Abstract
Recent investigations have indicated that altered expression of non-coding RNAs (ncRNAs) could be associated with human diseases such as type 2 diabetes (T2D). Circular RNAs (circRNAs) are a new discovered class of ncRNAs with unique structural characteristics that involved in several molecular and cellular functions. Exploring of the circulating circRNAs as a reliable non-invasive biomarker for monitoring and diagnosing of human diseases has grown significantly. However, the molecular functions and clinical relevance of circRNAs are not yet well clarified in T2D. Accordingly, in this review, the involvement of circRNAs in the β-cell function and T2D-related complications is highlighted. The study also shed light on the possibility of using circRNAs as a biomarker for T2D diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) refers to a group of metabolic disorders characterized by hyperglycemia and defect in metabolism of macromolecules [1]. According to the international diabetes federation (IDF) reports, about 415 million people suffered from diabetes worldwide in 2017, and this number will rise to 645 million people by 2040 [2, 3]. The etiology of DM varies from the defect in insulin production to defect in response to insulin or both [4]. Type 2 diabetes (T2D) is the most common form of diabetes that mainly develops in adults [5, 6]. T2D starts with postprandial hyperglycemia and insulin resistance followed by the compensatory response of pancreatic β-cells for insulin production, which in turn, lead to decreased β-cell mass [4]. Therefore, it can be concluded that T2D is a combination of insulin resistance and insulin deficiency conditions. T2D is associated with several complications that mainly include: hyperosmolar coma, nephropathy, neuropathy, retinopathy, and cardiovascular disease [7]. Therefore, this metabolic disorder is one of the main factors involved in mortality and morbidity worldwide, and out of the 56.4 million deaths in 2015, about 1.6 million were due to T2D [8]. Since T2D develops as a result of the interaction between genes and environment [9], investigation of gene regulating factors is among the issues of interest to researchers.
It is documented that the majority of the mammalian genome is transcribed to the non-coding RNAs (ncRNAs) that play an important role in the regulation of gene expression [10]. Usually, ncRNAs are categorized according to their size into two major groups: long non-coding RNAs (lncRNAs, length > 200 bp) and small non-coding RNAs (small ncRNA, length < 200 bp) [11, 12]. lncRNAs can be also categorized by their structure, function and genomic location. Structurally, lncRNAs are divided into linear RNAs and circular RNAs (CircRNAs). Furthermore, small ncRNA are also composed of two groups that involved in translation, transcription and gene regulation (Fig. 1) [13, 14]. An increasing number of experimental investigations are providing evidence that altered expression of ncRNAs patterns is associated with several human diseases such as T2D [15, 16] and human malignancies [17, 18].
Schematic representation of ncRNAs classification. ncRNAs are categorized according to their size into lncRNAs and small ncRNAs. LncRNAs can be also categorized by their structure, function and genomic location. Structurally, they are divided into linear RNAs and CircRNAs. tRNA transfer RNA, piRNA piwi RNA, siRNA small interfering RNA, rRNA ribosomal RNA, cisRNA cis-acting RNA, transRNA trans-acting RNA, ceRNA competing endogenous RNA, eRNA enhancer- derived RNA, NAT natural antisense transcript, TUCRNA transcribed ultraconserved RNAs, lincRNA long intergenic noncoding RNA
CircRNAs are a new class of RNAs that in contrast to linear RNAs have a covalently closed loop in their structure that protects them against RNA exonucleases [19]. The history of circRNAs dates back to 1976, when RNAs with covalently closed loop were discovered in some plant viroids [20]. Further investigations showed that the genome of the hepatitis delta virus is composed from circRNAs, and therefore it was thought that circRNAs are viroid [21]. Later research on human tumor suppressor genes showed transcripts with different exon arrangement compared to parent gene that were actually circRNAs [22]. However, due to the technical limitations as well as structural characteristics of circRNAs, many aspects of these molecules have remained unknown, but with the advent of new techniques such as advanced sequencing and bioinformatics tools, circRNAs have become one of the interesting research topics. CircRNAs are involved in gene expression regulation through mimicking of several roles including: microRNA (miRNA) inhibition, the sequestering of RNA-binding protein, and nuclear transcriptional regulators [23, 24].
In this narrative review, a systematically literature review was performed by Pubmed, Google Scholar, and Web of Science for English up to 4 June 2019; using the terms “T2D”, “diabetes” and “circRNAs”. Actually, we discussed the properties and molecular functions of circRNAs and their clinical relevance as potential biomarkers for T2D. Moreover, we highlighted the involvement of circRNAs in the β-cell function and T2D-related complications.
Biogenesis and classification of circRNAs
In eukaryotic systems, messenger RNA (mRNA) is first synthesized as pre-mRNA and subsequently undergoes a further process by splicing which removes its introns. In the canonical splicing process, there are three strategic positions that include: GU sequence at the 5′ end of the intron that plays the role of donor site, the AG sequence at the 3′ end of intron as an acceptor site, and a highly conserved adenosine, located 20–50 nucleotides upstream of the acceptor position [25]. In summary, the splicing procedure starts with the formation of a ribonucleoproteins complex from the five small nuclear RNAs (snRNAs) and their related proteins followed by enzymatic cleavage, thereby the intron is deleted and adjacent exons are joined together [26].
The circRNAs are transcribed by RNA polymerase II mainly from coding regions of genes [27]. In addition, circRNAs may be produced by introns, 5′ untranslated region (5′ UTR), and 3′ untranslated region (3′ UTR) or intergenic regions of genes [27, 28]. Indeed, circRNAs are formed by the process known as back-splicing. In this process, 5′ splice site (donor site in downstream) is linked to the 3′ splice site (acceptor site in upstream) [29]. Previous evidence revealed that inhibition of canonical spliceosome complex by isoginkgetin, decreases the levels of pre-mRNA and circRNAs [30]. Therefore, it can be concluded that circRNA production depends on canonical spliceosome.
There are three models that describe the circRNA formation, namely intron pairing-driven circularization [31], lariat-driven circularization (exon skipping) [31], and RNA binding proteins (RBPs) [32, 33] (Fig. 2). The intron pairing-driven circularization model is based on the pairing between complementary sequences (mainly Alu elements) within the introns that results in the correct positioning of splicing site and followed by nucleophilic attack and circRNA release [34]. There is evidence that approximately 20% of the total circRNAs loci have intron with complementary sequences [31], so other mechanisms are involved in the circularization process. Lariat-driven circularization known as the exon skipping model is proceeding by rotation of downstream exon and interaction with the upstream exon that subsequently release an exon-containing lariat. In the next step, the formed lariat undergoes back-splicing and produces circRNAs [34]. One of the key factors in circularization of RNAs is RBPs. In this model, RBPs interact with their binding sites within the flanking introns followed by intron pairing and subsequent circularization [34]. Generally, the back-splicing process can be affected by several genomic features. For example, the length of exons, especially in single-exon circRNA, as well as the presence of reverse complementary sequence, induces the circRNA formation [35]. However, the stability of intron base pairing has a negative effect on the circRNA production [36]. Furthermore, previous studies have indicated that RNA-binding proteins -as a transregulators- can stimulate or inhibit the circRNA formation [37].
Different models of circRNA biogenesis. a Lariat-driven circularization (or exon skipping model) proceed by interaction between downstream and upstream exons that result in exon-containing lariat. In the next step, the formed lariat undergoes back-splicing and circRNA was formed. b Intron pairing-driven circularization model is based on the pairing between introns that continued by nucleophilic attack in splicing site and release of circRNA. c Circularization model based on the RBPs. In this model interaction between introns are mediated by RBP and thereby back-splicing is facilitated
Since circRNAs are produced by different regions of genes, they can be classified as: exonic circRNAs (ecircRNAs) [27, 31, 38], circular intronic RNAs (ciRNAs) [28], retained-intron or exon–intron RNAs (EIciRNAs) [39, 40] and intergenic circRNAs [27]. EcircRNAs are the most common form of circRNAs that involve approximately up to 80% of the total circRNAs and are mainly located in the cytoplasm [27, 35].
Molecular functions of circRNAs in human genome
There are several functions proposed for circRNAs that are mainly categorized into five types, including: (I) regulation of gene splicing and transcription, (II) miRNA sponging, (III) protein sponging, (IV) affecting the protein function and (V) regulation of protein translation [41].
Regulation of gene splicing and transcription
In general, the mechanism of transcription regulation by circRNAs is different from that by many linear lnRNAs, in which lncRNAs are involved in assembling chromatin-regulatory complexes in specific gene loci and also interact with several proteins simultaneously. However, these mechanisms have not been described for circRNAs [41]. An in vitro study on Arabidopsis thaliana revealed that the circRNAs bind to the sequence from which it has been transcribed, opens DNA locally and is then paired with one strand of DNA. This scenario leads to the formation of a circRNA:DNA R-loop that inhibits the transcription process in elongation phase [42].
In mammalian models, circRNAs are among the factors that regulate the transcription of linear RNAs through affecting RNA polymerase II activity in initiation and elongation phases [43]. It has been documented that intronic circRNAs, such as ciRNAs and EIciRNAs can affect the expression of their host genes. Accordingly, c-sirt7, produced by lariat mechanism, can affect the polymerase II and thereby decrease parental gene ankyrin repeat domain 52 (ANKRD52) as well as sirtuin 7 (SIRT7) [44].
miRNA sponge
miRNAs are referred to small non-coding RNAs (18-24 nucleotides) that are involved in gene expression regulation at the post-transcriptional regulation stages [45]. The miRNAs interact with the 3′ UTR of the target mRNA and subsequently leads to translation suppression, degradation or deadenylation of mRNAs [46, 47]. Recently, studies have shown that circRNAs have several binding sites for miRNAs and thereby can suppress their inhibitory effects or sponge them [48]. Accordingly, there is several pieces of evidence that overexpression of circRNAs could induce the expression of miRNAs targets and vice versa [49].
One of the well-known circRNAs that has sponging effects for mir-7 is human cerebellar degeneration related protein 1 antisense (CDR1as). This circRNA contains over 70 binding sites for mir-7 and plays an important role in neuronal development (Fig. 3) [49]. Therefore, CDR1as (has-circ-0001946) overexpression is followed by inhibition of the miR-7 activity and subsequently increased expression of miR-7 target genes [49].
Binding energy for all binding sites within the CDR1as/miR-7 sponge. Red points show the binding energy for each predicted binding site in the CDR1as, as predicted by RIsearch2 [128]. The lower the binding energy is, the stronger the binding site. The box plot shows the distribution of those predicted binding energies for miRNAs with experimentally verified targets. (Color figure online)
Another example for miRNAs sponging is cirSRY, a testis specific circRNA. This circRNA has 16 conserved binding sites for miR-138 [24, 50, 51]. Furthermore, cir-ZNF609, mm9-circ-012559 and circ-BIC6 are other examples of circRNAs with sponging activity for miRNAs [52,53,54]. On the other hand, accumulating evidence suggests that circRNAs are more effective than linear RNAs in miRNAs sponging [55, 56].
Protein sponging
CircRNAs can participate in sponging of RBPs and thereby affect the levels of proteins. The MUSCLEBLIND protein (MBL) is encoded by mbl gene of Drosophila and is required for differentiation and development of muscle and eye photoreceptor cells [57]. Increased concentration of MBL protein promotes back-splicing in parental pre-mRNA and produces circ-MBL. Low concentrations of MBL, on the other hand, induce the canonical splicing of Mbl mRNA. Therefore, it can be concluded that MBL protein has self-regulating properties [51]. The interesting point in this regard is MBL binding sites in the structure of circMBL that enable it to sequester MBL. In other words, circ-MBL regulates its own expression through MBL sponging.
Protein interaction
CircRNAs can also interact with proteins and thereby affect several phenotypic properties. The PeBoW complex is involved in the maturation process of pre-60 ribosome [58, 59]. Circ-ANRIL interferes with this process and thereby decreases the translation potential of cells [60].
HuR protein is one of the best known of RBPs participate in RNAs splicing and nuclear export [61]. One of the main targets of HuR is polyadenylate-binding nuclear protein 1 (PABPN1) mRNA. PABPN1 protein is required for poly adenylation of 3′ terminal of mRNA in eukaryotic species and therefore is involved in mRNAs stability. There is evidence that circPABPN1 bind to the linear mRNA of PABPN1 in competition with HuR, followed by inhibition of PABPN1 translation as well as other mRNAs dependent on PABPN1 [61].
Circ-FOXO3 is encoded by Foxo3 gene and is involved in cell cycle. Indeed, cyclin-dependent kinase2 (CDK2) as a member of CDK family is essential for the transition from G1 to S phase in the cell cycle. The p21 can bind to CDK2 protein and inhibit the cell cycle in G1 stage. Circ-FOXO3 could interfere with the CDK2-p21 interaction, release CDK2, and subsequently induce cell cycle [62, 63].
Regulation of protein translation
A challenging question that could be posed at this juncture is: Are circRNAs translated? Generally, 7-methylguanosine structure in 5′ end (5′ cap) and polyA tail in 3′ end of mRNAs are required for the translation process [41]. CircRNAs do not contain these structures and their translation is beyond expectation. However, recently it has been shown that some of the circRNAs can be translated into proteins. This was suggested in a study in 1995, where internal ribosome entry site (IRES) of encephalomyocarditis virus was shown to be able to translate an artificial circRNA [64]. The human circ-ZNF609 is highly expressed in myoblasts and participates in myogenesis regulation. Circ-ZNF609 contains an open reading frame with 753nt length and can be translated into a protein [65]. Indeed, untranslated region of circ-ZNF609 mimics the IRES and promotes 5′ cap-independent translation [66].
One of the most common modifications of eukaryotic RNAs is methylation of adenosine into N6-methyladenosine (m6A) by methyltransferase complex [67, 68]. This modification has several effects on the metabolism of mRNAs, such as localization, translation, splicing and degeneration of mRNAs [69, 70]. Furthermore, previous studies have revealed that m6A in 5′ UTRs increase the potential of translation through the 5′-cap independent process [71, 72].
Molecular functions and clinical relevance of circRNAs in T2D
Recent studies suggest that the deregulation of circRNAs could be associated with human disorders such as coronary artery disease [73], neurodegenerative and autoimmune diseases [74, 75], and several types of cancer [76]. In this section, we will highlight the involvement of circRNAs in the β-cell function and T2D-related complications. We will also discuss the possibility of using circRNAs as a biomarker for T2D diagnosis.
CircRNAs and β cell function
Genome wide association studies have shown that most risk loci in type 2 are implicated in islet function [77]. circRNAs are widely expressed in human cells including both α- and β-cells of pancreas and could act as novel regulators of β-cell activities [78]. It has been reported that 10,830 high-confidence circRNAs expressed in human α, β, and exocrine cells. The most highly expressed candidates are MAN1A2 (hsa_circ_0000118), RMST, and circ-HIPK3 (hsa_circ_0000284) [79]. Lisa Stoll et al. reported that the expression of circ-HIPK3 and ciRS-7/CDR1as were reduced in the islets of diabetic db/db mice and knock-downing of circ-HIPK3 resulted in increased apoptosis and decreased β-cell proliferation [78]. Moreover, their transcriptomic analysis revealed that circ-HIPK3 acts by sequestering a group of miRNAs, including miR-124-3p and miR-338-3p, and by regulating the expression of key β-cell genes, such as Slc2a2, Akt1, and Mtpn [78]. It has been also demonstrated that both insulin content and secretion were significantly increased by overexpression of CDR1as in islet cells [80]. CDR1as was recently revealed to act as a powerful sponge for miR-7, therefore could upregulate miR-7 target genes such as Myrip in islet cells and thereby regulates insulin granule secretion [80]. Figure 4 summarizes the molecular roles of circ-HIPK3 and CDR1as circRNAs in β-cell function.
Schematic presentation of the circRNAs role in β-cell functions. Circ-HIPK3 acts as a sponge for miR-124-3p and miR-338-3p, and thereby regulating the expression of key β -cell genes including Slc2a2, Akt1, and Mtpn. In addition, Cdr1as acts as a powerful sponge for miR-7, and up-regulates miR-7 target genes such as Myrip and Pax6 in islet cells
Association of circRNA expressions with T2D pathogenesis and its related complications
Complications of diabetes including diabetic nephropathy, diabetic retinopathy, and the risk of cardiovascular diseases are the leading causes of increased morbidity and mortality of diabetes patients and posing a huge burden on the health care system [81]. Recent data have demonstrated that circRNAs play crucial roles in vascular endothelial cell dysfunction and diabetic neuropathic pain [82, 83]. Accordingly, deregulation of circRNAs could be associated with T2D pathogenesis and related complications (Fig. 5).
Cardiovascular complications
Impaired proliferation of vascular smooth muscle cells (VSMCs) plays an important role in the atherosclerotic promotion in diabetes [82]. It has been reported that the expression of circ-WDR77 in high glucose treatment VSMCs is significantly up-regulated compared to the normal group. This circRNA regulates VSMCs proliferation and migration via targeting miR-124/FGF2 axis [83].
Several investigations have shown that increased blood glucose impairs vascular endothelial cells and could develop cardiovascular complications in diabetic patients [84, 85]. Fei-Fei et al. demonstrated that high glucose level dramatically altered expression profiles of 95 circRNA including NC_000007.14:149492859_149558839 and has-circ-0004182 in endothelial cells [86]. This indicates the contribution of circRNAs in the pathogenesis of hyperglycemia-induced endothelial cells injury and diabetic cardiovascular diseases.
Interestingly, it has been reported that cZNF609 silencing decreases retinal vessel loss and suppresses pathological angiogenesis in vivo, and also protects endothelial cell against oxidative stress [87]. Furthermore, down-regulation of cZNF609 could elevate endothelial cell migration and tube formation, and also protected endothelial cell against oxidative stress. This circRNA could act as an endogenous miR-615-5p sponge, which led to the increased expression of MEF2A level [87]. MEF2A activation is strongly correlated with vascular endothelial cell function [88].
CircRNA-000203 was shown to be up-regulated in the diabetic mouse myocardium and in Angiotensin II (Ang II)-induced mouse cardiac fibroblasts [89]. Moreover, over-expression of this RNA could increase expression levels of Col1a2, Col3a1 and α-SMA in mouse cardiac fibroblasts [89]. Indeed, circRNA-000203 could block the interactions of miR-26b-5p and 3′UTRs of these genes by molecular sponge formation [89]. Taken together, that suppressing the function of miR-26b-5p contributes to the pro-fibrosis effect of circRNA-000203 in cardiac fibroblasts. Furthermore, Zhou B et al. reported that circRNA-010567 was markedly up-regulated in diabetic mice myocardium and cardiac fibroblasts treated with Ang II [90]. They revealed that circRNA-010567 silencing could up-regulate miR-141 and down-regulate TGF-β1 expression, and suppress fibrosis-associated protein resection in cardiac fibroblasts, including Col I, Col III and α-SMA [90]. These findings show that the circRNA-010567/miR-141/TGF-β1 axis plays an important regulatory role in the diabetic mice myocardial fibrosis model.
Neuropathic pain and diabetic retinopathy
Increasing evidence has verified that neuropathic pain is one of the most common diabetic complications. Its incidence is about 30% in T2D cases and is growing with the duration of diabetes [91]. It has been reported that circ-HIPK3 knockdown alleviated neuropathic pain in STZ-induced diabetic rats. Circ-HIPK3 could interact with miR-124 and negatively regulated its expression. Indeed, this circRNA regulates neuropathic pain progression through miR-124 [92]. Moreover, circ-HIPK3 silencing or overexpressing changed retinal endothelial cell viability, proliferation, migration, and tube formation in vitro. It has been revealed that circ-HIPK3 silencing in vivo alleviated retinal vascular dysfunction, as shown by decreased retinal acellular capillaries, vascular leakage, and inflammation. Circ-HIPK3 could serve as endogenous miR-30a-3p sponge retinal endothelial cells and inhibit its activity [93]. Inhibition of miR-30a-3p led to increased Vascular Endothelial Growth Factor-C (VEGF-C), FZD4, and WNT2 expression which have been shown to be involved in diabetic retinopathy [94, 95].
The circRNAs could also play a critical role in the responses of human umbilical vein endothelial cells (HUVEC) to the glucose stress [96]. It has been reported that circRNA-001175 could be involved in the protection of HUVECs against high glucose stress. Xiaoyan Pei et al. showed that the HUVECs glucose treatments gradually decreased the expressions of circRNA-00117 [97]. Moreover, they reported that the up-regulation of circRNA-001175 inhibits the HUVECs apoptosis and also increases the tubule formation ability of that under high glucose situation [97]. Furthermore, it has been recently observed that high glucose treatment could induce the expression of circ-0054633 and miR-218 levels in HUVEcs. In addition, circ-0054633 expression suppressed the injurious effects of high glucose to HUVEcs by targeting miR-218 [98]. Long Pan et al. reported that overexpression of circ-0054633 could reverse high glucose-induced cell proliferation, migration and angiogenesis inhibition by targeting the miR-218/ROBO1 and miR-218/HO-1 signals. Indeed, elevated expression of circ-0054633 increased HO-1 level by targeting miR-218, which resulted in reducing the apoptosis of HUVEcs [98].
A current investigation reported that circ-0005015 facilitated retinal endothelial angiogenic function via regulating endothelial cell proliferation, migration, and tube formation [99]. Circ-0005015 could act as miR-519d-3p sponge, leading to increased MMP-2, STAT3 and XIAP expression [99]. These genes are involved in many pathways, including regulation of vascularization [100], diabetic retinopathy [101], and neuroprotection [102].
T2D related-chronic inflammation and oxidative stress
T2D is a metabolic disorder characterized by chronic, low-grade inflammation which can lead to insulin resistance and impaired glucose tolerance [103]. The expression of circRNAs, and their association with chronic inflammation in T2D was evaluated in some investigations [98, 104]. It has been reported that circ-ANKRd36 expression level is positively correlated with IL-6 and TNF-α in diabetic patients, suggesting an association between circ-ANKRD36 and inflammation in T2D [104]. Yuan Fang et al. prediction analysis showed that circ-ANKRd36 may be involved in T2D and inflammation-associated pathways via interaction whit miR-3614-3p, miR-498 and miR-501-5p [104]. Moreover, it has been indicated that circ-0054633 expression may suppress cellular oxidative stress and inflammation [98]. This circRNA could target miR‑218 and inhibits its expression [98]. A previous study showed that miR-218 accelerated high glucose-induced podocyte apoptosis through directly downregulating HO-1 [105]. HO-1 has been demonstrated to have a protective effect against high glucose-induced cell toxicity, including oxidative stress and inflammation [106]. Furthermore, Jun-Hui Yang et al. revealed that deregulated expression of mmu-circRNA-34132, mmu-circRNA_017077 and mmu-circRNA-015216 in the Substantia Nigra and Corpus Striatum of Nrf2-Knockout Mice models could be involved with Nrf2-mediated neuroprotection against oxidative stress [107]. They reported that mmu-circRNA-34132 may be a potential regulator of Nrf2-mediated protection for diabetes mellitus and Nrf2-mediated defense against reactive oxygen species (ROS) in hearts [107]. In addition, it has been reported that the has-circ-0068087 level is up-regulated in high glucose treated-HUVECs. Down-regulation of this circRNA, suppressed the inflammation and endothelial cell dysfunction by sponging miR-197 and as a result, suppression of the TLR4/NF-κB/NLRP3 signaling pathway [108].
Diabetic nephropathy
Diabetic nephropathy is one of the most common causes of chronic renal failure in diabetic patients [109]. Previous investigations demonstrated that a large number of circulating circRNAs can be reliably detected in blood of acute kidney injury patients and in renal tissues [110, 111]. It has been validated that circRNA-15698 was up-regulated in both db/db mice and high glucose-exposed mouse mesangial cells [112]. CircRNA-15698 could act as a molecular sponge of miR‐185, and then positively regulated the transforming growth factor‐β 1 (TGF‐β 1) protein expression [112].
CircRNAs and wound healing
Impaired wound healing has been a major public health issue in patients with DM [113]. Currently, a study shows that the circ-Amotl1 can accelerate wound healing by facilitated Stat3 nuclear translocation and binding to Dnmt3a promoter, which enhanced Dnmt3a expression [114]. Zhen-Guo Yang et al. showed that the increased Dnmt3a level could methylated the promoter of miR-17 and thereby decreasing its levels. Interestingly, they reported that Stat3, similar to Dnmt3a, was a target of miR-17-5p. Therefore, decreased miR-17-5p levels would increase expression of Dnmt3a and Stat3, which led to increased cell adhesion, migration, proliferation, survival, and wound repair [114].
CircRNAs as a potential biomarkers in T2D
Most of the classical biomarkers for T2D diagnosis are useful only after the establishment of disease and fails to predict the prediabetic cases or diabetes complications at early stage of disease. Accordingly, the novel and more sensitive and complications-related biomarkers are needed [115, 116]. Due to the circular structure of circRNAs and lacking of free 5′ and 3′ ends, these molecules are highly resistant to exonuclease RNase R [117]. Indeed, circRNAs are stable molecules in body fluids with an average half-life of 48 h in plasma, much longer than 10 h of the average value of mRNAs [118]. Moreover, circRNAs are tissue and cell specific, additionally; the abundance of circRNA is higher in body fluid including blood, cerebrospinal fluid, saliva and urine [119]. Therefore, there is a growing trend in exploring the use of circulating and tissue profiling of circRNAs as a potential biomarker for the diagnosis and prognosis of a range of human disorders including diabetes [93, 119]. Table 1 represents the deregulated levels of important circRNAs in T2D.
Circ-HIPK3
It has been shown that the expression level of circ-HIPK3 is highly abundant in serum from diabetes patients who suffered from neuropathic pain compared to controls. Moreover, the circ-HIPK3 expression was up-regulated in serum and dorsal root ganglion from STZ-induced diabetes rats compared with control rats [92]. Kun Shan et al. showed that retinal level of circ-HIPK3 in diabetic mice was significantly higher than that in the non-diabetic controls [93]. Thus, circ-HIPK3 has great potential to become a diagnostic or predictive biomarker for neuropathic pain of T2D.
cZNF609
Chang Liu et al. revealed that cZNF609 expression level in the fibrovascular membranes of diabetic patients was significantly higher than that in the idiopathic epiretinal membranes of non-diabetic patients. In addition, they reported that cZNF609 expression was up-regulated in the plasma fraction of peripheral blood of diabetic patients compared with non-diabetic controls [87].
Circ-0054633
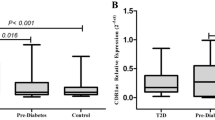
Zhenzhou Zhao et al. evaluated the expression levels of some circRNAs in the peripheral blood from control, pre-diabetes and T2D subjects [120]. They reported that the levels of circ-0124636 and circ-0139110 expression among the three groups presented no significant differences. However, the expression levels of circ-0018508 in the pre-diabetes and T2D groups were higher than that of the control group. They also revealed that, the expression levels of circ-0054633 and circ-0068087 were increased gradually from the control group to the T2D subjects. After ROC curve analysis, they demonstrated that circ-0054633 has the potential to be used as a diagnostic biomarker for pre-diabetes and T2D in clinical practice [120]. In the line with this finding, Sally et al. reported that plasma level of circ-0054633 was significantly increased gradually from controls to the T2D patients [121].
Circ-RNA11783-2
Xuejie Li et al. determined the expression profile of circRNAs in the peripheral blood of coronary artery disease and T2D patients [122]. They did not observe a significant difference for the expression levels of circ-0009036 and circ-0054129 between groups. However, they showed that the levels of circ-RNA11806-28, circ-RNA6510-1 and cir-cRNA11783-2 were lower in both the T2D and CAD groups compared with the controls. Subsequently, they validated the significance of differential circ-RNA11783-2 expression in the third cohort including 60 control, 64 T2D and 81 CAD subjects. Their result showed that circRNA11783-2 was down-regulated in both the CAD and T2D groups compared with the controls [122].
Circ-0005015
Shu-Jie Zhang et al. performed circRNAs microarray to investigate differential expression profile of that between diabetic and non-diabetic human retinas [99]. They identified 529 differentially expressed circRNAs, including 356 up-regulated and 173 down-regulated circRNAs compared with non-diabetic controls. In addition, they revealed that circ-0005015 expression was significantly up-regulated in the plasma fraction of diabetic retinopathy patients compared to cataract patients, and healthy controls [99].
Circ-ANKRD36
It has been reported that there is an association between the expression profiles of circRNAs in peripheral leucocytes of patients with T2D and inflammatory cytokines [104]. The RNAseq analysis revealed 220 differentially expressed circRNAs, including 107 up-regulated and 113 down-regulated circRNAs in diabetic subjects compared with non-diabetic controls. Among these, the expression of circ-ANKRD36 was up-regulated in patients with T2D and its expression level was positively correlated with IL-6, glucose and glycosylated hemoglobin levels [104].
Other circRNAs
Yonghao et al. reported that thirty circRNAs were significantly unregulated in the serum of T2D retinopathy patients compared with the serum from both T2D and control subjects [123]. Further, the expression of 7 circRNAs including circRNA-063981, circRNA-404457, circRNA-100750, circRNA-406918, circRNA-104387, circRNA-103410, and circRNA-100192 were significantly elevated in T2D retinopathy patients relative to the T2D or control groups [123].
Several investigations have shown that T2D is closely associated with the onset and progression of depression [124, 125]. Guangjian Jiang et al. showed that 183 circRNAs were significantly up-regulated, whereas 64 were down-regulated in the whole blood samples from T2D with depression group compared with that in the T2D group [126]. They suggested that differentially expressed circRNAs could clarify the pathogenesis of depression in patients with T2D [126]. Interestingly, it has been reported that Baduanjin, a traditional Chinese exercise therapy, could effectively ameliorate the symptoms of depression and blood glucose levels in diabetic patients with depression by regulating the expression of circRNAs [127].
Conclusions and perspectives
CircRNAs are involved in the genome regulation through mimicking of several roles including: regulation of gene splicing and transcription, miRNA and protein sponging and affecting the protein function and translation. Therefore, an increasing number of experimental investigations are providing evidence that the altered expression of circRNAs could be associated with several human diseases such as T2D. However, the molecular functions and clinical relevance of circRNAs are not yet well elucidated in T2D and there is still no available clinical relevance of circRNAs in diabetic patients.
This review provides current knowledge about the properties and functions of circRNAs and their clinical relevance as potential biomarkers for T2D. In addition, we highlight the involvement of circRNAs in the β-cell function and T2D-related complications. Further in vitro and in vivo studies are required to confirm and elucidate the functional roles of circRNAs in the establishment and development of T2D pathogenesis and the possibility using of these ncRNAs as a potential therapeutic targets and biomarkers for T2D.
References
Zarrinkalam E et al (2018) Resistance training and hawthorn extract ameliorate cognitive deficits in streptozotocin-induced diabetic rats. Biomed Pharmacother 97:503–510
Ogurtsova K et al (2017) IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50
Ramezankhani A et al (2018) Diabetes mellitus: findings from 20 years of the Tehran lipid and glucose study. Int J Endocrinol Metab 16(4 Suppl):e84784
Kumar V et al (2005) Robbins and Cotran pathologic basis of disease. Elsevier Saunders, Philadelphia
Wild S et al (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5):1047–1053
Alberti KGMM, Zimmet PF (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetes Med 15(7):539–553
Baynes H (2015) Classification, pathophysiology, diagnosis and management of diabetes mellitus. J Diabetes Metab 6(5):1–9
World Health Organization (2014) The top 10 causes of death. World Health Organization, Geneva
Murea M, Ma L, Freedman BI (2012) Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev Diabetic Stud: RDS 9(1):6
Palazzo AF, Lee ES (2015) Non-coding RNA: what is functional and what is junk? Front Genet 6:2
Adams BD et al (2017) Targeting noncoding RNAs in disease. J Clin Investig 127(3):761–771
Matsui M, Corey DR (2017) Non-coding RNAs as drug targets. Nat Rev Drug Discov 16(3):167
Amin N, McGrath A, Chen YPP (2019) Evaluation of deep learning in non-coding RNA classification. Nat Mach Intell 1(5):246
Dahariya S, Paddibhatla I, Kumar S, Raghuwanshi S, Pallepati A, Gutti RK (2019) Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol Immunol 112:82–92
Alipoor B et al (2018) The rs2910164 variant is associated with reduced miR-146a expression but not cytokine levels in patients with type 2 diabetes. J Endocrinol Invest 41(5):557–566
Alipoor B et al (2017) Association of miR-146a expression and type 2 diabetes mellitus: a meta-analysis. Int J Mol Cell Med 6(3):156
Ghaedi H et al (2019) Co-expression profiling of plasma miRNAs and long noncoding RNAs in gastric cancer patients. Gene 687:135–142
Zare A et al (2019) Decreased miR-155-5p, miR-15a, and miR-186 expression in gastric cancer is associated with advanced tumor grade and metastasis. Iran Biomed J
Qu S et al (2015) Circular RNA: a new star of noncoding RNAs. Cancer Lett 365(2):141–148
Sanger HL et al (1976) Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci 73(11):3852–3856
Kos A et al (1986) The hepatitis delta (δ) virus possesses a circular RNA. Nature 323(6088):558
Nigro JM et al (1991) Scrambled exons. Cell 64(3):607–613
Chen L-L (2016) The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol 17(4):205
Salzman J (2016) Circular RNA expression: its potential regulation and function. Trends Genet 32(5):309–316
Burset M, Seledtsov I, Solovyev V (2000) Analysis of canonical and non-canonical splice sites in mammalian genomes. Nucleic Acids Res 28(21):4364–4375
Suñé-Pou M et al (2017) Targeting splicing in the treatment of human disease. Genes 8(3):87
Memczak S et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495(7441):333
Zhang Y et al (2013) Circular intronic long noncoding RNAs. Mol Cell 51(6):792–806
Wang Y, Wang Z (2015) Efficient backsplicing produces translatable circular mRNAs. RNA 21(2):172–179
Starke S et al (2015) Exon circularization requires canonical splice signals. Cell Rep 10(1):103–111
Jeck WR et al (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19(2):141–157
Souii A, M’hadheb-Gharbi MB, Gharbi J (2015) Cellular proteins act as bridge between 5′ and 3′ ends of the Coxsackievirus B3 mediating genome circularization during RNA translation. Curr Microbiol 71(3):387–395
Ivanov A et al (2015) Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep 10(2):170–177
Yang Z et al (2017) Circular RNAs: regulators of cancer-related signaling pathways and potential diagnostic biomarkers for human cancers. Theranostics 7(12):3106
Salzman J et al (2012) Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 7(2):e30733
Liang D, Wilusz JE (2014) Short intronic repeat sequences facilitate circular RNA production. Genes Dev 28(20):2233–2247
Ashwal-Fluss R et al (2014) circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56(1):55–66
Du WW et al (2017) Identifying and characterizing circRNA-protein interaction. Theranostics 7(17):4183
Li Z et al (2015) Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 22(3):256
Salzman J et al (2013) Cell-type specific features of circular RNA expression. PLoS Genet 9(9):e1003777
Lei K et al (2018) The mechanism and function of circular RNAs in human diseases. Exp Cell Res 368(2):147–158
Chédin F (2016) Nascent connections: R-loops and chromatin patterning. Trends Genet 32(12):828–838
Holdt LM, Kohlmaier A, Teupser D (2018) Molecular functions and specific roles of circRNAs in the cardiovascular system. Non-Coding RNA Res 3(2):75–98
Rong D et al (2017) An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget 8(42):73271
Saadatian Z et al (2014) Single-nucleotide polymorphisms within micrornas sequences and their 3′utr target sites may regulate gene expression in gastrointestinal tract cancers. Iran Red Crescent Med J 16(7):e16659
Baek D et al (2008) The impact of microRNAs on protein output. Nature 455:64–71
Selbach M et al (2008) Widespread changes in protein synthesis induced by microRNAs. Nature 455(7209):58
Liu J et al (2017) Circles reshaping the RNA world: from waste to treasure. Mol Cancer 16(1):58
Hansen TB et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384
Chen Y et al (2016) Circular RNAs: a new frontier in the study of human diseases. J Med Genet 53(6):359–365
Barrett SP, Salzman J (2016) Circular RNAs: analysis, expression and potential functions. Development 143(11):1838–1847
Peng L et al (2017) Circular RNA ZNF609 functions as a competitive endogenous RNA to regulate AKT3 expression by sponging miR-150-5p in Hirschsprung’s disease. Oncotarget 8(1):808
Wang K et al (2016) A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J 37(33):2602–2611
Guo JU et al (2014) Expanded identification and characterization of mammalian circular RNAs. Genome Biol 15(7):409
Ebert MS, Neilson JR, Sharp PA (2007) MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4(9):721
Tay FC et al (2015) Using artificial microRNA sponges to achieve microRNA loss-of-function in cancer cells. Adv Drug Deliv Rev 81:117–127
Begemann G et al (1997) Muscleblind, a gene required for photoreceptor differentiation in Drosophila, encodes novel nuclear Cys3His-type zinc-finger-containing proteins. Development 124(21):4321–4331
Lapik YR et al (2004) Physical and functional interaction between Pes1 and Bop1 in mammalian ribosome biogenesis. Mol Cell 15(1):17–29
Rohrmoser M et al (2007) Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit. Mol Cell Biol 27(10):3682–3694
Holdt LM et al (2016) Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun 7:12429
Abdelmohsen K et al (2017) Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol 14(3):361–369
Du WW et al (2016) Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res 44(6):2846–2858
Du WW et al (2017) Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ 24(2):357
Chen C-Y, Sarnow P (1995) Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 268(5209):415–417
Legnini I et al (2017) Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell 66:22–37
Costelli P (2017) Circ-ZNF609: a novel regulator of myogenesis. Non-Coding RNA Investig 1:2
Li S, Mason CE (2014) The pivotal regulatory landscape of RNA modifications. Annu Rev Genom Hum Genet 15:127–150
Wei C-M, Gershowitz A, Moss B (1975) Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell 4(4):379–386
Yue Y, Liu J, He C (2015) RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev 29(13):1343–1355
Meyer KD, Jaffrey SR (2014) The dynamic epitranscriptome: N 6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol 15(5):313
Zhou J et al (2015) Dynamic m 6 A mRNA methylation directs translational control of heat shock response. Nature 526(7574):591
Meyer KD et al (2015) 5′ UTR m 6 A promotes cap-independent translation. Cell 163(4):999–1010
Pan R-Y et al (2017) Circular RNAs promote TRPM3 expression by inhibiting hsa-miR-130a-3p in coronary artery disease patients. Oncotarget 8(36):60280
Lukiw W (2013) Circular RNA (circRNA) in Alzheimer’s disease (AD). Front Genet 4:307
Zheng F et al (2017) Circular RNA expression profiles of peripheral blood mononuclear cells in rheumatoid arthritis patients, based on microarray chip technology. Mol Med Rep 16(6):8029–8036
Zhang Z et al (2018) Circular RNA: new star, new hope in cancer. BMC Cancer 18(1):834
Thurner M et al (2018) Integration of human pancreatic islet genomic data refines regulatory mechanisms at Type 2 Diabetes susceptibility loci. Elife 7:e31977
Stoll L et al (2018) Circular RNAs as novel regulators of β-cell functions in normal and disease conditions. Mol Metab 9:69–83
Kaur S, Mirza A, Pociot F (2018) Cell type-selective expression of circular RNAs in human pancreatic islets. Non-Coding RNA 4(4):38
Xu H et al (2015) The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells. Sci Rep 5:12453
Jacobs E et al (2017) Burden of mortality attributable to diagnosed diabetes: a nationwide analysis based on claims data from 65 million people in Germany. Diabetes Care 40(12):1703–1709
Reddy MA et al (2016) Regulation of vascular smooth muscle cell dysfunction under diabetic conditions by miR-504. Arterioscler Thromb Vasc Biol 36(5):864–873
Chen J et al (2017) Circular RNA WDR77 target FGF-2 to regulate vascular smooth muscle cells proliferation and migration by sponging miR-124. Biochem Biophys Res Commun 494(1–2):126–132
Balijepalli C et al (2017) Hypoglycemia: a review of definitions used in clinical trials evaluating antihyperglycemic drugs for diabetes. Clin Epidemiol 9:291
Bertoluci MC, Rocha VZ (2017) Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr 9(1):25
Shang F-F et al (2018) Alterations of circular RNAs in hyperglycemic human endothelial cells. Biochem Biophys Res Commun 499(3):551–555
Liu C et al (2017) Silencing of circular RNA-ZNF609 ameliorates vascular endothelial dysfunction. Theranostics 7(11):2863
Hamik A, Wang B, Jain MK (2006) Transcriptional regulators of angiogenesis. Arterioscler Thromb Vasc Biol 26(9):1936–1947
Tang C-M et al (2017) CircRNA_000203 enhances the expression of fibrosis-associated genes by derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac fibroblasts. Sci Rep 7:40342
Zhou B, Yu J-W (2017) A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-β1. Biochem Biophys Res Commun 487(4):769–775
Liebl A et al (2002) Complications, co-morbidity, and blood glucose control in type 2 diabetes mellitus patients in Germany-results from the CODE-2TM study. Exp Clin Endocrinol Diabetes 110(01):10–16
Wang L et al (2018) Intrathecal circHIPK3 shRNA alleviates neuropathic pain in diabetic rats. Biochem Biophys Res Commun 505(3):644–650
Shan K et al (2017) Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation 136(17):1629–1642
Robitaille J et al (2002) Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet 32(2):326
Munoz-Chapuli R, Quesada A, Medina MA (2004) Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci CMLS 61(17):2224–2243
Lukiw WJ, Rogaev EI, Zhao Y (2016) Circular RNA (circRNA) ciRS-7 targets miRNA-7 trafficking and ubiquitin-conjugase E2A (UBE2A)-mediated protein degradation in Alzheimer’s disease (AD) and age-related macular degeneration (AMD). Invest Ophthalmol Vis Sci 57(12):5778
Pei X et al (2018) Overexpression of circRNA-001175 promotes proliferation and angiogenesis and inhibits apoptosis of the human umbilical vein endothelial cells (HUVECs) induced by high glucose. Int J Clin Exp Pathol 11(1):359–366
Pan L et al (2018) Human circular RNA-0054633 regulates high glucose-induced vascular endothelial cell dysfunction through the microRNA-218/roundabout 1 and microRNA-218/heme oxygenase-1 axes. Int J Mol Med 42(1):597–606
Zhang S-J et al (2017) Identification and characterization of circular RNAs as a new class of putative biomarkers in diabetes retinopathy. Invest Ophthalmol Vis Sci 58(14):6500–6509
Rojiani MV et al (2010) Expression of MMP-2 correlates with increased angiogenesis in CNS metastasis of lung carcinoma. Int J Clin Exp Pathol 3(8):775
Yun JH et al (2017) Endothelial STAT3 activation increases vascular leakage through downregulating tight junction proteins: implications for diabetic retinopathy. J Cell Physiol 232(5):1123–1134
Zadro-Lamoureux LA et al (2009) Effects on XIAP retinal detachment-induced photoreceptor apoptosis. Invest Ophthalmol Vis Sci 50(3):1448–1453
Khodabandehloo H et al (2016) Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res 167(1):228–256
Fang Y et al (2018) Screening of circular RNAs and validation of circANKRD36 associated with inflammation in patients with type 2 diabetes mellitus. Int J Mol Med 42(4):1865–1874
Yang H, Wang Q, Li S (2016) MicroRNA-218 promotes high glucose-induced apoptosis in podocytes by targeting heme oxygenase-1. Biochem Biophys Res Commun 471(4):582–588
Mahmoud AM et al (2017) Commiphora molmol resin attenuates diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis by modulating oxidative stress, inflammation, angiogenesis and Nrf2/ARE/HO-1 signaling. Chem Biol Interact 270:41–50
Yang J-H et al (2018) The differentially expressed circular RNAs in the substantia nigra and corpus striatum of Nrf2-knockout mice. Cell Physiol Biochem 50(3):936–951
Cheng J, Liu Q, Hu N, Zheng F, Zhang X, Ni Y et al (2019) Downregulation of hsa_circ_0068087 ameliorates TLR4/NF-κB/NLRP3 inflammasome-mediated inflammation and endothelial cell dysfunction in high glucose conditioned by sponging miR-197. Gene 709:1–7
Parveen A, Jin M, Kim SY (2018) Bioactive phytochemicals that regulate the cellular processes involved in diabetic nephropathy. Phytomedicine 39:146–159
Kölling M et al (2018) The circular RNA ciRs-126 predicts survival in critically ill patients with acute kidney injury. Kidney Int Rep 3(5):1144–1152
Cheng X, Joe B (2017) Circular RNAs in rat models of cardiovascular and renal diseases. Physiol Genom 49:484–490
Hu W et al (2019) Circular RNA circRNA_15698 aggravates the extracellular matrix of diabetic nephropathy mesangial cells via miR-185/TGF-β1. J Cell Physiol 234(2):1469–1476
Dangwal S et al (2015) Impairment of wound healing in patients with type 2 diabetes mellitus influences circulating microRNA patterns via inflammatory cytokines. Arterioscler Thromb Vasc Biol 35(6):1480–1488
Yang Z-G et al (2017) The circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Mol Ther 25(9):2062–2074
Vaishya S, Sarwade RD, Seshadri V (2018) MicroRNA, proteins, and metabolites as novel biomarkers for prediabetes, diabetes, and related complications. Front Endocrinol 9:180
Guay C, Regazzi R (2013) Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol 9(9):513
Enuka Y et al (2015) Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res 44(3):1370–1383
Jeck WR, Sharpless NE (2014) Detecting and characterizing circular RNAs. Nat Biotechnol 32(5):453
Zhang Z, Yang T, Xiao J (2018) Circular RNAs: promising biomarkers for human diseases. EBioMedicine 34:267–274
Zhao Z et al (2017) Hsa_circ_0054633 in peripheral blood can be used as a diagnostic biomarker of pre-diabetes and type 2 diabetes mellitus. Acta Diabetol 54(3):237–245
Sally M et al (2017) Plasma circular RNA (0054633) expression as a biomarker for prediabetes and type 2 diabetes mellitus. Bull Egypt Soc Physiol Sci 38(1):77–88
Li X et al (2017) Hsa-circRNA11783-2 in peripheral blood is correlated with coronary artery disease and type 2 diabetes mellitus. Diabetes Vasc Dis Res 14(6):510–515
Gu Y et al (2017) Altered expression profile of circular RNAs in the serum of patients with diabetic retinopathy revealed by microarray. Ophthalmic Res 58(3):176–184
Wang X et al (2017) Investigating factors associated with depressive symptoms of chronic kidney diseases in china with type 2 diabetes. J Diabetes Res 2017:7
Vancampfort D et al (2015) Type 2 diabetes in patients with major depressive disorder: a meta-analysis of prevalence estimates and predictors. Depress Anxiety 32(10):763–773
Jiang G et al (2017) Relationships of circular RNA with diabetes and depression. Sci Rep 7(1):7285
An T, He Z-C, Zhang X-Q, Li J, Chen A-L, Tan F et al (2019) Baduanjin exerts anti-diabetic and anti-depression effects by regulating the expression of mRNA, lncRNA, and circRNA. Chin Med 14(1):3
Alkan F et al (2017) RIsearch2: suffix array-based large-scale prediction of RNA-RNA interactions and siRNA off-targets. Nucleic Acids Res 45(8):e60
Acknowledgements
This work was financially supported by a grant (98U-567) from the Deputy of Research, Abadan Faculty of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghasemi, H., Sabati, Z., Ghaedi, H. et al. Circular RNAs in β-cell function and type 2 diabetes-related complications: a potential diagnostic and therapeutic approach. Mol Biol Rep 46, 5631–5643 (2019). https://doi.org/10.1007/s11033-019-04937-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04937-x