Abstract
Rhodotorula spp. and Trichosporon spp. are opportunistic pathogens, and although an association between these two species in the same infection appears to be uncommon, it has been reported. This is the first study that aimed to evaluate the pathogenesis of a co-infection by R. mucilaginosa and T. asahii, using a new in vivo model, the Zophobas morio larvae. Suspensions from planktonic and biofilm-recovered cells were injected in the larvae as in monospecies as mixed (a ratio of 1:1 for both agents of a of 105 inoculum). Individual and mixed biofilms of R. mucilaginosa and T. asahii were produced for 24 and 48 h, and they were partially characterized by crystal violet and reduction of tetrazolium salt. When evaluating the impact of the planktonic suspension in vivo we verified that the fungi in monoculture were more able to kill the larvae than those from planktonic mixed suspension. On the other hand, regarding biofilm-recovered cells, there was an increase in the death of larvae infected for mixed suspensions. Moreover, the death rate was more pronounced when the larvae were infected with 48 h biofilm-recovered cells than the 24 h ones. T. asahii was the best producer of total biomass, mainly in 48 h. The metabolic activity for both yeasts organized in biofilm maintained the same pattern between 24 and 48 h. The present study proves a synergistic interaction between R. mucilaginosa and T. asahii after an experience in a mixed biofilm. Our results suggest that both species were benefited from this interaction, acquiring a greater potential for virulence after passing through the biofilm and this ability was acquired by the cells released from the biofilm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhodotorula spp. and Trichosporon spp. are basidiomycetous yeasts, opportunistic pathogens that reside commensally on the human skin and are considered emerging pathogens [1, 2]. Any alteration of the surrounding environment of these fungi may activate their virulence potential [3,4,5]. R. mucilaginosa was found to be the third most commonly isolated yeast from blood cultures and the most common microorganism isolated from the hands of hospital employees and patients [6]. In regards to onychomycosis, fungal infection of the nail, R. mucilaginosa has been little reported, present mainly in mixed infections [7, 8]. Trichosporon spp. is the second most common non-Candida yeast infection, and species of this genus are mainly reported in blood or urine infections [4, 9], with few reports of it being present in cases of onychomycosis. Although onychomycosis caused by these two species together appears to be uncommon [10], an unusual case was recently reported that identified an association of R. mucilaginosa and T. asahii [11]. The virulence factors of these fungal genera specifically have been little investigated. Despite this, the factors described are generally related to morphological switching, thermotolerance, the expression of cell wall components, enzyme production and secretion, and the ability to adhere to surfaces to produce biofilms [1, 2, 4].
Biofilms are complex microbial communities adhered to a surface and surrounded by a matrix of polymeric substances [12], which can be formed by one or more species of microorganisms, being for example, bacteria and fungi [13] or fungi and fungi [11, 14, 15]. In mixed biofilms, both species may exhibit different behaviors compared to the biofilms of the monospecies, and this can alter the pathogenesis of an infection [11, 16].
Onychomycosis is a major health problem due to its frequency, chronicity and resistance to therapy. In recent years, onychomycosis, as with other fungal infections, has been reported to occur due to fungi naturally forming biofilm on nails [17]. Yeasts are among the principal onychomycosis causative organisms [17, 18], with species from the genus Candida being the most frequently isolated [19]. On the other hand, R. mucilaginosa and T. asahii are scarcely reported, despite a case suggesting an association of the two agents [11]. As little is known about the co-habitation of these two microorganisms, this study aimed to evaluate the pathogenesis of co-infection by R. mucilaginosa and T. asahii from biofilm, using a new in vivo model, the Zophobas morio larvae.
Materials and Methods
Microorganisms
This study was conducted with R. mucilaginosa ATCC 64,684 and T. asahii CBS 2630. Briefly, yeasts were subcultured in chromogenic medium CHROMagar™ Candida (Difco™, USA), to check the culture purity. These yeasts were deposited in the Mycology and Culture Collection of Fungi (Micoteca) of the Medical Mycology Laboratory, Laboratório de Ensino e Pesquisa em Análises Clínicas of Universidade Estadual de Maringá (LEPAC). The yeasts were stored in Sabouraud Dextrose Broth (SDB; Difco™, USA) with glycerol at –80 °C. All samples were cultured on Sabouraud Dextrose Agar (SDA; Difco™, USA) and incubated at 25 °C for up to 2 days before all tests [1].
Standardization of the Inoculum
An ideal inoculum for all experiments was standardized according to Jarros et al. (2020) and validated by infection of Z. morio larvae, each weighing approximately 800–900 mg, using a total of 10 larvae per group. Four concentrations of inoculum with ATCC 64,684 R. mucilaginosa and three concentrations of inoculum with CBS 2630 T. asahii were evaluated for standardization of the highest lethality inoculum: 1–3 × 103, 104, 105 and 106 and 1–3 × 103, 104 and 105 respectively, determined by counting on a Neubauer chamber. Aliquots of 20 μL of each in sterile RPMI 1640 medium (Roswell Park Memorial Institute, Gibco) were injected, using a Hamilton syringe (1702 N, 22 s gauge, 25 μL capacity), into the hemocoel of the larvae, on the second or third sternite visible above the legs and the ventral portion. The negative control included sterile RPMI medium only. The larvae were placed in sterile Petri dishes containing a rearing diet and kept in darkness at 28 °C. Mortality was monitored once a day for 10 days. To establish larvae death, according to de Souza et al. (2015), visual confirmation of melanization and response to physical stimuli by gently touching them was carried out.
Impact of Fungal Planktonic Cells from Simple and Mixed Cultures on the Infection of Z. morio Larvae
Using the same standardized inoculum (1–3 × 105) for the two fungal species, the virulence of the yeasts in their planktonic form was evaluated, both for individual and mixed suspensions. Four larvae groups were infected as described above: control RPMI medium, R. mucilaginosa (105 inoculum), T. asahii (105 inoculum), and mixed suspension (105 R. mucilaginosa + 105 T. asahii). Larvae death was monitored for 10 days.
In Vitro Monospecies and Mixed Biofilm Production and Evaluation
Individual R. mucilaginosa and T. asahii monospecies biofilms, and a mixed biofilm (MB) using a ratio of 1:1 of the same inoculum, were first formed in sterile 96-well polystyrene flat-bottom plates (TPP®, Trasadingen, Switzerland) with 200 μL of inoculum adjusted to a concentration (1–3 × 105), as previously described by Jarros et al. (2020). The plates were then incubated with agitation at 110 rpm and 28 °C for 24 and 48 h.
Biofilm Characterization
The total biomass and metabolic activity of biofilms formed for 24 and 48 h were evaluated: total biofilm biomass by crystal violet staining (CV; Sigma-Aldrich, USA) and metabolic activity by the tetrazolium salt 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-(phenylamino)-carbonyl-2H-tetrazoliumhydroxid (XTT; Sigma-Aldrich, USA) reduction assay. The determinations were made by reading the plates after reagent incubation and well washing as according to Negri et al. (2016). The absorbance values at 492 nm for the XTT assay and at 620 nm for the CV assay were standardized per unit area of well (absorbance/cm2). The absorbance values of the negative control wells were subtracted from the values of the test wells to account for any background absorbance [21].
Biofilm-Recovered Cells
After incubation for biofilm formation (24 and 48 h), the wells were washed with 0.01 mol/L phosphate-buffered saline (PBS), pH 7.4, and the biofilm scraped and suspended in 1 mL RPMI medium. Subsequently, each suspension was transferred to a new tube and sonicated (Sonic Dismembrator Ultrasonic Processor, Fisher Scientific, USA) for 50 s at 30% amplitude. After dissociating the yeast cells from the biofilm, counting and adjusting the number of cells to 1–3 × 105 was performed using a Neubauer chamber.
Impact of fungal cells after biofilm formation on the infection of Z. morio larvae
The inoculation of the biofilm-recovered cells in the larvae was performed as previously described, and larvae death was evaluated over 10 days. The infection groups were as follows: control RPMI medium, biofilm-recovered cells from the R. mucilaginosa monospecies, from the T. asahii monospecies and from a MB.
Statistical analysis
All tests were performed in triplicate, on three independent days. For in vivo pathogenicity, Kaplan–Meier survival plots were used as according to de Souza et al. (2015). Data with a non-normal distribution were expressed as the mean ± standard deviation (SD) of at least three independent experiments. Significant differences among means were identified using the Two-way ANOVA test followed by Bonferroni multiple-comparison test. The data were analyzed using Prism 8.1 software (GraphPad, San Diego, CA, USA). Values of p < 0.05 were considered statistically significant.
Results and discussion
Standardization of the inoculum to be used in the Z. morio larvae was first carried out for this novel in vivo fungal infection model by determining the lethality to the larvae. The results are summarized in Fig. 1. For R. mucilaginosa (Fig. 1a), an inoculum of 106 led to a larger number of dead larvae, with only approximately 30% survival after 10 days of infection. The lowest evaluated inoculums (103 and 104) resulted in survival curves similar to that for the inoculum of 105, and for this reason they are not represented in the figure. While for T. asahii (Fig. 1b), the 105 inoculum caused the death of all the larvae of this group by the end of the evaluated period. The remaining groups, infected with the 103 and 104 inoculums, resulted in approximately 40% and 60% survival, respectively.
From these results, the inoculum of 105 was adopted for all subsequent experiments. Although the 106 inoculum for R. mucilaginosa could offer better results in vivo for this species on its own, in order to enable the effect of co-infection to be investigated, a 1:1 ratio of the microorganisms from the mixed cultures and biofilms was necessary so the 105 inoculum was chosen. Interestingly, this concentration corresponded to the highest mortality rate for Tenebrio molitor larvae infected with R. mucilaginosa in a previous study [1]. Z. morio larvae have already been used in bacterial studies [22,23,24], but the current study is the first to propose using this alternative model with fungi. Invertebrate animals such as Z. morio, as well as T. molitor, produce several antimicrobial peptides for defense against microbial pathogens [20, 22], including fungi, thus different inoculums can result in different survival curves. A 1:1 ratio for MB formation and infection was adopted in a study using Candida spp. and Trichophyton rubrum [13]. We believe this would be the ideal way to assess the virulence potential of each fungus investigated here, as the initial relative cell densities in a co-culture biofilm can strongly influence the MB properties and the prevalence of each species may change over time due to differences in the metabolism [15]. Olson et al. (2018) concluded the predominance of each species has a significant impact on the biofilm formation, as they showed that Candida glabrata in monocultures form a weaker biofilm than the biofilms of Candida albicans.
Figure 2 shows Kaplan–Meier plots of the survival rates of Z. morio larvae infected with monocultures or a mixed culture of planktonic cells of R. mucilaginosa and T. asahii cells before and after biofilm production. From these, we can highlight some key times in this experiment: 120 h (5 days), 168 h (7 days) and 240 h (10 days), for both the planktonic suspension (before biofilm production) and biofilm-recovered cells infections. When evaluating the impact in vivo of planktonic suspension (Fig. 2a), we found that fungi in monocultures seem to be more virulent to the larvae than the 1:1 mixed suspension, with the cells from the T. asahii monoculture causing death from 96 h of infection. Only after 192 h did the mixed culture begin to cause larval mortality.
Impact on the survival of Zophobas morio larvae infected with suspensions from Rhodotorula mucilaginosa or Trichosporon asahii monospecies, or a 1:1 mix of R. mucilaginosa and T. asahii. a. Planktonic cells; b. 24 h biofilm-recovered cells; c. 48 h biofilm-recovered cells. Results from trials performed in triplicate, on three independent days
Regarding the 24-h biofilm-recovered cells, the MB showed an increment in larvae death in 120 h, while the biofilm-recovered cells from the R. mucilaginosa or T. asahii monospecies were less efficient (Fig. 2b). Furthermore, from 216 h onwards, the biofilm-recovered cells from the MB began to cause significantly higher mortality. Thus, we infer that especially R. mucilaginosa became more virulent after the MB experience, since infection with the biofilm-recovered cells from the MB presents a trend of increasing the mortality in comparison to the infection with those of the monospecies. It is known that fungi rarely exist in isolated form, and the interactions between different species can benefit one or more of them [25]. Thus, in the current study it is possible to suppose that R. mucilaginosa received nutrients or complement from metabolism of T. asahii during the MB experience. As found in a study with a murine model of disseminated hematogenic candidiasis, dispersed biofilm cells demonstrated greater virulence compared to their planktonic cells. Dispersed cells of C. albicans retain their high virulence over several generations, ensuring greater adhesion, filamentation and virulence of dispersed cells [26].
For the 48-h biofilm-recovered cells (Fig. 2c), it was possible to observe that T. asahii had characteristics of a higher immediate mortality, while R. mucilaginosa caused death later. It was clear that, after the MB experience, both yeasts had increased their ability to provoke larvae death in comparison to the monospecies infections; similarly, but greater than, that which occurred for infections with the 24-h biofilm-recovered cells. The biofilm-recovered cells from the R. mucilaginosa monospecies indicate potential time-dependent virulence. A previous study showed that this species produces a mature biofilm in 48 h, with a significant and progressive increase in the number of cells according to the biofilm age [1]. The survival curve after infection with the cells from the MB displays the same trend, but with greater efficiency, than the cells from the monospecies, thus we can infer the T. asahii in the MB is important for the virulence potential of R. mucilaginosa.
With these results, we show that being in a biofilm favors and increases the virulence of both yeasts, but particularly for R. mucilaginosa. This hypothesis is reinforced by the behavior of the 24-h MB biofilm-recovered cells, where T. asahii apparently maintained its own virulence potential, whereas for R. mucilaginosa the passage through a MB seems to have stimulated this ability. It has already been described that many bacteria acquire mutual benefits when organized in mixed biofilms, from sharing strategies to prevent the host's immunological clearance and better protection against environmental threats such as antimicrobials [27]. Indeed, the association of different microorganisms can be advantageous for at least one of them, for instance, in the case of C. albicans with bacteria, the bacterial cells could bind directly to the hyphae of the yeast in the biofilm architecture [28]. This fungus-bacterial interaction can be synergistic, providing physical protection, secretion and diffusion of signaling molecules, and environmental changes such as in pH, oxygen and carbon dioxide levels [28, 29]. Moreover, it is essential to consider intercellular communications that occur through quorum sensing molecules, which allow one species to identify and react to the presence of another, coordinating the collective behavior of these microorganisms in communities [30].
R. mucilaginosa, apparently less virulent, seems to have benefitted from being in a MB with T. asahii, this has also been reported to occur between C. albicans and C. glabrata, which are frequently co-isolated from human infection [15]. In that study the increased hyphae length of C. albicans contributed to a more complex biofilm structure, increasing both its thickness and hyphal surface area for C. glabrata, which allowed the cells of this yeast to attach and cluster in the biofilm. We can hypothesize that R. mucilaginosa may also have taken advantage of the T. asahii filamentous forms. According to the authors, the changing Candida polymicrobial culture dynamics during biofilm formation may alter the infection course [15]. Thus, it is possible that cases involving R. mucilaginosa and T. asahii may be more frequent than has been reported [11, 31].
Based on the knowledge of Uppuluri et al. (2010), we hypothesize that the increase in mortality due to the mixed suspension of planktonic cells (Fig. 2a) may be the result of a biofilm formed in vivo in the larvae after 192 h. These authors claim that scattered cells from mature biofilms appear to be more virulent and have a greater ability to adhere to surfaces to form new biofilms [26].
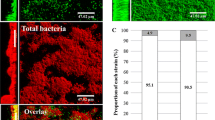
This is the first study that evaluates the effect of T. asahii and R. mucilaginosa biofilm-recovered cells on Z. morio larvae. It is worth mentioning that for all stages of the experiment, the same inoculum concentration (105) was used, in order to assess the virulence potential that fungal cells acquire when passing through the biofilm. There are many ways to characterize a fungal biofilm [13], however, in the current study we chose the total biomass and metabolic activity (Fig. 3). We found that T. asahii was the best producer of total biomass, more so in 48 h. Unfortunately, there are no studies in the literature that provide a characterization of T. asahii isolates to confirm whether this is typical for the species. While for R. mucilaginosa, the production of biomass was similar to results already published by our research group [1]. Intriguingly, in the current study, the extracellular matrix did not seem to be responsible for increasing the virulence of the isolates. In addition, the metabolic activity of yeasts organized in biofilm maintained the same pattern of activity when comparing between 24 and 48 h.
The R. mucilaginosa monospecies at 48 h had significantly increased metabolic activity compared to that of 24 h, and we infer that this could be reflected in the increase of its virulence. This result is apparently inconsistent with previously published results on R. mucilaginosa biofilm formation [1], but we attribute this to differences in protocol details, such as incubation temperature and culture media, which are critical factors, as described and discussed by Garcia et al. (2020).
Other factors may explain the increase in virulence of R. mucilaginosa, since the results of total biomass production and metabolic activity were not sufficient for this conclusion. A limiting factor in our study was not having determined the colony-forming units of the biofilm-recovered cells, as well as the structural architecture of the biofilms [13]. Furthermore, crystal violet is only able to quantify biomass without distinguishing between living and dead cells [13, 32]. Another point is that it is not possible to differentiate the cellular metabolic activity of a monospecies within a MB based only on the XTT reduction assay, as the metabolic behavior of microorganisms in a MB can vary compared to a monospecies biofilm [13, 25]. Nevertheless, Fig. 2 shows irrefutable data on the effect of MB between R. mucilaginosa and T. asahii, indicating the association of both microorganisms in the etiology of infectious diseases.
Conclusion
The present study proves a synergistic interaction between R. mucilaginosa and T. asahii after an experience in MB. Our results suggest that R. mucilaginosa benefitted from this interaction, acquiring a greater potential for virulence after passing through the biofilm, and the cells released from the biofilm likely gained this ability.
References
Jarros IC, Veiga FF, Corrêa JL, Barros ILE, Gadelha MC, Voidaleski MF, et al. Microbiological and virulence aspects of Rhodotorula mucilaginosa. EXCLI J. 2020;19:687–704.
Singh S, Capoor MR, Varshney S, Gupta DK, Verma PK, Ramesh V. Epidemiology and antifungal susceptibility of infections caused by spec- and non-yeast worldwide. Indian J Med Microbiol. 2019;37:536–41. https://doi.org/10.4103/ijmm.IJMM_19_146.
Ioannou P, Vamvoukaki R, Samonis G. Rhodotorula species infections in humans: a systematic review. Mycoses. 2019;62:90–100. https://doi.org/10.1111/myc.12856.
Colombo AL, Padovan ACB, Chaves GM. Current knowledge of Trichosporon spp and Trichosporonosis. Clin Microbiol Rev. 2011;24:682–700. https://doi.org/10.1128/CMR.00003-11.
Zhang E, Sugita T, Tsuboi R, Yamazaki T, Makimura K. The opportunistic yeast pathogen Trichosporon asahii colonizes the skin of healthy individuals: analysis of 380 healthy individuals by age and gender using a nested polymerase chain reaction assay. Microbiol Immunol. 2011;55:483–8. https://doi.org/10.1111/j.1348-0421.2011.00341.x.
Gomez-Lopez A, Mellado E, Rodriguez-Tudela JL, Cuenca-Estrella M. Susceptibility profile of 29 clinical isolates of Rhodotorula spp and literature review. J Antimicrob Chemother. 2005;55:312–6. https://doi.org/10.1093/jac/dki020.
Ge G, Li D, Mei H, Lu G, Zheng H, Liu W, et al. Different toenail onychomycosis due to Rhodotorula mucilaginosa and Candida parapsilosis in an immunocompetent young adult. Medical Mycology Case Reports. 2019. p. 69–71. http://dx.doi.org/https://doi.org/10.1016/j.mmcr.2019.04.012
Pârvu M, Moţ CA, Pârvu AE, Mircea C, Stoeber L, Roşca-Casian O, et al. Allium sativum extract chemical composition, antioxidant activity and antifungal effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa causing onychomycosis. Molecules. 2019. p. 3958. http://dx.doi.org/https://doi.org/10.3390/molecules24213958
Francisco EC, de Almeida Junior JN, de Queiroz TF, Aquino VR, Mendes AVA, de Andrade Barberino MGM, et al. Species distribution and antifungal susceptibility of 358 Trichosporon clinical isolates collected in 24 medical centres. Clin Microbiol Infect. 2019;25:909.e1-909.e5. https://doi.org/10.1016/j.cmi.2019.03.026.
Vasconcellos C, Pereira CQM, Souza MC, Pelegrini A, Freitas RS, Takahashi JP. Identification of fungi species in the onychomycosis of institutionalized elderly. An Bras Dermatol. 2013;88:377–80. https://doi.org/10.1590/abd1806-4841.20131884.
Idris NFB, Huang G, Jia Q, Yuan L, Li Y, Tu Z. Mixed infection of toe nail caused by Trichosporon asahii and Rhodotorula mucilaginosa. Mycopathologia. 2019. https://doi.org/10.1007/s11046-019-00406-y.
Solano C, Echeverz M, Lasa I. Biofilm dispersion and quorum sensing. Current Opinion in Microbiology. 2014. p. 96–104. http://dx.doi.org/https://doi.org/10.1016/j.mib.2014.02.008
Garcia LM, Costa-Orlandi CB, Bila NM, Vaso CO, Gonçalves LNC, Fusco-Almeida AM, et al. A two-way road: antagonistic interaction between dual-species biofilms formed by Candida albicans/Candida parapsilosis and Trichophyton rubrum. Front Microbiol. 2020;11:1980. https://doi.org/10.3389/fmicb.2020.01980.
Harris MR, Coote PJ. Combination of caspofungin or anidulafungin with antimicrobial peptides results in potent synergistic killing of Candida albicans and Candida glabrata in vitro. International Journal of Antimicrobial Agents. 2010. p. 347–56. http://dx.doi.org/https://doi.org/10.1016/j.ijantimicag.2009.11.021
Olson ML, Jayaraman A, Kao KC. Relative abundances of Candida albicans and Candida glabrata in coculture biofilms impact biofilm structure and formation. Appl Environ Microbiol. 2018. https://doi.org/10.1128/AEM.02769-17.
Trejo-Hernández A, Andrade-Domínguez A, Hernández M, Encarnación S. Interspecies competition triggers virulence and mutability in Candida albicans-Pseudomonas aeruginosa mixed biofilms. ISME J. 2014;8:1974–88. https://doi.org/10.1038/ismej.2014.53.
Gupta AK, Foley KA. Evidence for biofilms in onychomycosis. G Ital Dermatol Venereol. 2019;154:50–5. https://doi.org/10.23736/S0392-0488.18.06001-7.
Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003;149(Suppl 65):1–4. https://doi.org/10.1046/j.1365-2133.149.s65.4.x.
Paškevičius A, Švedienė J, Kiverytė S, Bridžiuvienė D, Vaitonis G, Jablonskienė V. Candida distribution in onychomycosis and in vitro susceptibility to antifungal agents. Acta Dermatovenerol Cro At. 2020;28:204–9.
de Souza PC, Morey AT, Castanheira GM, Bocate KP, Panagio LA, Ito FA, et al. Tenebrio molitor (Coleoptera: Tenebrionidae) as an alternative host to study fungal infections. J Microbiol Methods. 2015;118:182–6. https://doi.org/10.1016/j.mimet.2015.10.004.
Negri M, Silva S, Capoci IRG, Azeredo J, Henriques M. Candida tropicalis biofilms: biomass, metabolic activity and secreted aspartyl proteinase production. Mycopathol. 2016;181:217–24. https://doi.org/10.1007/s11046-015-9964-4.
Du M, Liu X, Xu J, Li S, Wang S, Zhu Y, et al. Antimicrobial effect of Zophobas morio hemolymph against bovine mastitis pathogens. Microorganisms. 2020. p. 1488. http://dx.doi.org/https://doi.org/10.3390/microorganisms8101488
Tokarev YS, Malysh SM, Volodartseva YV, Gerus AV, Berezin MV. Molecular identification of a densovirus in healthy and diseased Zophobas morio (Coleoptera, Tenebrionidae). Intervirol. 2019;62:222–6. https://doi.org/10.1159/000508839.
Carvalho MC, Tomazini A, Prado RA, Viviani VR. Selective inhibition of Zophobas morio (Coleoptera: Tenebrionidae) luciferase-like enzyme luminescence by diclofenac and potential suitability for light-off biosensing. Luminescence. 2021;36:367–76. https://doi.org/10.1002/bio.3952.
dos Santos JD, dos Santos JD, Piva E, Vilela SFG, Jorge AOC, Junqueira JC. Mixed biofilms formed by C albicans and non-albicans species: a study of microbial interactions. Brazilian Oral Res. 2016. https://doi.org/10.1590/1807-3107bor-2016.vol30.0023.
Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Köhler JR, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010;6: e1000828. https://doi.org/10.1371/journal.ppat.1000828.
Thet NT, Wallace L, Wibaux A, Boote N, Jenkins ATA. Development of a mixed-species biofilm model and its virulence implications in device related infections. J Biomed Mater Res B Appl Biomater. 2019;107:129–37. https://doi.org/10.1002/jbm.b.34103.
Lohse MB, Gulati M, Johnson AD, Nobile CJ. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. 2018;16:19–31. https://doi.org/10.1038/nrmicro.2017.107.
Iñigo M, Del Pozo JL. Fungal biofilms: From bench to bedside. Rev Esp Quimioter. 2018;31(Suppl 1):35–8.
Demuyser L, Jabra-Rizk MA, Van Dijck P. Microbial cell surface proteins and secreted metabolites involved in multispecies biofilms. Pathog Dis. 2014;70:219–30. https://doi.org/10.1111/2049-632X.12123.
Biegańska MJ, Rzewuska M, Dąbrowska I, Malewska-Biel B, Ostrzeszewicz M, Dworecka-Kaszak B. Mixed infection of respiratory tract in a dog caused by Rhodotorula mucilaginosa and Trichosporon jirovecii: a case report. Mycopathologia. 2018. p. 637–44. http://dx.doi.org/https://doi.org/10.1007/s11046-017-0227-4
Pantanella F, Valenti P, Natalizi T, Passeri D, Berlutti F. Analytical techniques to study microbial biofilm on abiotic surfaces: pros and cons of the main techniques currently in use. Ann Ig. 2013;25:31–42. https://doi.org/10.7416/ai.2013.1904.
Acknowledgements
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES)—Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) no. 421620/2018-8 and Fundação de Amparo à Pesquisa do Estado do Paraná (Fundação Araucária).
Author information
Authors and Affiliations
Contributions
Isabele Carrilho Jarros: Conceived of or designed study, Performed research, Analyzed data and Wrote the paper. Isabella Letícia Esteves Barros: Performed research and Analyzed data. Andressa Prado: Performed research. Jakeline Luiz Corrêa: Performed research. Amanda Milene Malacrida: Analyzed data and Wrote the paper. Melyssa Negri: Analyzed data and Wrote the paper. Terezinha Inez Estivalet Svidzinski: Conceived of or designed study, Analyzed data and Wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Damien Costa.
Rights and permissions
About this article
Cite this article
Jarros, I.C., Barros, I.L.E., Prado, A. et al. Rhodotorula sp. and Trichosporon sp. are more Virulent After a Mixed Biofilm. Mycopathologia 187, 85–93 (2022). https://doi.org/10.1007/s11046-021-00606-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-021-00606-5