Abstract
The circadian timing system of mammals is synchronized in concert with a central clock, but is also influenced by additional stimuli, including nutrients. However, little research has been done on polyphenols other than resveratrol and there seem to be no studies on their influence on young and old cells. The purpose of this study was to analyse the potential effects of quercetin, caffeic acid, and resveratrol on young and old fibroblast cells in the expressions of different clock genes and aging-related genes, and further investigate the mechanism. The mRNA expression of different clock genes and aging-related genes was assessed by quantitative real-time PCR. The protein levels of clock genes (BMAL1, PER1 and SIRT1) and glucocorticoid receptor α (GRα) were assessed by ELISA. Quercetin and caffeic acid in old fibroblast cells showed higher clock gene expression than resveratrol, quercetin increased Sirt1 expression, and caffeic acid increased Sirt6 expression indicating the possibility of an anti-aging effect. Also, quercetin and caffeic acid showed higher clock-controlled gene (Sirt1 and NR1D1) expression than resveratrol in young fibroblast cells. It appears that caffeic acid acts on NRF2 expression, and in turn to the actions of GRα, GDF11, Sirt1, and Sirt6. Regarding the increased expression of Per1, the activation effect on NR1D1 was confirmed only for caffeic acid in young fibroblast cells. Our results have confirmed the interplay of the circadian clock genes and cellular aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In mammals, the circadian system is composed of several endogenous clocks [1]. The main component of this system is the suprachiasmatic nucleus of the hypothalamus (central clock) [2], but circadian clocks are also present in lung, heart, and other tissue [3]. These are synchronized in accord with the central clock, but are also influenced by additional stimuli, including that of food [4], and hormones [5]. Recent research has demonstrated relationships between the desynchronization of clock gene and the development of certain diseases [6].
Plant in foods contains diverse secondary metabolites viz., quercetin, caffeic acid, and resveratrol, which are thought to provide health benefits by decreasing the risk of certain disease (such as cancer, cardiopathy and encephalopathy) [7,8,9]. The effects of these compounds on circadian rhythm in cells have been previously studied. Resveratrol regulates the expression of clock genes Period1 (Per1), Per2, and aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL=Bmal1) [10], as well as clock-controlled genes sirtuin 1 (Sirt1) and nuclear receptor subfamily 1 group D member 1 (NR1D1) [11] in fibroblast cells [10, 12]. However, little research has been done on polyphenols other than resveratrol. In addition, no studies of their influence on young and old cells were found.
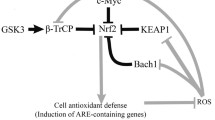
Recent research indicates that the dimer CLOCK:BMAL1 regulates the gene expression of the transcriptional regulator, nuclear factor E2-related factor 2 (NRF2) in the lung tissue, which in turn, promotes the transcription of antioxidant genes. Thus, the clock machinery controls the circadian antioxidant response in the lung tissue [13]. Importantly, oxidative stress has been concerned with a variety of age-related diseases. In addition, the response to oxidative stress decreases with aging. NRF 2 signaling regulates the gene expression of many antioxidant enzymes [14]. The altered NRF 2 signaling in a variety of aging models accomplishes lifespan extension via nutritional, genetic, and epigenetic approaches [15]. Results have indicated the interplay of the circadian clock and aging. Particularly, cellular nutrient sensory mechanisms seem to be critical in both circadian rhythm and the aging process [16].
A previous study in mice demonstrated that growth differentiation factor 11 (GDF11) levels decrease during aging and that this contributes to the development of age-related hypertrophic cardiomyopathy [17]. GDF11 retards skeletal muscle and brain aging in mice [18]. On the other hand, expression of aging biomarkers, including SIRT1 and SIRT6, was down-regulated by passaging of human fibroblast cells [19]. Therefore, in the present study, GDF11, Sirt 1 and Sirt 6 were used as aging-related genes.
Nagy et al. demonstrated that periodic oscillations of clock genes are influenced by glucocorticoid receptor α (GRα) [20]. Glucocorticoid treatment caused rapid PER1, PER2, and CRY1 response in a GRα-dependent manner. Glucocorticoid stimulation induced suppression of NR1D1. Treatment of a GR antagonist disrupted the circadian rhythm of clock genes and inhibited the quick changes in PER1 and CRY1 levels. Overexpression of the GRß isoform failed to modify the expression of the clock genes. However, it inhibited the GRα-related suppression of NR1D1. Therefore, we conducted GRα measurements.
Previously, we published two papers describing the characteristics of broad beans [21, 22] indicating that extracts from broad beans possess antioxidant properties. We furthered this research by examining dried seeds of plants marketed in Japan, to determine whether or not they also exhibit the above-mentioned activities [23, 24]. The dried seeds of plants often contain quercetin or caffeic acid. It is highly likely that the antioxidant activity of plant seeds is also due to these compounds. Thus, the purpose of the present study was to analyse the potential effects of quercetin, caffeic acid, and resveratrol on young and old lung fibroblast cells in the expressions of different clock genes and aging-related genes, and further investigate the mechanism.
Materials and methods
Reagents
We purchased cells and reagents from the following sources: Human fibroblast cells (JCRB0501; TIG-1-20; young cells & JCRB0505;TIG-1-60; old cells) from JCRB Cell Bank (Osaka, Japan). Cells (TIG-1-20 and TIG-1-60) are derived from the same cell and differ only in the number of subcultures; FibroLife® Fibroblast Serum Free Medium Complete Kit from Lifeline Cell Technology (Frederick, MD); Period Circadian Protein 1 (PER1) ELISA Assay Kit and Glucocorticoid receptor α(GRα) (Human) ELISA Assay Kit from CLOUD-CLONE CORP. (Katy, TX, USA); Aryl hydrocarbon receptor nuclear translocator (ARNT; BMAL1) Bioassay™ ELISA Kit (Human) from United States Biological (Salem, MA, USA); Human SIRT1 ELISA from RayBiotech, Inc. (Norcross, GA, USA). We obtained all other analytical grade chemicals from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Cell culture
Human lung fibroblast cells (TIG-1-20; young cells & TIG-1-60; old cells) were cultured for 4 days in a 96-well plate in FibroLife Serum-Free Medium and were maintained at 37 °C in a 5% CO2 incubator. On day 4 or after the preculture of cells, cells were maintained at 37 °C in a 5% CO2 incubator for 7 days without changing the medium. This was done in order to eliminate circadian rhythms in whole cells. Then, the cells were further cultured for 4 h in 96-well plates in mediums with or without samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) under 5% CO2 at 37 °C. The concentrations of these three compounds were determined with reference to the papers [10, 25,26,27]. Similarly, according to the experimental results of Oike and Kobori, the sample processing time was set to 4 h [10]. All samples were dissolved with dimethyl sulfoxide, and the final concentration was 0.1% or less without cytotoxicity.
Quantitative real time RT-PCR analysis (qRT-PCR)
Total RNA was extracted from cultured cells using an RNA purification kit (SuperPrep® Cell Lysis; Toyobo, Japan). Total RNA was reverse-transcribed using a SuperPrep® RT Kit for qPCR (Toyobo) in accordance with the manufacturer’s instructions. The resulting first-strand cDNA samples were diluted and used as a template in subsequent quantitative real-time PCR (qRT-PCR) analyses.
These analyses were performed using a Thunderbird SYBR qPCR Mix (Toyobo) using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, USA). The efficiency and specificity of the amplification were controlled by generating standard curves and carrying out melting curves, respectively.
Primers used were as follows: for Per1 (GenBank accession number NM_002616.2), forward, 5′-gtgctccaggatcccatttg-3′ and reverse, 5′-tcctgcttcagcacagaggtc-3′; for Bmal1 (GenBank Accession Number NM_001030272.2), forward, 5′-gcctactatcaggccaggctca-3′ and reverse, 5′-agccattgctgcctcatcattac-3′; for Sirt1 (GenBank Accession Number NM_012238.4), forward, 5′-cccagaacatagacacgctgga-3′ and reverse, 5′-atcagctgggcacctaggaca-3′; for Sirt6 (GenBank Accession Number NM_016539.2), forward, 5′- ctggtcagccagaacgtgga-3′ and reverse, 5′- cacgactgtgtctcggacgta-3′; for GDF11 (GenBank Accession Number NM_005811.4), forward, 5′- gtcattagcatggcccagga-3′ and reverse, 5′- agctgggccttcagtacctttg-3′; for NRF2 (GenBank Accession Number NM_001145412.3), forward, 5′- tgggcccattgatgtttctg-3′ and reverse, 5′- tgccacactgggacttgtgttta-3′; for NR1D1 (GenBank Accession Number NM_021724.4), forward, 5′-gtgaagacatgacgaccctgga-3′ and reverse, 5′-ggagccactggagccaatgta-3′; and for 18S ribosomal RNA (18S rRNA; GenBank Accession Number X03205), forward, 5′-actcaacacgggaaacctca-3′ and reverse, 5′-aaccagacaaatggctccac-3′. Each gene was normalized to the housekeeper mRNA of 18S rRNA. The ratio of the mRNA of a specific gene divided by the mRNA of 18S rRNA at t = 0 was defined as 1.00 and ratios of every sample were calculated in relation to t = 0.
PER1, BMAL1, SIRT1 ELISA kit assay procedure
The samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the culture cells in 96-well plates. The microplate was then incubated at 37 °C for 4 h. At the end of the treatment, the supernatant was collected, and sample supernatants were centrifuged for 20 min at 1000×g. We removed particulates and placed the sample into each well conjugated with a monoclonal antibody to the PER1, BMAL1 or SIRT1. Then, the detection of PER1, BMAL1, and SIRT1 was analyzed with a commercial kit according to instructions provided by the manufacturers. The PER1, BMAL1 or SIRT1 levels measured A450 nm.
Assessment of glucocorticoid receptor α (GRα) concentration
The samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the culture cells in 96-well plates. The microplate was then incubated at 37 °C for 4 h. After 4 h incubation, we obtained 100 μL of supernatants, and distributed some of the sample into each well coated with a monoclonal antibody specific to the NH2-terminus region of human GRα. Detected GRα was analyzed with a commercial kit according to instructions provided by the manufacturer. The detection limit of the assay was 0.118ng/mL for GRα. The GRα level measured A450 nm.
Statistical analysis
We present all data as mean ± SE. A statistical comparison between the groups was carried out using ANOVA or Student’s t test. P values < 0.05 were considered statistically significant.
Results
Young and old fibroblast cells exhibit significantly increased expression of clock genes and aging-related genes following resveratrol treatment
Although Bmal1, Sirt1, and NR1D1 mRNA levels were found to significantly increase in response to resveratrol in young fibroblast cells, resveratrol treatment demonstrated significant decreases in Per1, Sirt6, NRF2, and GDF11 mRNA levels (Fig. 1a, young). On the other hand, old fibroblast cells (Fig. 1b, Old) treated with resveratrol demonstrated a significant increase in Bmal1, Per1, Sirt6, and GDF11 mRNA levels; in old fibroblast cells in particular, significant increases in Per1 and GDF11 (about 2-fold increases) were observed following resveratrol treatment (Fig. 1b, old) compared to the control. NR1D1 mRNA was not expressed at all in old fibroblast cells.
Expression of mRNA in response to resveratrol treatment. a Gene expression profiling by qRT-PCR was performed for Bmal1, Per1, Sirt1, Sirt6, NR1D1, NRF2 and GDF11 in human young lung fibroblast cells (PDL20) grown for 4 h under normal conditions (Control) or under 100 μM resveratrol (Res) treatment. Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t-test. *p < 0.05 and **p < 0.01 were considered as significantly different from control. b Gene expression profiling by qRT-PCR was performed for Bmal1, Per1, Sirt1, Sirt6, NR1D1, NRF2 and GDF11 in human old lung fibroblast cells (PDL60) grown for 4 h under normal conditions (Control) or under 100 μM resveratrol (Res) treatment. Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. *p < 0.05, **p < 0.01 and *** p < 0.001 were considered as significantly different from control
Quercetin treatment differentially affects mRNA clock genes and aging-related genes in young and old fibroblast cells
Young fibroblast cells incubated with quercetin (Fig. 2a, young) demonstrated an inverse dose-response up-regulation of Bmal1 compared to the control (p < 0.05). Similarly, at the lowest concentration of quercetin (2 μM), young fibroblast cells also demonstrated up-regulation of Sirt1 (1.4-fold), Sirt6 (1.5-fold) or NR1D1 (2.3-fold), respectively (Fig. 2a, young). However, in young cells quercetin reduced Perl expression. In contrast, old fibroblast cells demonstrated significant increases in Bmal1 (2.3-fold), Per1 (5.4-fold) Sirt1 (1.3-fold) or GDF11 (1.2-fold) mRNA expression at 2 μM quercetin concentration (Fig. 2b, old). Similarly, 20 μM quercetin treatment affected Bmal1 and Per1 mRNA levels (about 2~2.5-fold increases).
Expression of mRNA in response to quercetin treatment. a Gene expression profiling by qRT-PCR was performed for Bmal1, Per1, Sirt1, Sirt6, NR1D1, NRF2 and GDF11 in human young lung fibroblast cells (PDL20) grown for 4 h under normal conditions (Control) or under quercetin (Que) (2 or 20 μM) treatment. Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered as significantly different from control. b Gene expression profiling by qRT-PCR was performed for Bmal1, Per1, Sirt1, Sirt6, NR1D1, NRF2 and GDF11 in human old lung fibroblast cells (PDL60) grown for 4 h under normal conditions (Control) or under quercetin (Que) (2 or 20 μM) treatment. Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered as significantly different from control
mRNA expression of clock genes and aging-related genes in young and old fibroblast cells is significantly up-regulated in response to caffeic acid treatment
Young fibroblast cells demonstrated significant increases in Per1 (1.3-fold), Sirt6 (1.6-fold) or NR1D1 (5.4-fold) mRNA expression at 2.5 μM caffeic acid and in Sirt6 (2.5-fold) mRNA expression at 25 μM caffeic acid (Fig. 3a, young). Similarly, old fibroblast cells (Fig. 3b, old) demonstrated dose-response up-regulation of Bmal1 and NRF2 mRNA in response to caffeic acid treatment (p < 0.01), with Per1 and Sirt6 also exhibiting a trend towards an increase. The Sirt1 and GDF11 mRNA expressions in young and old fibroblast cells were a significant decrease in the expression levels.
Expression of mRNA in response to caffeic acid treatment. a Gene expression profiling by qRT-PCR was performed for Bmal1, Per1, Sirt1, Sirt6, NR1D1, NRF2 and GDF11 in human young lung fibroblast cells (PDL20) grown for 4 h under normal conditions (Control) or under caffeic acid (Caff A) (2.5 or 25 μM) treatment. Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered as significantly different from control. b Gene expression profiling by qRT-PCR was performed for Bmal1, Per1, Sirt1, Sirt6, NR1D1, NRF2 and GDF11 in human old lung fibroblast cells (PDL60) grown for 4 h under normal conditions (Control) or under caffeic acid (Caff A) (2.5 or 25 μM) treatment. Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered as significantly different from control
Protein expression in young and old fibroblast cells
In the young fibroblast cells, GRα protein expression was significantly reduced by exposure to phytochemicals (Figure 4a, young). Similarly, in the old fibroblast cells, the expression of GRα protein demonstrated significant responses to the three phytochemicals (Fig. 4b, old). In addition, the expression of PER1 and SIRT1 proteins was not significantly affected by the three phytochemicals, with the exception of PER1 treated with 2.5 μM caffeic acid and 2 μM quercetin, and SIRT1 treated with resveratrol in the young fibroblast cells (Figs. 6, 7). In the young fibroblast cells, expression of BMAL1 protein was affected by the three phytochemicals, demonstrating a trend towards a dose-response (Fig. 5a). In the old fibroblast cells, significant up-regulation of BMAL1 protein expression was observed in response to quercetin and caffeic acid, while BMAL1 expression did not increase in response to resveratrol (Fig. 5b).
Effects of resveratrol, quercetin and caffeic acid on the glucocorticoid receptor α (GRα) level. a To clarify the underlying mechanisms of these effects, the samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the human young lung fibroblast cells (PDL20) in 96-well plates. The microplate was then incubated at 37°C for 4 h. After 4 h incubation, we obtained 100 μL of supernatants. The GRα in supernatants was analyzed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. *p < 0.05 was considered as significantly different from control. b To clarify the underlying mechanisms of these effects, the samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the human old lung fibroblast cells (PDL60) in 96-well plates. The microplate was then incubated at 37°C for 4 h. After 4 h incubation, we obtained 100 μL of supernatants. The GRα in supernatants was analyzed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. *p < 0.05 was considered as significantly different from control
Effects of resveratrol, quercetin and caffeic acid on the BMAL1 level. a To clarify the underlying mechanisms of these effects, the samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the human young lung fibroblast cells (PDL20) in 96-well plates. The microplate was then incubated at 37°C for 4 h. After 4 h incubation, we obtained 100 μL of supernatants. The BMAL1 in supernatants was analyzed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. ***p < 0.001 was considered as significantly different from control. b To clarify the underlying mechanisms of these effects, the samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the human old lung fibroblast cells (PDL60) in 96-well plates. The microplate was then incubated at 37°C for 4 h. After 4 h incubation, we obtained 100 μL of supernatants. The BMAL1 in supernatants was analyzed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. **p < 0.01 and ***p < 0.001 were considered as significantly different from control
Effects of resveratrol, quercetin and caffeic acid on the PER1 level. a To clarify the underlying mechanisms of these effects, the samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the human young lung fibroblast cells (PDL20) in 96-well plates. The microplate was then incubated at 37°C for 4 h. After 4 h incubation, we obtained 100 μL of supernatants. The PER1 in supernatants was analyzed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. *p < 0.05 was considered as significantly different from control. b To clarify the underlying mechanisms of these effects, the samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the human old lung fibroblast cells (PDL60) in 96-well plates. The microplate was then incubated at 37°C for 4 h. After 4 h incubation, we obtained 100 μL of supernatants. The PER1 in supernatants was analyzed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test
Effects of resveratrol, quercetin and caffeic acid on the SIRT1 level. a To clarify the underlying mechanisms of these effects, the samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the human young lung fibroblast cells (PDL20) in 96-well plates. The microplate was then incubated at 37°C for 4 h. After 4 h incubation, we obtained 100 μL of supernatants. The SIRT1 in supernatants was analyzed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test. *p < 0.05 was considered as significantly different from control. b To clarify the underlying mechanisms of these effects, the samples (100 μM resveratrol, 2 or 20 μM quercetin, and 2.5 or 25 μM caffeic acid) were added to the human old lung fibroblast cells (PDL60) in 96-well plates. The microplate was then incubated at 37°C for 4 h. After 4 h incubation, we obtained 100 μL of supernatants. The SIRT1 in supernatants was analyzed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD. Statistical significance was assessed by one-way ANOVA and Student t test
Assessment of the relationship between clock genes and aging-related genes in young fibroblast cells
We evaluated the correlation coefficients for the genes individually. The correlation coefficients are shown in Table 1. The expression of GDF11 mRNA measured by real-time PCR correlated with the most parameters (Sirt1, Sirt6, and GRα). The relationship between Bmal1 mRNA expression and Sirt1 mRNA expression had the highest level of correlation with the parameters examined (r = 0.965). For the most part, Bmal1, Per1, and Sirt1 mRNA values (which were based on relative changes in mRNA derived from sample treatments; Figs. 1, 2, 3) were positively correlated with assessment parameters (Table 1). However, the Sirt6 mRNA values indicated an inverse relationship with many parameters (Bmal1, Sirt1, and GDF11). Table 1 also shows the correlation coefficients produced by protein expression alone. The correlation coefficients for BMAL1 and Bmal1 were as low as 0.187 compared with 0.405 for PER1 and Per1. In addition, the correlation coefficients for SIRT1 and Sirt1 were as high as 0.492 compared with BMAL1 and PER1 (Table 1).
Assessment of the relationship between clock genes and aging-related genes in old fibroblast cells
The correlation coefficients for clock genes and aging-related genes in old fibroblast cells are shown in Table 1. The SIRT1 protein measured by ELISA kit correlated with the most parameters (Sirt6 and BMAL1). The expression of Bmal1 mRNA had the highest level of correlation with the expression of NRF2 mRNA (r = 0.976). For the half part, Bmal1 and NRF2 mRNA values (which were based on relative changes in mRNA derived from sample treatments; Figs. 1, 2, 3) were positively correlated with assessment parameters (Table 1). Table 1 also shows the correlation coefficients produced by protein expression alone. The correlation coefficients for BMAL1 and Bmal1 were as well as 0.264 compared with 0.291 for SIRT1 and Sirt1. In addition, the correlation coefficients for PER1 and Per1 were as high as 0.608 compared with BMAL1 and SIRT1 (Table 1).
Discussion
Circadian clock genes control several pathways involved in life span regulation and organ aging. For instance, mouse models defective in specific clock genes exhibited a shortened life span and had features of accelerated organ aging [28, 29]. In recent years, the relationship between clock genes and aging-related genes has been reported. The two major circadian regulators, CLOCK/BMAL1 binds to the SIRT1 promoter to enhance its expression [30]. Chang et al. showed that SIRT1 in the brain governs central circadian control by activating transcription of BMAL1 and CLOCK [31]. Also, Masri et al. describe that SIRT6 and SIRT1 regulate different subdomains of the circadian genome through different mechanisms. They show that SIRT6 interacts with CLOCK: BMAL1 and controls chromatin recruitment to the circadian gene promoter [32].
Moreover, Kalfalah et al. [33] identified that the determination of core clock gene expressions in primary human skin fibroblast cells indicated an age-related deregulation of a subset of genes. Particularly, several genes of the negative and interconnecting types of the clock (Per1 and NR1D1) showed age-related down-regulation, while Bmal1, a member of the positive type, was the only gene indicating an increase in expression in aged fibroblast cells. Therefore, it may be deregulation of individual clock genes as a whole, which contributes to the decrease of cellular function in aged fibroblast cells. As these many reports show, clock genes and aging-related genes are connected to each other and the circadian system is controlled.
We demonstrated that quercetin, caffeic acid, and resveratrol alter parameters related to clock genes and aging-related genes in human lung fibroblast cells. Figs. 1, 2, 3 represent clock-controlled genes (Sirt1and NR1D1) mRNA levels, expressed as values relative to the controls, in young and old fibroblast cells in resveratrol, quercetin and caffeic acid treatments. Resveratrol is a well-known entrainer of circadian clocks [10]. We demonstrated that resveratrol up-regulated mRNA expression of clock genes, especially Bmal1 and Per1, within 4 h in fibroblast cells except for Per1 in young cells (Fig. 1a and b). This result was similar to the results of Oike and Kobori with the exception of Perl in young cells [10]. From the results of Figs. 1, 2, 3, given the pattern of these three compounds, quercetin (2 or 20 μM) and caffeic acid (2.5 or 25 μM) appeared to regulate the circadian clock genes by a mechanism different from resveratrol treatment. Moreover, quercetin (2 μM) and caffeic acid (2.5 μM) showed higher clock gene regulation than was seen with resveratrol, which up-regulated mRNA expression of Per1 and Bmal1 in old fibroblast cells in the first 4 h following stimulation.
In addition, we indicated that NR1D1 mRNA expression was inversely correlated with in vitro cellular aging (PDL20 → 60) (Figs 1, 2, 3). That is, NR1D1 expression was not observed in the old cells. The reason is unknown, but NR1D1 may be related to cellular senescence. However, quercetin and caffeic acid exhibited higher NR1D1 gene expressions than resveratrol in young fibroblast cells. This suggests that the up-regulation of NR1D1 gene by treatment with quercetin and caffeic acid may contribute to suppression of cellular aging by passaging.
Sirt1and Sirt6 have been shown to play multiple functions in oxidative stress resistance, apoptosis and aging. Kim et al. [19] showed that expression of aging biomarkers, including Sirt1 and Sirt6, were down-regulated by passaging. Aging biomarkers showed a strong correlation with the cellular aging of human fibroblast cells. Our results suggest that 2 μM quercetin increases Sirt1 expression of PDL 60 old fibroblast cells, which may indicate anti-aging potential. Similarly, 2 μM quercetin in young fibroblast cells showed increased Sirt1 expression. Wang et al. indicated that quercetin delays postovulatory aging of mouse oocytes by regulating Sirt expression [34]. From their results, quercetin appears to suppress cellular senescence by controlling Sirt1.
On the other hand, D’Onofrio et al. [35] state as follows: Aging processes, by blocking all cellular signals controlled by Sirt1and Sirt6, lead to vascular aging. Sirt1and Sirt6 deacetylate their specific and common molecules, including histone and nonhistone, thus improving genome stability and inhibiting cellular senescence. Our results suggest that quercetin and caffeic acid increase Sirt6 expression of PDL 20 young fibroblast cells. Moreover, 25 μM caffeic acid exhibited higher aging-related gene regulation than that seen with resveratrol, which demonstrated up-regulated mRNA expression of Sirt6 in young and old fibroblast cells (Fig. 3). Especially, 25 μM of caffeic acid increased Sirt6 expression of old fibroblast cells and implies the possibility of an anti-aging effect. Our results may have the potential to suppress the aging process described by D’Onofrio et al.
Quercetin did not increase NRF2 expression (Fig. 2a, b). Pekovic-Vanghan et al. describe that the circadian transcription factors CLOCK and BMAL1 positively regulate Nrf2 transcription through temporal control of the E-box element of the Nrf2 gene promoter [13]. And, Lee et al. showed that quercetin treatment led to up-regulation of NRF2 at both the mRNA and protein levels in malignant mesothelioma cells [36]. Our results did not agree with those of Lee et al. However, there are no reports on the effects of quercetin on NRF2 expression in normal cells. The difference from our results may be due to differences in cell lines. Alternatively, it may be due to the concentration of quercetin. Furthermore, caffeic acid seems to have a different action mechanism than quercetin. Wu et al. indicated that caffeic acid enhanced the transcriptional activity of NRF2 gene [37]. Our results showed that 25 μM caffeic acid increased NRF2 expression in old cells. Our results agreed with those of Wu et al.
GDF11 levels in mice have been positively correlated with longevity [38] and decreased during aging in mice [17]. In addition, GDF11 retards skeletal muscle and brain aging in mice [18]. Our results indicated that 2 μM quercetin and 100 μM resveratrol showed significant increases in GDF11 mRNA expression in old fibroblast cells (Figs. 1b and 2b). Although GDF 11 is not reported in fibroblast cells, 2 μM quercetin and 100 μM resveratrol may indicate an anti-aging effect.
Next, in order to investigate the relationship between clock genes and aging-related genes in young fibroblast cells, we evaluated the correlation coefficients for the genes individually. The correlation coefficients are shown in Table 1.
The relationship between Bmal1 mRNA expression and Sirt1 mRNA expression seems to have the highest level of correlation with the parameters examined (r = 0.965). It is reported that Sirt1 interacts with Bmal1 and acts in a circadian rhythm. For example, Yang et al. [39] indicated that Bmal1 is a key clock gene involved in Sirt1-mediated cartilage homeostasis. In addition, Zhang et al. indicated that both BMAL1 and SIRT1 had a mutually positive regulation [40]. In this respect, our results were similar to those of Zhang et al.
On the other hand, the correlation coefficient between Bmal1 and Sirt6 was − 0.807. In addition, the correlation coefficient for Sirt6 and Sirt1 was − 0.795. As previously mentioned, Masri et al. state that SIRT6 interacts with the dimer CLOCK:BMAL1 and, differently from SIRT1, controls its chromatin recruitment to circadian clock gene promoters [32].
Also, Masri et al. demonstrated that when CLOCK and BMAL1 are ectopically expressed in SIRT6 alone or in both SIRT6 and SIRT1, SIRT6 only interacts with CLOCK and BMAL1, not SIRT1 [32]. This represents the difference in the function of Sirt1 and Sirt6 for Bmal1. Our results show a negative correlation (r = − 0.795), which may indicate a difference in the action of the two sirtuins.
By the way, the core circadian clock mechanism consists of two linked negative feedback loops [41]. In the primary loop, circadian target genes, Bmal1 and Clock activate transcription of Per and Cry genes. PER and CRY proteins repress the transcriptional activity of Bmal1 and Clock. This loop also regulates rhythmic expression of the nuclear receptors genes, NR1D1and NR1D2, which in turn suppress the expression of Bmal1 and Clock as the second loop. We indicated that the correlation coefficient for Per1 and NR1D1was 0.796. That is, this correlation coefficient may explain the above loop.
Although there is tissue specificity in many reports, young cells have higher expression levels of Nrf2 genes and NRF2 proteins than old cells [42,43,44,45], whereas Bmal1 is expressed at a lower level in young cells [33]. In young cells, our results indicated that the correlation coefficient for BMAL1and NRF2 was − 0.789. This negative correlation coefficient seems to represent the above relationship.
Nader et al. [46] indicated that transactivational activity of GR oscillated spontaneously in circadian rhythms in reverse phase with Bmal1/Clock mRNA expression. Moreover, glucocorticoid regulates NRF2 activity via GR-NRF2 interaction. Alam et al. [47] showed that GR signaling inhibits NRF2-dependent transcriptional activation and attenuates cellular protection from NRF2-mediated oxidative stress. Our results showed that the correlation coefficient between BMAL1 and GRα was − 0.906, and the correlation between GRα and NRF2 was 0.869. Especially, the relationship between GRα and NRF2 does not show the same trend as the result of Alam et al. The reason for this differing result is not clear. However, we cannot guess because there are no reports on aging cells.
On the other hand, the correlation coefficients between GDF 11 and Sirt 1, 6 or GR were 0.796, − 0.828 and 0.786, respectively. There is no report showing the relationship between GDF 11 and Sirt 1, 6 or GR. Therefore, these relationships seem to be new findings.
Next, we describe old cells. The correlation coefficients are shown in Table 1. The relationship between Bmal1 mRNA expression and NRF2 mRNA expression had the highest level of correlation with the parameters examined (r = 0.976). NRF2 is known to decrease with aging [42,43,44,45]. On the other hand, Bmal1 has been reported to increase with aging [33]. It is not clear why the relationship between Bmal1 and NRF2 showed a positive correlation in old cells. We infer that, it may be the timing of the measurement. In other words, the timing after 4 h of sample treatment may be the cause. The time of 4 h is derived from the highest expression level of Bmal1 and Per1 in the previous study [10]. Or it may be due to the variance of circadian rhythm in old cells.
As mentioned in the section on young cells, Bmal1 and Clock activate transcription of Per and Cry genes [41]. Also, SIRT1 has been reported to interact with BMAL1 and function in a circadian manner [40]. In old cells, our results showed that the correlation coefficient between Bmal1 and Per1 is 0.928. It has been reported that the expression level of Per1 decreases with aging [33], and the expression level of Bmal1 increases with aging [33]. However, our results do not reflect the above relationship. The reason for this is not clear, but the same reason described in Bmal1 and NRF2 above can be considered. On the other hand, SIRT1 protein and BMAL1 protein showed a positive correlation (r = 0.841) in old cells. This appeared to be consistent with the correlation between the Bmal1 and Sirt1 genes.
Furthermore, BMAL1 protein expression was increased in primary fibroblast cells from old (age: over 60) donors [33]. Also, GDF11 levels decline during aging [17]. Our correlation coefficient between BMAL1 and GDF 11 is − 0.882. From this negative correlation coefficient, we believe that our results reflect the above relationship.
No report shows a direct relationship between Sirt1 and PER1. However, Baburski et al. [48] showed this relationship in Leydig cells isolated from adult (3 month) and aged (18–24 month) rats. They reported that the majority of examined clock genes displayed circadian rhythm in expression, but only Bmal1, Per1 and NR1D1 were reduced in the aged rats. Further, it was reported that aging reduced the oscillatory expression pattern of Sirt1 gene [48]. Our results indicated that the correlation coefficient for Sirt1 and PER1 was − 0.812. Their results and our results differ. This may be due to tissue specificity or to the difference between the PER1 protein and Per1 gene, both of which may be caused by time lag. Further consideration is of this point is necessary.
Fig. 8a indicated the correlation map of gene expression and protein levels on resveratrol, quercetin and caffeic acid treatment in young fibroblast cells. As shown in Table 1, this relationship is estimated from the correlation coefficient in young cells. The correlation suggests that the increased expression of Bmal1 by resveratrol may be due to suppression of Sirt6 expression. A different mechanism seems to be at work in the case of resveratrol and quercetin. Resveratrol may up-regulate the expression of Bmal 1 by suppressing Sirt 6. On the other hand, quercetin may up-regulate the expression of Bmal1 by activating Sirt1. In addition, considering the correlation, we infer that caffeic acid acts on NRF2 expression, which seems to lead to the action of GR, GDF11, Sirt1, and / or Sirt6. On the other hand, regarding the increased expression of Per1, the activation effect on NR1D1 was only confirmed for caffeic acid. Given the correlation, we infer an increase in Per1 expression associated with increased expression of NR1D1 by caffeic acid.
a Correlation map of gene expression and protein levels on caffeic acid, quercetin and resveratrol treatment in young fibroblast cells. BMAL1 and GR represent protein (yellow mark). Other characters (blue mark) represent genes. The orange line shows a positive correlation, and the blue line shows a negative correlation. b Correlation map of gene expression and protein levels on caffeic acid, quercetin and resveratrol treatment in old fibroblast cells. BMAL1, PER1 and SIRT1 represent protein (yellow mark). Other characters (blue mark) represent genes. The orange line shows a positive correlation, and the blue line shows a negative correlation. (Color figure online)
Fig. 8b shows the correlation map of gene expression and protein levels on resveratrol, quercetin and caffeic acid treatment in old fibroblast cells. Judging from the correlation coefficient, the increase in expression of Bmal1 in old cells by caffeic acid may be related to an increase in the expression of NRF2. NRF2 is activated by caffeic acid [37, 49]. As we showed, activation of NRF2 by caffeic acid appears to be similar to our young cell results. Increased expression of Bmal1 by resveratrol and quercetin was not involved in NRF2 expression. The mechanism of the increase in Per1 expression in old cells by resveratrol, quercetin and caffeic acid cannot be known from the correlation coefficient relationship. However, caffeic acid may up-regulate the expression of Per1 by acting on the NRF2 gene in old cells.
Finally, in the genes examined in this study, resveratrol may target the Sirt6 gene. Quercetin may target the Sirt1 gene. Caffeic acid also target the NR1D1 and NRF2 genes in young cells and NRF2 gene in old cells. The three compounds target different genes and seem to have different mechanisms.
Conclusions
This study showed that resveratrol, quercetin, and caffeic acid activate clock genes and aging-related genes. We demonstrated that quercetin and caffeic acid activated more genes than resveratrol and that their mechanism may differ from that of resveratrol. The three compounds target different genes and seem to have different mechanisms. Therefore, the ingestion of foods containing resveratrol, quercetin, and caffeic acid may contribute to clock gene and aging-related gene control.
References
Cagampang FR, Bruce KD (2012) The role of the circadian clock system in nutrition and metabolism. Br J Nutr 108:381–392
Girardet C, Becquet D, Blanchard MP, Francois-Bellan AM, Bosler O (2010) Neuroglial and synaptic rearrangements associated with photic entrainment of the circadian clock in the suprachiasmatic nucleus. Eur J Neurosci 32:2133–2142
Bendova Z, Sumova A (2006) Photoperiodic regulation of PER1 and PER2 protein expression in rat peripheral tissues. Physiol Res 55:623–632
Mukherjee S, Maitra SK (2015) Effects of starvation, re-feeding and timing of food supply on daily rhythm features of gut melatonin in carp (Catla catla). Chronobiol Int 32:1264–1277
Tischkau SA, Howell RE, Hickok JR, Krager SL, Bahr JM (2011) The luteinizing hormone surge regulates circadian clock gene expression in the chicken ovary. Chronobiol Int 28:10–20
Engin A (2017) Circadian rhythms in diet-induced obesity. Adv Exp Med Biol 960:19–52
Joseph SV, Edirisinghe I, Burton-Freeman BM (2016) Fruit polyphenols: a review of anti-inflammatory effects in humans. Crit Rev Food Sci Nutr 56:419–444
Chagas VT, Franca LM, Malik S, Paes AM (2015) Syzygium cumini L. skeels: a prominent source of bioactive molecules against cardiometabolic diseases. Front Pharmacol 6:259
Kulkarni SS, Canto C (2015) The molecular targets of resveratrol. Biochim Biophys Acta 1852:1114–1123
Oike H, Kobori M (2008) Resveratrol regulates circadian clock genes in Rat-1 fibroblast cells. Biosci Biotechnol Biochem 72:3038–3040
Miranda J, Portillo MP, Madrid JA, Arias N, Macarulla MT, Garaulet M (2013) Effects of resveratrol on changes induced by high-fat feeding on clock genes in rats. Br J Nutr 110:1421–1428
Park I, Lee Y, Kim HD, Kim K (2014) Effect of resveratrol, a SIRT1 activator, on the interactions of the CLOCK/BMAL1 complex. Endocrinol Metab 29:379–387
Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, Yamamoto M, Chan J, van der Horst GT, Fukada Y, Meng QJ (2014) The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev 28:548–560
Kubben N, Zhang W, Wang L, Voss TC, Yang J, Qu J, Liu GH, Misteli T (2016) Repression of the antioxidant NRF2 pathway in premature aging. Cell 165:1361–1374
Bruns DR, Drake JC, Biela LM, Peelor FF 3rd, Miller BF, Hamilton KL (2015) Nrf2 signaling and the slowed aging phenotype: evidence from long-lived models. Oxid Med Cell Longev 2015:732596
Qi G, Mi Y, Fan R, Zhao B, Ren B, Liu X (2017) Tea polyphenols ameliorates neural redox imbalance and mitochondrial dysfunction via mechanisms linking the key circadian regular Bmal1. Food Chem Toxicol 110:189–199
Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT (2013) Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153:828–839
Patel VK, Demontis F (2014) GDF11/myostatin and aging. Aging 6:351–352
Kim KS, Park HK, Lee JW, Kim YI, Shin MK (2015) Investigate correlation between mechanical property and aging biomarker in passaged human dermal fibroblasts. Microsc Res Tech 78:277–282
Nagy Z, Marta A, Butz H, Liko I, Racz K, Patocs A (2016) Modulation of the circadian clock by glucocorticoid receptor isoforms in the H295R cell line. Steroids 116:20–27
Okada Y, Okada M (1998) Scavenging effect of water soluble proteins in broad beans on free radicals and active oxygen species. J Agric Food Chem 46:401–406
Okada M, Okada Y, Inaba R, Iwata H (1998) Scavenging effects of methanolic extracts of broad beans on free-radical species. Environ Health Prev Med 3:6–11
Okada Y, Okada M, Sagesaka Y (2010) Screening of dried plant seed extracts for adiponectin production activity and tumor necrosis factor-alpha inhibitory activity on 3T3-L1 adipocytes. Plant Foods Hum Nutr 65:225–232
Okada Y, Okada M (2013) Protective effects of plant seed extracts against amyloid beta-induced neurotoxicity in cultured hippocampal neurons. J Pharm Bioallied Sci 5:141–147
Jing Z, Wang Z, Li X, Li X, Cao T, Bi Y, Zhou J, Chen X, Yu D, Zhu L, Li S (2016) Protective effect of quercetin on posttraumatic cardiac injury. Sci Rep 29(6):30812. https://doi.org/10.1038/srep30812
Moosavi F, Hosseini R, Rajaian H, Silva T, eSilva DM, Saso L, Edraki N, Miri R, Borges F, Firuzi O (2017) Derivatives of caffeic acid, a natural antioxidant, as the basis for the discovery of novel nonpeptidic neurotrophic agents. Bioorg Med Chem 25(12):3235–3246. https://doi.org/10.1016/j.bmc.2017.04.026
Hosseini R, Moosavi F, Silva T, Rajaian H, Hosseini SY, Bina S, Saso L, Miri R, Borges F, Firuzi O (2018) Modulation of ERK1/2 and Akt pathways involved in the neurotrophic action of caffeic acid alkyl esters. Molecules 23(12):3340. https://doi.org/10.3390/molecules23123340
Dubrovsky YV, Samsa WE, Kondratov RV (2010) Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging 2(12):936–944
Liu F, Chang HC (2017) Physiological links of circadian clock and biological clock of aging. Protein Cell 8(7):477–488. https://doi.org/10.1007/s13238-016-0366-2
Zhou B, Zhang Y, Zhang F, Xia Y, Liu J, Huang R, Wang Y, Hu Y, Wu J, Dai C, Wang H, Tu Y, Peng X, Wang Y, Zhai Q (2014) CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology 59(6):2196–2206. https://doi.org/10.1002/hep.26992
Chang HC, Guarente L (2013) SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153(7):1448–1460. https://doi.org/10.1016/j.cell.2013.05.027
Masri S, Rigor P, Cervantes M, Ceglia N, Sebastian C, Xiao C, Roqueta-Rivera M, Deng C, Osborne TF, Mostoslavsky R, Baldi P, Sassone-Corsi P (2014) Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158:659–672
Kalfalah F, Janke L, Schiavi A, Tigges J, Ix A, Ventura N, Boege F, Reinke H (2016) Crosstalk of clock gene expression and autophagy in aging. Aging 8:1876–1895
Wang H, Jo YJ, Oh JS, Kim NH (2017) Quercetin delays postovulatory aging of mouse oocytes by regulating SIRT expression and MPF activity. Oncotarget 8:38631–38641
D’Onofrio N, Servillo L, Balestrieri ML (2018) SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal 28:711–732
Lee YJ, Lee DM, Lee SH (2015) Nrf2 expression and apoptosis in quercetin-treated malignant mesothelioma cells. Mol Cells 38:416–425
Wu YL, Chang JC, Lin WY, Li CC, Hsieh M, Chen HW, Wang TS, Wu WT, Liu CS, Liu KL (2018) Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway. Free Radic Biol Med 115:309–317
Zhou Y, Jiang Z, Harris EC, Reeves J, Chen X, Pazdro R (2016) Circulating concentrations of growth differentiation factor 11 are heritable and correlate with life span. J Gerontol A Biol Sci Med Sci 71:1560–1563
Yang W, Kang X, Liu J, Li H, Ma Z, Jin X, Qian Z, Xie T, Qin N, Feng D, Pan W, Chen Q, Sun H, Wu S (2016) Clock gene bmal1 modulates human cartilage gene expression by crosstalk with sirt1. Endocrinology 157:3096–3107. https://doi.org/10.1210/en.2015-2042
Zhang J, Liu J, Zhu K, Hong Y, Sun Y, Zhao X, Du Y, Chen ZJ (2016) Effects of BMAL1-SIRT1-positive cycle on estrogen synthesis in human ovarian granulosa cells: an implicative role of BMAL1 in PCOS. Endocrine 53(2):574–584. https://doi.org/10.1007/s12020-016-0961-2
Lande-Diner L, Boyault C, Kim JY, Weitz CJ (2013) A positive feedback loop links circadian clock factor CLOCK-BMAL1 to the basic transcriptional machinery. Proc Natl Acad Sci USA 110:16021–16026
Shih PH, Yen GC (2007) Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology 8(2):71–80
Zhang G, Li S, Kang Y, Che J, Cui R, Song S, Cui H, Shi G (2016) Enhancement of dopaminergic activity and region-specific activation of Nrf2-ARE pathway by intranasal supplements of testosterone propionate in aged male rats. Horm Behav 80:103–116. https://doi.org/10.1016/j.yhbeh.2016.02.001
Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA (2009) Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc Natl Acad Sci USA. 106(8):2933–2938. https://doi.org/10.1073/pnas.0813361106
Liddell JR (2017) Are astrocytes the predominant cell type for activation of Nrf2 in aging and neurodegeneration? Antioxidants 6(3):65. https://doi.org/10.3390/antiox6030065
Nader N, Chrousos GP, Kino T (2009) Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J 23:1572–1583
Alam MM, Okazaki K, Nguyen LTT, Ota N, Kitamura H, Murakami S, Shima H, Igarashi K, Sekine H, Motohashi H (2017) Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2. J Biol Chem 292:7519–7530
Baburski AZ, Sokanovic SJ, Bjelic MM, Radovic SM, Andric SA, Kostic TS (2016) Circadian rhythm of the Leydig cells endocrine function is attenuated during aging. Exp Gerontol 73:5–13
Kim H, Kim W, Yum S, Hong S, Oh JE, Lee JW, Kwak MK, Park EJ, Na DH, Jung Y (2013) Caffeic acid phenethyl ester activation of Nrf2 pathway is enhanced under oxidative state: structural analysis and potential as a pathologically targeted therapeutic agent in treatment of colonic inflammation. Free Radic Biol Med 65:552–562
Acknowledgments
Research was supported by the Grant-in-Aid for Scientific Research (Grant No. 17K00863) from the Japanese Ministry of Education, Culture, Sports, Science and Technology We thank Ms. Wanda Miyata for help with critical review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okada, Y., Okada, M. Quercetin, caffeic acid and resveratrol regulate circadian clock genes and aging-related genes in young and old human lung fibroblast cells. Mol Biol Rep 47, 1021–1032 (2020). https://doi.org/10.1007/s11033-019-05194-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05194-8