Abstract
In this study, poly ethylene glycol 100 stearate (PEG 100-S) was used to prepare coated solid lipid nanoparticles with loading levothyroxine sodium (levo-loaded PEG 100-S-coated SLNs) by microemulsification technique. Evaluation of the release kinetic of prepared colloidal carriers was conducted. The particle size and zeta potential of levo-loaded PEG 100-S-coated SLNs have been measured to be 187.5 nm and −23.0 mV, respectively, using photon correlation spectroscopy (PCS). Drug entrapment efficiency (EE) was calculated to be 99 %. Differential scanning calorimetry indicated that the majority of drug loaded in PEG 100-S-coated SLNs were in amorphous state which could be considered desirable for drug delivery. The purpose of this study was to develop a new nanoparticle system, consisting lipid nanoparticles coated with PEG 100-S. The modification procedure led to a reduction in the zeta potential values, varying from −40.0 to −23.0 mV for the uncoated and PEG-coated SLNs, respectively. Stability results of the nanoparticles in gastric and intestinal media show that the low pH of the gastric medium is responsible for the critical aggregation and degradation of the uncoated lipid nanoparticles. PEG 100-S-coated SLNs were more stable due to their polymer coating layer which prevented aggregation of SLNs. Consequently, it is possible that the PEG surrounds the particles reducing the attachment of enzymes and further degradation of the triglyceride cores. Shape and surface morphology of particles were determined by transition electron microscopy and scanning electron microscopy that revealed spherical shape of nanoparticles. In vitro drug release of PEG 100-S-coated SLNs was characterized using diffusion cell which showed a controlled release for drug.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid lipid nanoparticles (SLNs) have been focused since last two decades as a relatively new carrier system for drug delivery (Uner and Yener 2007; Kashanian et al. 2011; Parhi and Suresh 2010). The unique properties of SLNs such as controllable size, significant large surface area, high drug loading capacity, biocompatibility, and hydrophobicity of lipids make them an appropriate mean to carry hydrophobic drug throughout the body fluids (Cavalli et al. 2002; Chimmiri et al. 2012; Khan 2012; Ekambaram et al. 2012; Garcia-Fuentes et al. 2005a, b; Muller et al. 2000). Among various drug delivery routes, the oral way is more attractive, comfortable and rather low cost for hydrophobic drugs (Garcia-Fuentes et al. 2002). There are several works investigating the SLNs to improve the oral absorption of drugs such as camptothecin, cyclosporine A, idarubicin, tobramycin, and nystatin (Yang et al. 1999; Zhang et al. 2000; Zara et al. 2002; Cavalli et al. 2003; Khalil et al. 2013). Several studies have also increasingly focused on improving their stability in body fluids after administration by coating particles with hydrophilic polymers like poly ethylene glycol (PEG) derivatives (Uner and Yener 2007).
Levothyroxine, the same as many other hydrophobic drugs, is poorly transported through the intestinal epithelium. Levothyroxine is a drug with a narrow therapeutic index for which dosage must be titrated for each individual patient in order to achieve the necessary therapeutic effect (Blakesley 2005). Thyroid hormones affect protein, lipid and carbohydrate metabolism, influencing growth and development (Lilja et al. 2005). Diminished or absent thyroid function may result from functional deficiency, primary atrophy, partial or complete absence of the thyroid gland, or the effects of surgery, radiation, or antithyroid agents (Lilja et al. 2005). Levothyroxine may also be used for replacement or supplemental therapy in patients with secondary (pituitary) or tertiary (hypothalamic) hypothyroidism (Lilja et al. 2005; Svensson et al. 2006). The development of more convenient extended release methods for levothyroxine sodium delivery may encounter an innate challenge: the vehicle must also provide a controlled and defined dose within the narrow therapeutic window across the entire time period of drug release (Lilja et al. 2005).
The main advantages of this study is to modify the surface of SLNs for increasing intestinal absorption and decreasing nanoparticles uptake by immune system as a result of partial neutralization of strong negative or positive charge on nanocarrier surface (Uner and Yener 2007; Fang et al. 2012; Bocca et al. 1998). It is notable, surface modifications of SLNs by coating a hydrophilic polymer, i.e., PEG lead to increase in surface hydrophilicity of nanoparticles which results in increment of nanoparticles transmucosal transport for drug delivery by nasal, oral, and ocular way (Heurtault et al. 2003; Garcia-Fuentes et al. 2005c). Controlled release efficiency and intestinal wrapped drug destruction in stomach and intestine and probable absorption of drug through the intestinal mucosa are two different models for drug distribution and absorption (Damge et al. 1990; Lowe and Temple 1994; Desai et al. 1996; Jani et al. 1990; Florence 1997). The intestinal absorption of drug as a well-known and preferential way was assumed. Up to now, there is no report about using PEG-stearate coated SLNs carrier for levothyroxine oral administration therefore future clarification of the parameters governing particle uptake will surely lead to the design of new and more efficient colloidal carriers. The main approach of this study is to develop a new nanoparticulate carrier for a hydrophobic drug to be used in oral administration. The new carriers are composed of hydrophilic polymer and lipid core aimed to protect and control the release of the hydrophobic drug which is supposed to facilitate the interaction of the carrier with the intestinal mucosa and the further transport of the hydrophobic drug.
Materials and methods
Materials
Nano-suspension was prepared using the micro-emulsification technique. The following materials were obtained from the indicated sources and used in our study without further purification. Tripalmitin glyceride was purchased from Alfa Aesar (Germany). Palmitic acid and polyethylene glycol 100 stearate (PEG 100-S) were purchased from Sigma-Aldrich (St Louis, MO). Polysorbate 80 and sucrose were obtained from Merck (Darmstadt, Germany). Levothyroxine sodium was provided by Iran Hormone, Tehran, Iran. High-pressure liquid chromatography (HPLC) grade methanol and analytical grade chloroform and ethanol were also purchased from Merck. Other reagents were of analytical or HPLC grade. Double-distilled water was prepared in our laboratory.
Preparation of PEG 100-S-coated solid lipid nanoparticles
Recently, researchers developed SLN preparation techniques which are based on the dilution of microemulsions (Cavalli et al. 2002; Parhi and Suresh 2010; Subedi et al. 2009; Lai et al. 2007). Microemulsions were made by stirring an optically transparent mixture at 65–70 °C which is typically composed of a low melting fatty acid, tripalmitin glyceride (0.80 % w/w), palmitic acid (0.40 % w/w), 0.80 % w/w of PEG 100-S, and levothyroxine sodium (0.05 % w/w). Then, 5.00 ml distilled water at 65 °C containing an emulsifier poly sorbate 80 (2.00 % w/w) and co-emulsifiers (butanol) (1.00 % w/w) was added to organic phase. The hot microemulsion was dispersed in cold water (2–3 °C) under stirring (750 rpm) (Li et al. 2008). The microemulsion was dispersed in ice-cold water at a constant speed (2 ml/min) using a syringe with a needle gage of 25. Typical volume ratio of the hot microemulsion to the cold water was adjusted 1:10 (Li et al. 2008). The SLN mixture was then washed twice with water using a Dialysis bag (cut off 8,000 Da) in ultrasonic cleaning tank in order to remove as much of the remaining surfactant applied for obtaining the microemulsion (Subedi et al. 2009; Lai et al. 2007). Next, the nanoparticles suspension was centrifuged by ultracentrifuge at 40,000 rpm and 25 °C for 90 min. The separated nanoparticles were redispersed and centrifuged three times in distilled water in order to remove free drug and excess surfactant completely (Derakhshandeh et al. 2007; Souto et al. 2004, Subedi et al. 2009; Abdelbary and Fahmy 2009). A two-step method is applied to prepare the aqueous PEG 100-S coated SLN dispersions: first formulation of an oil-in-water microemulsion and second preparation of the PEG 100-S coated SLN by dispersing the warm microemulsion into cold water. The lipid including co-surfactant and the water phase were heated separately, mixed and subsequently titrated with the surfactant until preparing a microemulsion (Cavalli et al. 2002; Parhi and Suresh 2010).
Stability of PEG 100-S-coated SLN in simulated gastric media
A 1.00 % (w/v) solution of particles was incubated in acid medium (0.063 M) and 0.32 % w/v of pepsin to obtain a suspension at pH 1.2. Evaluation of the stability of coated and uncoated SLNs, which were incubated in HCl medium for 1 h, was also performed. Subsequently, SLNs were separated from the medium using centrifugation at 3,000×g for 5 min. The size of SLNs in the upper solution was reexamined by photon correlation spectroscopy (PCS). Relative turbidity is the ratio between the absorbance [optical density (OD)] at λ = 225 nm after 1 h and before incubation to estimate the degradation of coated and uncoated nanoparticles in HCl (0.063 M) (Garcia-Fuentes et al. 2002, 2005b).
Morphological characterization of PEG 100-S-coated SLNs
Size and zeta potential were measured by PCS (Nano ZS4700 nanoseries, Malvern Instruments, UK). The transmission electron microscopy (TEM) (Zeiss, EM 10C, Germany) images were taken following staining of the isolated nanoparticles with 2 % (w/v) phosphotungstic acid. Investigation of surface morphology of coated NP was performed by scanning electron microscopy (SEM, KYKY-EM3200, China) (Derakhshandeh et al. 2010; Dadashzadeh et al. 2008).
Differential scanning calorimetry
The thermal characteristics of the PEG 100-S-coated SLNs and each component were separately determined using a differential scanning calorimeter (DSC; Shimadzu DSC-60, single heating ramp 0–300 °C, 10 °C/min) under dry N2 atmosphere. 5 mg of each sample was placed in an aluminum pan. Then, the temperature was increased with the rate of 10 °C/min and the thermo-grams were recorded.
Determination of entrapment efficiency and drug loading capacity
The efficiency of drug entrapment was investigated by measuring the concentration of drug in the supernatant of ultracentrifuged PEG 100-S-coated SLNs solution. To achieve this purpose, PEG 100-S-coated SLNs solution was ultracentrifuged at 40,000 rpm for 45 min. The absorption of upper solution was determined by UV spectrophotometer at 225 nm (Agilent 8453). Levothyroxine has a sharp absorption at 225 nm, which is acceptable and reliable for UV OD measurements (Collier et al. 2011; Volpato et al. 2004; Gregorini et al. 2013; Rapaka et al. 1982; Shah et al. 2008). By this way, the quantity of free drug could be obtained. The drug entrapment efficiency and drug loading in the coated and uncoated SLNs were calculated as follows:
where EE is entrapment efficiency, DL is drug loading, W a, W s, and W L are the weight of drug added into the system, analyzed weight of drug in the supernatant, and weight of lipid added into the system, respectively (Derakhshandeh et al. 2010; Hu et al. 2005).
In vitro release of levothyroxine from PEG 100 stearate-coated solid lipid nanoparticles
The experiment was conducted using a static horizontal Franz diffusion cell to evaluate the amount of released levothyroxine (Liu et al. 2008). A cellulose acetate membrane with a molecular weight cutoff of 10,000–12,000 Da and a surface area of 2 cm2 was fixed on the diffusion cell. The receptor medium was 50 ml in volume and composed of an aqueous solution of phosphate buffer, which was stirred by a magnetic stirrer at 750 rpm to homogenize the medium (pH 7.4). 2 ml aliquots of coated and uncoated nanoparticles suspension were separately loaded onto the cellulose acetate membrane in the donor compartment. The temperature of medium was controlled at 37 °C. 1 ml of the release medium was sampled using a syringe needle and the same volume of fresh receptor medium was replaced at certain time intervals. The samples were analyzed using a spectroscopic method as described previously (Zhang et al. 2005). The experiments were repeated three times to diminish the errors.
In vitro release kinetics calculation
Various release kinetic models, presented in Table 1, were employed to explore the fitness of the obtained data (Barzegar-Jalali et al. 2008; Singhvi and Singh 2011).
Results and discussion
Drug entrapment efficiency and drug loading
The requirement to obtain a sufficient drug loading is a high solubility of the drug in the molten lipid. Relatively higher EE is considered as one of the major advantages of SLNs. To calculate the EE and loading capacity, calibration curves for the UV detection of levothyroxine sodium were prepared using five standard solutions in the concentration range of 1.3 × 10−3–1.0 × 10−5 mol/l; encapsulation efficiency and drug loading were 99.00 and 33.17 %, respectively. The high EE of the drug is thought to be the result of the lipophilic characteristics and high compatibility between the drug and the mixed lipid. Moreover, selection of appropriate mixture of tripalmitin and palmitic acid is another factor to increase loading capacity. It is observed that the type and concentration of either the lipid material or the surfactant used had a noticeable influence on the entrapment efficiencies of prepared SLNs. Addition of lipid increased drug loading %. On the other hand, the imperfection (lattice defects) of the lipid structure could offer more loading space to accommodate drugs. As a result, the structure of less ordered arrangement in the nanoparticles would be beneficial to the drug loading capacity (Abdelbary and Fahmy 2009), which in turn affects on the drug release process. According to DSC data, this may be due to the stability of emulsion droplets and the method of preparation and mixing of the drug and lipid matrix (Kashanian et al. 2011; Derakhshandeh et al. 2007; Subedi et al. 2009).
Characterization of uncoated SLNs and PEG 100-S-coated SLNs
The modification of SLNs led to a reduction in zeta potential values varying from −40.0 mV for the uncoated particles to −23.0 mV for those prepared with PEG-stearate (Table 2). The zeta potential results show that a strong negative charge of SLN surface is partially neutralized by coating.
The size of SLNs plays an important role in their absorption by the intestinal mucosa. Therefore, SLNs aggregation may reduce convenience of absorption (Jani et al. 1990). According to Table 2, the size of the uncoated SLNs increases after incubation in gastric medium, while the PEG 100-S-coated SLNs are weakly enlarged. These results indicate that the gastric medium compromised the stability of the particles investigated in a different manner. Different behaviors were observed for uncoated and coated particles. Whereas, the uncoated nanoparticles showed an increase in their particle size during the experiment, the PEG-stearate coated formulations underwent a small size increase. The clear size increase observed for the uncoated particles could be related to the known destabilizing effect that the acidic medium and enzymes have on lipid nanoparticles. It is possible that the PEG brush around the particles reduces the attachment of the enzymes and the further degradation of the triglycerides core (Hans and Lowman 2002; Gref et al. 2000; Prego et al. 2006). As we observed (Table 2) and also indicated in the literature uncoated lipid nanoparticles displayed an instantaneous aggregation following their incubation in gastric medium, whereas protective coatings such as PEG-stearate reduced this process (Vila et al. 2002, 2004; Garcia-Fuentes et al. 2002). Hence, the absence of degradation observed in all the PEG S-coated nanoparticles suggests that neither acidity nor enzyme was able to cleave the triglyceride ester bonds. The zeta potential results show that modification with PEG decreases the surface negative charge from −40.0 to −23.0 mV. In other words, the PEG layer can partially cover the negative surface charge of nanoparticles (Uner and Yener 2007; Gref et al. 2000). Furthermore, from particle size data, it can be concluded that the resistance of nanoparticles against aggregation and the enzymes is increased by decreasing the particle size (Garcia-Fuentes et al. 2005b; Sarmento et al. 2007). The results of stability of the nanoparticles in gastric media indicate that the low pH of the gastric medium is responsible for the aggregation (Table 2) and degradation (Fig. 1) of the uncoated SLNs to some extent. PEG 100-S-coated SLNs were more stable due to their polymer coating layer, which prevented aggregation of SLNs in gastrointestinal media (Uner and Yener 2007; Garcia-Fuentes et al. 2002, 2005a; Prego et al. 2006: Sarmento et al. 2007). In order to investigate whether pH is an important factor in the stability of the uncoated and PEG-coated nanoparticles observed in the gastric medium, the stability of uncoated SLNs in an acidic medium (pH 1.2) was measured by estimating the relative turbidity at time 0 and 1 h post-incubation. Increment in the relative turbidity demonstrates increase in levothyroxine concentration after incubation in acidic medium due to the degradation of nanoparticles (Garcia-Fuentes et al. 2002, 2005b). Uncoated SLNs showed an increase in relative turbidity during the experiment period, while a little change in relative turbidity was observed in the case of PEG 100-S-coated SLNs (Fig. 1).
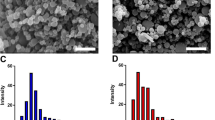
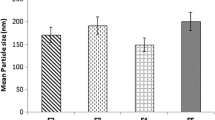
TEM image of the samples containing drug loaded PEG 100-S-coated SLNs is shown in Fig. 2. The image showed spherical shape and smooth surface with a particle size in nanometric scale. The particle size of levothyroxine-loaded PEG 100-S-coated SLNs depicted in TEM images is in agreement with the results obtained by SEM microphotographs (Fig. 3). Furthermore, the images show that the particles exhibit a spherical shape, and a dense lipid matrix without aggregation (Sarmento et al. 2007). All employed techniques confirm that the PEG 100-S-coated SLNs are circular to ellipsoidal in shape and well dispersed on the surface. It can be seen that the sizes of the PEG 100-S-coated SLNs, determined by PCS, are in agreement with TEM results. The average diameter determined by both PCS and TEM was found to be around 170 nm. Differential scanning calorimetry (DSC) thermograms for levothyroxine sodium, tripalmitin glyceride, a physical mixture of levothyroxine sodium, tripalmitin glyceride, palmitic acid, PEG 100-Stearate, and lyophilized PEG 100-S-coated SLNs are shown in Fig. 4. Levothyroxine sodium powder had a sharp melting peak at 232.72 °C. Melting peak for levothyroxine sodium in the lyophilized PEG 100-S-coated SLNs suspension was not depicted from DSC data suggesting that levothyroxine sodium could exist in an amorphous state (Dadashzadeh et al. 2008). However, we observed the melting peak of levothyroxine sodium in the physical mixture. Therefore, the absence of the levothyroxine melting peak suggested that it (levothyroxine) had better solubility in both tripalmitin and palmitic acid in lyophilized PEG 100-S-coated SLN as compared to bulk lipid. Solubility of the drug in the lipid melt is known to be an important precondition to obtain sufficient EE and drug loading (Subedi et al. 2009; Bhaskar et al. 2009). Comparing the melting peak temperature of tripalmitin glyceride in bulk lipid and lyophilized SLNs showed no significant change in melting point, which can confirm the absence of surfactant in the lyophilized SLNs and no significant interaction occurring between the drug and the lipid (Derakhshandeh et al. 2007; Kashanian et al. 2011). Finally it should be added, the sharpness of lyophilized PEG 100-S-coated SLN is not as much as that of the bulk lipid which indicates a slight transition from crystalline to amorphous state.
In vitro release characterization
According to Fig. 5, a comparison between the two release percent plots of PEG 100-S-coated SLNs and uncoated SLNs indicates appearance of a slight reduction for PEG-coated SLNs, due to covering effect of coating layer. Coating the surface leads to a decrease in the burst release effect compared to uncoated SLNs because the adsorbed levothyroxine on lipid surface gradually releases from PEG 100-S-coated SLNs. Following the initial burst, the systems provided a continuous and slow release of the levothyroxine. This slow release was attributed to the affinity of the hydrophobic drug for the lipids as well as the absence of degradation of the lipid matrix under the in vitro release conditions (Garcia-Fuentes et al. 2002, 2005a, b). Indeed, the coating layer resistance against diffusion of levothyroxine leads to a reduction in drug release. The initial burst release decreases as a result of higher thickness of modified SLNs based on Fickian diffusion (Garcia-Fuentes et al. 2002, 2005b). Drug release is 68.9 % for PEG-coated and 75.9 % for uncoated SLN. During SLN fabrication, a small portion of the drug can easily diffuse in the lipid core which usually cannot be released. Therefore, in such cases there is only partial drug release (Liu et al. 2007; Derakhshandeh et al. 2007; Zhang et al. 2013).
In vitro drug release kinetic characterization
The regression coefficient (R 2) values of release data obtained by the curve fitting method for zero-order, first-order, Higuchi model, and Hixson–Crowell model are reported in Table 3. The release data were analyzed using the following Higuchi kinetic equation. Regarding the release model of PEG 100-S-coated SLNs, it was found that the prolonged release characteristic of levothyroxine was fitted to Higuchi’s square root model, as reported for drug-loaded PEG-coated SLN systems. This result can be considered as supporting evidence for the fact that drug release mechanism obeys the Fick’s law (Kashanian et al. 2011; Hu et al. 2005).
The regression coefficient (R 2) of release data of PEG 100-S-coated SLN obtained by curve fitting method on various kinetic models is reported in Table 3.
Linear fits confirm that the release profile of levothyroxine from homogenous and granular matrix systems are diffusion-controlled. Based on the R 2 values in Table 3, the best data fitness was offered by Higuchi model 0.998 (nearer to 1) proposing a diffusion-controlled mechanism for drug release (Kashanian et al. 2011).
Conclusion
The specific aim of our study was to develop new lipid nanoparticulate carriers that might be suitable for oral administration of hydrophobic drugs. Surface modification of tripalmitin carrier was aimed to protect, fewer uptakes by the immune system, and increase the action of hydrophobic drugs. PEG modification of SLNs increased stability against pH variation in gastric medium. Consequently the interaction of these particles with intestinal mucosa will be increased which leads to facilitated transport of levothyroxine, as a hydrophobic drug. The modification showed a reduction in the zeta potential values from −40.0 to −23.0 mV for uncoated and PEG 100-S-coated SLNs, respectively, which could partially cover the negative surface charge. Moreover, the initial burst release decreased as a result of higher thickness of modified SLNs based on Fickian diffusion. Regarding the release model of all formulations, it was found that the prolonged release characteristic of levothyroxine was well fitted to Higuchi’s square root model, as has been reported for drug-loaded PEG-coated SLN systems as well.
As a result, using a proper hydrophilic polymer, e.g., PEG 100-S can help obtaining more stability, lower zeta potential, reduced uptake by immune system for SLNs as well as possibility of easier oral consumption of drug in syrup form.
References
Abdelbary G, Fahmy RH (2009) Diazepam-loaded solid lipid nanoparticles: design and characterization. AAPS Pharm SciTech 10(1):211–219
Barzegar-Jalali M, Adibkia K, Valizadeh H, Siahi-Shadbad MR, Nokhodchi A, Omidi Y, Mohammadi G, Hallaj-Nezhad S, Hasan M (2008) Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci 11(1):167–177
Bhaskar K, Anbu J, Ravichandiran V, Venkateswarlu V, Rao YM (2009) Lipid nanoparticles for transdermal delivery of flurbiprofen: formulation, in vitro, ex vivo and in vivo studies. Lipids Health Dis 8(6):1–15
Blakesley VA (2005) Current methodology to assess bioequivalence of levothyroxine sodium products is inadequate. AAPS J 7(1):122–128
Bocca C, Caputo O, Cavalli R, Gabriel L, Miglietta A, Gasco MR (1998) Phagocytic uptake of fluorescent stealth and non-stealth solid lipid nanoparticles. Int J Pharm 175(2):185–193
Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF (2002) Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm 238(1–2):241–245
Cavalli R, Bargoni A, Podio V, Muntoni E, Zara GP, Gasco MR (2003) Duodenal administration of solid lipid nanoparticles loaded with different percentages of tobramycin. J Pharm Sci 92(5):1085–1094
Chimmiri P, Rajalakshmi R, Mahitha B, Ramesh G, Noor Ahmed VH (2012) Solid lipid nanoparticles: a novel carrier for cancer therapy. Int J Biol Pharm Res 3(3):405–413
Collier JW, Shah RB, Bryant AR, Habib MJ, Khan MA, Faustino PJ (2011) Development and application of a validated HPLC method for the analysis of dissolution samples of levothyroxine sodium drug products. J Pharm Biomed Anal 54(3):433–438
Dadashzadeh S, Derakhshandeh K, Shirazi FH (2008) 9-Nitrocamptothecin polymeric nanoparticles: cytotoxicity and pharmacokinetic studies of lactone and total forms of drug in rats. Anticancer Drugs 19(8):805–811
Damge C, Michel C, Aprahamian M, Couvreur P, Devissaguet JP (1990) Nanocapsules as carriers for oral peptide delivery. J Control Release 13(2–3):233–239
Derakhshandeh K, Erfan M, Dadashzadeh S (2007) Encapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: factorial design, characterization and release kinetics. Eur J Pharm Biopharm 66(1):34–41
Derakhshandeh K, Soheili M, Dadashzadeh S, Saghiri R (2010) Preparation and in vitro characterization of 9-nitrocamptothecin loaded long circulating nanoparticles for delivery of to cancer. Int J Nanomedicine 5(1):1–9
Desai MP, Labhasetwar V, Amidon GL, Levy RL (1996) Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res 13(12):1838–1845
Ekambaram P, Sathali AAH, Priyanka K (2012) Solid lipid nanoparticles: a review. Sci Rev Chem Commun 2(1):80–102
Fang YP, Wu PC, Huang YB, Tzeng CC, Chen YL, Hung YH, Tsai MJ, Tsai YH (2012) Modification of polyethylene glycol onto solid lipid nanoparticles encapsulating a novel chemotherapeutic agent (PK-L4) to enhance solubility for injection delivery. Int J Nanomedicine 7:4995–5005
Florence AT (1997) The oral absorption of micro- and nanoparticulates: neither exceptional nor unusual. Pharm Res 14(3):259–266
Garcia-Fuentes M, Torres D, Alonso MJ (2002) Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules. Colloids Surf B 27(2–3):159–168
Garcia-Fuentes M, Torres D, Alonso MJ (2005a) Design and characterization of a new drug nanocarrier made from solid–liquid lipid mixtures. J Colloid Interf Sci 285(2):590–598
Garcia-Fuentes M, Torres D, Alonso MJ (2005b) New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int J Pharm 296(1–2):122–132
Garcia-Fuentes M, Prego C, Torres D, Alonso MJ (2005c) A comparative study of the potential of solid glyceride nanostructures coated with chitosan or poly(ethylene glycol) as carriers for oral calcitonin delivery. Eur J Pharm Sci 25(1):133–143
Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller RH (2000) ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B 18(3–4):301–313
Gregorini A, Ruiz ME, Volonté MG (2013) A derivative UV spectrophotometric method for the determination of levothyroxine sodium in tablets. J Anal Chem 68(6):510–515
Hans ML, Lowman AM (2002) Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci 6(4):319–327
Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP (2003) Physico-chemical stability of colloidal lipid particles. Biomaterials 24(23):283–300
Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S (2005) Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B 45(3–4):167–173
Jani PU, Halbert GW, Langridge J, Florence AT (1990) Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol 42(12):821–826
Kashanian S, Azandaryani HA, Derakhshandeh K (2011) New surface modified solid lipid nanoparticles by using N-glutaryl phosphatidyl ethanolamine as outer shell. Int J Nanomedicine 6:1–9
Khalil R, Kassem M, Elbary AA, ElRidi M, AbouSamra M (2013) Preparation and characterization of nystatin-loaded solid lipid nanoparticles for topical delivery. Int J Pharm Sci Res 4(6):2292–2300
Khan S (2012) Solid lipid nanoparticles: a review. World J Pharm Pharm Sci 1(1):96–115
Lai F, Sinico C, De LA, Zaru M, Müller RH, Fadda AM (2007) SLN as a topical delivery system for Artemisia arborescens essential oil: in vitro antiviral activity and skin permeation study. Int J Nanomedicine 2(3):419–425
Li XW, Lin XH, Zheng L, Yu L, Lv FF, Zhang Q, Liu W (2008) Effect of poly (ethylene glycol) stearate on the phase behavior of monocaprate/tween80/water system and characterization of poly (ethylene glycol) stearate-modified solid lipid nanoparticles. Colloids Surf A 317(1–3):352–359
Lilja JJ, Laitinen K, Neuvonen PJ (2005) Effects of grapefruit juice on the absorption of levothyroxine. Brit J Clin Pharmacol 60(3):337–341
Liu J, Gong T, Wang C, Zhong Z, Zhang Z (2007) Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm 340(1–2):153–162
Liu W, Hu M, Liu W, Xue C, Xu H, Yang XL (2008) Investigation of the carbopol gel of solid lipid nanoparticles for the transdermal iontophoretic delivery of triamcinolone acetate. Int J Pharm 364(1):135–141
Lowe PL, Temple CS (1994) Calcitonin and insulin in isobutyl cyanoacrylate nanocapsules: protection against proteases and effect on intestinal absorption. J Pharm Pharmacol 46(7):547–552
Muller RH, Mader K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery—are view of the state of the art. Eur J Pharm Biopharm 50(1):161–177
Parhi R, Suresh P (2010) Production of solid lipid nanoparticles—drug loading and release mechanism. J Chem Pharm Res 2(1):211–227
Prego C, Torres D, Fernandez-Megia E, Novoa-Carballal R, Quiñoá E, Alonso MJ (2006) Chitosan–PEG nanocapsules as new carriers for oral peptide delivery. Effect of chitosan pegylation degree. J Controlled Release 111(3):299–308
Rapaka RS, Roth J, Brine GA, Prasad VK (1982) A simple HPLC method for the dissolution studies on levothyroxine sodium tablets. Int J Pharm 12(4):285–294
Sarmento B, Martins S, Ferreira D, Souto EB (2007) Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomedicine 2(4):743–749
Shah RB, Bryant A, Collier J, Habib MJ, Khan MA (2008) Stability indicating validated HPLC method for quantification of levothyroxine with eight degradation peaks in the presence of excipients. Int J Pharm 360(1–2):77–82
Singhvi G, Singh M (2011) Review: in vitro drug release characterization models. Int J Pharm Stud Res 2(1):77–84
Souto EB, Wissing SA, Barbosa CM, Müller RH (2004) Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm 278(1):71–77
Subedi RK, Kang KW, Choi HK (2009) Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur J Pharm Sci 37(3–4):508–513
Svensson J, Ericsson UB, Nilsson P, Olsson C, Jonsson B, Lindberg B, Ivarsson S (2006) Levothyroxine treatment reduces thyroid size in children and adolescents with chronic autoimmune thyroiditis. J Clin Endocrinol Metab 91(5):1729–1734
Uner M, Yener G (2007) Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine 2(3):289–300
Vila A, Sanchez A, Tobıo M, Calvo P, Alonso MJ (2002) Design of biodegradable particles for protein delivery. J Controlled Release 78(1–3):15–24
Vila A, Sanchez A, Evora C, Soriano I, Vila Jato JL, Alonso MJ (2004) PEG-PLA nanoparticles as carriers for nasal vaccine delivery. J Aerosol Med 17(2):174–185
Volpato NM, Silva RL, Brito APP, Gonçalves JCS, Vaisman M, Noël F (2004) Multiple level C in vitro/in vivo correlation of dissolution profiles of two l-thyroxine tablets with pharmacokinetics data obtained from patients treated for hypothyroidism. Eur J Pharm Sci 21(5):655–660
Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ (1999) Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on the brain. J Control Release 59(3):299–307
Zara GP, Bargoni A, Cavalli R, Fundaro A, Vighetto D, Gasco MR (2002) Pharmaco- kinetics and tissue distribution of idarubicin-loaded solid lipid nanoparticles after duodenal administration to rats. J Pharm Sci 91(5):1324–1333
Zhang Q, Yie G, Li Y, Yang Q, Nagai T (2000) Studies on the cyclosporine A loaded stearic acid nanoparticles. Int J Pharm 200(2):153–159
Zhang N, Ping QN, Huang GH, Xua WF (2005) Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int J Pharm 294(1–2):247–259
Zhang C, Gu C, Peng F, Liu W, Wan J, Xu H, Lam CW, Yang X (2013) Preparation and optimization of triptolide-loaded solid lipid nanoparticles for oral delivery with reduced gastric irritation. Molecules 18(11):13340–13356
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kashanian, S., Rostami, E. PEG-stearate coated solid lipid nanoparticles as levothyroxine carriers for oral administration. J Nanopart Res 16, 2293 (2014). https://doi.org/10.1007/s11051-014-2293-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2293-6