 90:354–361
Niranjan A, Kano H, Mathieu D, Kondziolka D, Flickinger JC, Lunsford LD (2010) Radiosurgery for craniopharyngioma. Int J Radiat Oncol Biol Phys 78:64–71
Kobayashi T (2009) Long-term results of gamma knife radiosurgery for 100 consecutive cases of craniopharyngioma and a treatment strategy. Prog Neurol Surg 22:63–76
Yomo S, Hayashi M, Chernov M, Tamura N, Izawa M, Okada Y, Hori T, Iseki H (2009) Stereotactic radiosurgery of residual or recurrent craniopharyngioma: new treatment concept using Leksell gamma knife model C with automatic positioning system. Stereotact Funct Neurosurg 87:360–367
Mokry M (1999) Craniopharyngiomas: a six year experience with Gamma Knife radiosurgery. Stereotact Funct Neurosurg 72(Suppl 1):140–149
Chung WY, Pan DH, Shiau CY, Guo WY, Wang LW (2000) Gamma knife radiosurgery for craniopharyngiomas. J Neurosurg 93(Suppl 3):47–56
Yu X, Liu Z, Li S (2000) Combined treatment with stereotactic intracavitary irradiation and gamma knife surgery for craniopharyngiomas. Stereotact Funct Neurosurg 75:117–122
Ulfarsson E, Lindquist C, Roberts M, Rahn T, Lindquist M, Thoren M, Lippitz B (2002) Gamma knife radiosurgery for craniopharyngiomas: long-term results in the first Swedish patients. J Neurosurg 97:613–622
Amendola BE, Wolf A, Coy SR, Amendola MA (2003) Role of radiosurgery in craniopharyngiomas: a preliminary report. Med Pediatr Oncol 41:123–127
Saleem MA, Hashim AS, Rashid A, Ali M (2013) Role of gamma knife radiosurgery in multimodality management of craniopharyngioma. Acta Neurochir Suppl 116:55–60
Lee CC, Yang HC, Chen CJ, Hung YC, Wu HM, Shiau CY, Guo WY, Pan DH, Chung WY, Liu KD (2014) Gamma Knife surgery for craniopharyngioma: report on a 20-year experience. J Neurosurg 121(Suppl):167–178
Alafaci C, Grasso G, Conti A, Caffo M, Salpietro FM, Tomasello F (2014) Cyberknife radiosurgery for cranial plasma cell tumor. Turk Neurosurg 24:272–275
Conti A, Pontoriero A, Iati G, Esposito F, Siniscalchi EN, Crimi S, Vinci S, Brogna A, De Ponte F, Germano A, Pergolizzi S, Tomasello F (2017) Frameless stereotactic radiosurgery for treatment of multiple sclerosis-related trigeminal neuralgia. World Neurosurg 103:702–712
Conti A, Pontoriero A, Midili F, Iati G, Siragusa C, Tomasello C, La Torre D, Cardali SM, Pergolizzi S, De Renzis C (2015) CyberKnife multisession stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for perioptic meningiomas: intermediate-term results and radiobiological considerations. Springerplus 4:37
Conti A, Pontoriero A, Ricciardi GK, Granata F, Vinci S, Angileri FF, Pergolizzi S, Alafaci C, Rizzo V, Quartarone A, Germano A, Foroni RI, De Renzis C, Tomasello F (2013) Integration of functional neuroimaging in CyberKnife radiosurgery: feasibility and dosimetric results. Neurosurg Focus 34:E5
Conti A, Pontoriero A, Salamone I, Siragusa C, Midili F, La Torre D, Calisto A, Granata F, Romanelli P, De Renzis C, Tomasello F (2009) Protecting venous structures during radiosurgery for parasagittal meningiomas. Neurosurg Focus 27:E11
Conti A, Pontoriero A, Siddi F, Iati G, Cardali S, Angileri FF, Granata F, Pergolizzi S, Germano A, Tomasello F (2016) Post-treatment edema after meningioma radiosurgery is a predictable complication. Cureus 8:e605
Pontoriero A, Conti A, Iati G, Mondello S, Aiello D, Rifatto C, Risoleti E, Mazzei M, Tomasello F, Pergolizzi S, De Renzis C (2016) Prognostic factors in patients treated with stereotactic image-guided robotic radiosurgery for brain metastases: a single-center retrospective analysis of 223 patients. Neurosurg Rev 39:495–504
Romanelli P, Conti A, Bianchi L, Bergantin A, Martinotti A, Beltramo G (2017) Image-guided robotic radiosurgery for trigeminal neuralgia. Neurosurgery
Romanelli P, Conti A, Pontoriero A, Ricciardi GK, Tomasello F, De Renzis C, Innocenzi G, Esposito V, Cantore G (2009) Role of stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of recurrent glioblastoma multiforme. Neurosurg Focus 27:E8
Adler JR Jr, Gibbs IC, Puataweepong P, Chang SD (2006) Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery 59:244–254 discussion 244–254
Pham CJ, Chang SD, Gibbs IC, Jones P, Heilbrun MP, Adler JR Jr (2004) Preliminary visual field preservation after staged CyberKnife radiosurgery for perioptic lesions. Neurosurgery 54:799–810 discussion 810-792
Puataweepong P, Dhanachai M, Hansasuta A, Dangprasert S, Swangsilpa T, Sitathanee C, Jiarpinitnun C, Vitoonpanich P, Yongvithisatid P (2016) The clinical outcome of hypofractionated stereotactic radiotherapy with CyberKnife robotic radiosurgery for perioptic pituitary adenoma. Technol Cancer Res Treat 15:NP10–NP15
Romanelli P, Bianchi L, Muacevic A, Beltramo G (2011) Staged image guided robotic radiosurgery for optic nerve sheath meningiomas. Comput Aided Surg 16:257–266
Romanelli P, Wowra B, Muacevic A (2007) Multisession CyberKnife radiosurgery for optic nerve sheath meningiomas. Neurosurg Focus 23:E11
Colombo F, Casentini L, Cavedon C, Scalchi P, Cora S, Francescon P (2009) Cyberknife radiosurgery for benign meningiomas: short-term results in 199 patients. Neurosurgery 64: A7–13
Marchetti M, Bianchi S, Milanesi I, Bergantin A, Bianchi L, Broggi G, Fariselli L (2011) Multisession radiosurgery for optic nerve sheath meningiomas--an effective option: preliminary results of a single-center experience. Neurosurgery 69:1116–1122 discussion 1122–1113
Iwata H, Tatewaki K, Inoue M, Yokota N, Baba Y, Nomura R, Shibamoto Y, Sato K (2012) Single and hypofractionated stereotactic radiotherapy with CyberKnife for craniopharyngioma. J Neuro-Oncol 106:571–577
Lee M, Kalani MY, Cheshier S, Gibbs IC, Adler JR, Chang SD (2008) Radiation therapy and CyberKnife radiosurgery in the management of craniopharyngiomas. Neurosurg Focus 24:E4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest and no competing interest.
Rights and permissions
About this article
Cite this article
Conti, A., Pontoriero, A., Ghetti, I. et al. Benefits of image-guided stereotactic hypofractionated radiation therapy as adjuvant treatment of craniopharyngiomas. A review. Childs Nerv Syst 35, 53–61 (2019). https://doi.org/10.1007/s00381-018-3954-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-018-3954-z