Abstract
Acute myeloid leukemia (AML) has the highest rate of mortality among the leukemias. Disruption in miRNAs level is involved in the pathogenesis of the disease. The miR-155 has a role in primary differentiation of myeloid progenitor. Meanwhile, there is little knowledge about the effects of sulforaphane against leukemia. The present study tried to evaluate pathologic effect of miR-155 in patients in various subgroups of AML, and then pioneered in assessing miR-155 levels by the effect of sulforaphane in different AML cell lines. The miR-155 level was significantly higher in patients with AML compared to the controls. Interestingly, the increase in miR-155 was converged with raising the subtype of AML (from M1 to M5). The miR-155 levels increased by 1.2 times in patients with M1, but this increase reached 2.5 times in the patients in the M5 subgroup. Sulforaphane reduced the number of live cells and increased the mortality rate of AML cells particularly by induction of apoptosis. However, the anti-proliferative effect of this agent was more dominant and could dose-dependently lessen miR-155 levels in myeloid leukemia cells. More or less, about 80% reduction in miR-155 expression was almost observed after 48 h treatment with 60 µM sulforaphane in all four studied cell lines. The obtained results indicated that miR-155 might function as an oncomir in AML and can potentially be considered as a prognosis biomarker for AML. The anti-cancer effects of sulforaphane can be correlated with reduction of miR-155 levels. These findings suggested that sulforaphane could induce more differentiation in myeloid progenitor cells through controlling the miR-155, thereby mitigating the progress of AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a malignancy in hematopoietic progenitor cells. A clear characteristic of this disease is the loss of normal differentiation and proliferation of myeloid precursors [1, 2]. It is the most common acute leukemia among adults and has the highest incidence of mortality among different types of leukemia in the United States, with an annual incidence of 3.7 per 100,000 people [3]. Survival rates are low in patients with AML in comparison with other leukemias and almost one out of every four adults will have a lifespan of more than 5 years [4].
Non-coding RNAs including microRNAs (miRNAs), which play a key role in numerous pathological and physiological pathways, regulate more than 30% of human protein-encoding genes [5]. MicroRNAs could be regulated and transcribed independently [6]. Several studies have indicated that any impairment in miRNAs levels could affect the regulation of cellular differentiation, proliferation, division, apoptosis, and other mechanisms, which consequently have a role in the development of cancer [7]. Hence, due to being involved in pathogenesis, microRNAs could be considered as a diagnostic and/or therapeutic biomarker [8, 9].
MicroRNA-155 is associated with differentiation of myeloid and erythroid cells. Indeed, it has an inhibitory effect on the primary differentiation of hematopoiesis. Studies have also shown that alterations in level of miR-155 in hematopoietic stem cells contribute to the development of myeloid disorders in the bone marrow [10]. Elevated levels of miR-155 have been reported in certain types of lymphoma [11]. It has also been found that any impairment in the expression of miR-155 can be associated with different types of cancer (with an oncogenic role), cardiovascular disease, and viral infection [12].
Sulforaphane is a sub-product derived from the metabolism of cabbage in the body. Meanwhile, the antimicrobial effects of cabbage are often attributed to isothiocyanates, which are the major compounds whose N=C=S group affects cancer cells [13]. It has also been shown that sulforaphane could activate apoptosis pathways in cancer cells. It seems that this compound can activate the pre-apoptotic signaling of caspase-8, caspase-9, and consequently caspase-3 [14]. Indeed, it has been found that sulforaphane as an antioxidant could affect the expression level of miRNAs [15].
Considering the importance of miR-155 in the development of cancer, particularly in AML, the present study was conducted to elucidate the anticancer effect of sulforaphane against AML. The research also tried to discover whether sulforaphane could control the growth rate of AML cells by regulating miR-155 levels. Accordingly, the results of this study can reveal another mechanism for the anticancer activity of sulforaphane in this study.
Materials and methods
Human samples (in vivo study)
Blood samples of people afflicted with AML were obtained from the Pathology Department of Shahid Mohammadi Hospital located in Bandar Abbas City, Iran. The present study was approved by the Ethics and Human Rights Committee of Hormozgan University of Medical Sciences (HUMS), Bandar Abbas, Iran (HUMS.REC.1394.84). Informed consent was obtained from the parents of all participants before the enrolment. Decisive diagnosis of the disease was made by pathologists and oncologists according to the results of flow cytometry, immunohistochemistry, and blood cell count as well as peripheral blood smear test. The control subjects were selected according to their blood cell count, peripheral blood smear test, and then matched according to their age, gender, and even ethnicity. Finally, 25 subjects (afflicted with AML) were assigned to the patient group and 25 to the control group. None of the included subjects had already received radiotherapy or chemotherapy. All those who had been treated some way prior to the study were excluded (Table 1). About 2 ml of blood was taken from both control and treatment patients. Subsequently, 600 µl of blood RNA buffer was mixed with 200 µl of blood and then stored in a deep freezer (− 70 °C).
Cell lines
All four cell-lines including acute myelogenous leukemia cells U937, KG-1, HL-60, and NB-4 were purchased from Pasteur Institute (Tehran, Iran). After passaging the cells, the desired cells were cultured according to their morphology and growth in RPMI-1640 (Gibco), FBS 10%, and penestrep 1% (Invitrogen) and then incubated at 37 °C with humidity 95% and CO2 5%.
MTT assay
In this step, 8000, 20,000, 15,000, and 10,000 cells of NB-4, U-937, KG-1, and HL-60 were cultured, after counting the cell numbers by Neobar lam. The cells were incubated for 2 h under standard conditions. Then, the cells were treated with different concentrations of sulforaphane (Cat No. S4441; Sigma; Germany) (15, 30, 45, and 60 µM) for 24 and 48 h. After incubation, 20 µl of MTT solution (with a final concentration of 0.5 mg/ml) was added to each well and the microplate was incubated at 37 °C for 4 h. After centrifuging the microplate for 10 min in 4000g, the formed crystals were dissolved in 150 µl of DMSO and then the absorbance was read by ELISA Reader (Anthos 2020.England) at 495 ƞm. The cell viability percentage was calculated through the formula OD(T)/OD(C) × 100, where OD(T) represents the absorbance of the cells treated by sulforaphane and OD(C) denotes the absorbance of the non-treated cells.
RNA extraction of cell lines
Four human specimens were recruited for the RNA extraction by TriPure Isolation Reagent kit (Cat No. 11667165001; Sigma; Germany). To investigate the expression of miR-155 in AML cell lines and the effect of sulforaphane on it, 200 µl of the cells were seeded in each well of a 6-well microplate. Next, 200 µl of media was added to each well. Each treated well contained 60, 30, 15, and 120 µM of sulforaphane. These steps were also performed at intervals of 24 and 48 h for each of the mentioned doses. The control well was not treated by sulforaphane.
Analysis of the quality and quantity of RNA
The absorbance of 1.9 to 2 at 280/260 ratio indicated the purity of extracted total RNA using Nanodrop device (ND-1000; Thermo). In order to quantify the extracted specimens, the concentration was calculated according to the absorbance at 260 nm.
To evaluate the quality of extracted total RNAs, the samples were run in agarose gel 1% (100 V). If the extracted RNA has a good quality, it will have three bands in gel including 28S, 18S, and 5S. In the present study, the extracted RNAs had a good quality.
RT-PCR assays
MiScript II RT Kit (Qiagen; Germany) was applied to synthesize cDNA from 1 µg of the extracted total RNA. The reaction mixture was prepared according to the instructions of the kit. Briefly, this mixture was incubated at 37 °C for 60 min and then at 95 °C for 5 min.
The primers applied were for miR-155 (Cat No. MS00031486; Qiagen; Germany) and RNU6-2-11 (Cat No. MS00033740; Qiagen). Briefly, 2 µl of the synthesized cDNA was used in real-time PCR by applying the Script SYBR Green PCR Kit (Qiagen) and the Rotor-Gene (Corbett 6000; Australia). The Real-Time PCR mixture was prepared according to the manufacturer’s instruction (miScript SYBR® Green PCR Kit; Qiagen; Germany). The real-time program was performed in accordance with the instructions as follows: initial shock at 95 °C for 5 min, followed by 40 cycles at 94 °C for 15 s (denaturation), 55 °C for 30 s (annealing), and 70 °C for 30 s (extension). Real-Time PCR was performed two times and every sample was run as triplicates in each time. Finally, the average Ct was totally obtained from the six collected Cts for each sample.
Evaluating the apoptosis of cells
The FITC/Annexin-V kit (Cat No. 640922, BioLegend; UK) was used to identify specific apoptosis and necrosis of the cells. In the present study, we evaluated two-cell lines including KG-1 and HL-60 among the mentioned samples, which were treated with four doses (15, 30, 45 and 60 µM) of sulforaphane within 24- and 48-h intervals.
Statistical analysis
The 2−∆∆CT method was used to evaluate gene expression. Statistical analysis was performed using GraphPad Prism-5, SPSS-21, and Microsoft Excel software packages. P-values less than 0.05 were considered statistically significant in our experiments. In addition, Kolmogorov–Smirnov test was used to check the data distribution and their normality for human specimens. Accordingly, appropriate parametric and non-parametric tests were run to compare the data. Further, t-student, Mann–Whitney U-test, and Wilcoxon two-sample tests were applied to detect significant differences of microRNA-155 levels.
Results
AML patients were categorized into subtypes given the intensity of the disease (according to the scoring) obtained from age, gender, white cell count, and cytogenetic characteristics reported by a pathologist and oncologist. In the present research, most patients were afflicted in M2 subgroup (48% of patients). The results were classified as M1, M4, and M5. The percentage of blast cells of selected patients was averagely more than 71%, which 36% and 64% of patients had respectively less and more than the average the percentage of blast cells, (71%). 48% of participated patients in this project were recognized in stage of M2, 16% in the lowest stage of M1, 20% in stage of M4 and 20% in the highest stage of M5. (Table 1). Totally, 60% of patients belongs to lower stage (M1 + M2) and 40% were in higher stage (M4 + M5).
Expression of miR-155 in patients with AML
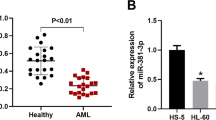
As presented in Fig. 1a, the expression level of miR-155 in patients with AML was higher compared with that in the controls (P = 0.0006). Indeed, at lower stages (subgroups M1 and M2), the expression of miR-155 was significantly lower (P = 0.0025) than that at more advanced stages (subgroups M4 + M5) (Fig. 1b). An increase in miR-155 level showed a significant correlation with the severity of AML. In the M1 subgroup of AML, the expression of miR-155 increased by 1.2 times compared to controls, but this increase reached 2.5 times in the patients in the M5 subgroup (Fig. 1c).
MTT assay
In order to investigate the toxicity of sulforaphane, MTT test was performed for all four cell-lines at 24 and 48 h intervals (Fig. 2). Totally, the percentage of live treated cells decreased compared to non-treated cells. Generally, in each of the four-cell lines treated with sulforaphane, with the increase in the dosage and time, the magnitude of live cells diminished while the mortality rate grew. For instance, the results obtained for HL-60 MTT assay are presented in Fig. 2. In this study, 3% and 8% reduction in cell viability was respectively observed after 24 and 48 h treatment with 15 µM sulforaphane as compared with control group, which treated with only DMSO. The percentage of viable cells reached to 65% and 61%, respectively, after 24 and 48-h treatment with 45 µM sulforaphane. The highest mortality rate across all four-cell lines was not significantly different between 45 and 60 µM.
The effect of sulforaphane in the induction of apoptosis
Apoptosis of HL-60 and KG-1 cells after 48 h treatment with 15, 30, 45, and 60 µM of sulforaphane was evaluated by a flow cytometer. In this study, untreated cells and doxorubicin were considered as negative and positive controls, respectively. The results revealed that elevation of the concentration of sulforaphane induces primary apoptosis of HL-60 (Fig. 3a), where the total apoptosis including early and late also rose. Further, the results indicated that augmenting the percentage of apoptotic cells was directly correlated with elevated dose of sulforaphane. Although at 60 µM (Fig. 3a.v) of sulforaphane, the rate of necrotic cells also grew, it was very low when compared with doxorubicin (a chemotherapeutic agent) (0.9% necrotic cells at 60 µM versus 7.5% by doxorubicin). Early apoptosis, was significantly increased after 48 h treatment with 60 µM sulforaphane as compared with other doses (Fig. 3b). Similar trends were obtained for other studied cell lines (data are not shown).
a Results of FITC-Annexin-V in HL60 cell lines after treatment with sulforaphane obtained by flow cytometry: (i) no treatment as control; (ii) 15 μM; (iii) 30 μM; (iv) 45 μM; (v) 60 μM; (vi) treated with doxorubicin. The graph in b shows the comparison the tabulated of the FITC-Annexin-V flow cytometry data in HL60 cell lines after treatment with different dose sulforaphane
miR-155 levels after treatment with sulforaphane
The expression of miR-155 at the mRNA level was investigated in HL60, KG1, NB4, and U937 cell lines treated with different concentrations (15, 30, 45 and 60 µM) of sulforaphane within 24 and 48 h intervals. The expression of miR-155 compared with non-treated cells was significantly reduced with the rise of sulforaphane concentrations. The results indicated a direct and significant correlation between the increase in sulforaphane concentration and reduction in the expression of miR-155 (Fig. 4).
The lowest expression of miR-155 was achieved after 48 h at sulforaphane concentration of 60 µM (0.14 fold, i.e around 80% reduction as compared to non-treated cells) (Fig. 4a). The same trend was observed for KG-1 cell lines. 85% reduction in miR-155 levels was observed in KG-1 cell line in effect of 48 h treatment with 60 µM, (Fig. 4b). The level of miR-155 reduced for 90% (0.09 fold) in both U-937 and NB-4 cell lines in effect of 48 h treatment with dose of 60 uM sulforaphane (Fig. 4c, d). Overall, increasing the dose of sulforaphane inversely diminished the miR-155 levels, but no significant difference was observed in the reduction of the miR-155 levels between 24 and 48-h treatments with varying doses of sulforaphane across all studied cell lines, except KG-1, where significant differences between 24 and 48 h treatment with different doses of sulforaphane was observed in reduction of miR-155 levels (for instance, 65 and 85% reduction respectively, in effect of 24 and 48 h treatment with dose of 60 µM) (Fig. 4b).
Discussion
Acute myeloid leukemia refers to accumulation of non-differentiated precursor cells in the bone marrow. Several factors contribute to the development of AML. Meanwhile, the use of natural compounds in the treatment of cancer is becoming more popular. In recent decades, numerous traditional medicine and herbal therapies have focused on the use of plant-based chemicals in the treatment of diseases such as cancer. Sulforaphane as an antioxidant has several roles in various stages of cancer. As the anticancer effects of sulforaphane, the role of blocking agents in the pathways of phase-I and II enzymes, cell cycle stopping, apoptosis, DNA protection by inhibiting histone deacetylase (HDAC), anti-proliferation and anti-tumor properties have been reported [16]. Nevertheless, most of its molecular mechanisms have remained unclear in cancer, particularly in AML.
miRNAs contribute to the physiological and pathological pathways involved in the regulation of proliferation and differentiation of myeloid precursor cells. O’Connell et al. [10] showed that changes in miR-155 expression in hematopoietic stem cells play a significant role in the development of myeloid disorders in the bone marrow, with the expression of miR-155 in AML growing excessively [12]. Different in vivo and in vitro studies have indicated that the expression of miR-155 may be affected by downstream signaling of genes involved in the hematopoiesis and leukemia process in AML cells. It was reported that moderate and high expression levels of this miRNA could impair different pathways in these hematopoietic cancer cells [17]. Also, in vitro studies have suggested that miR-155 inhibition can lead to inhibition of AML growth [18].
In this research project, an in vivo study was first conducted to evaluate the level of miR-155 in patients as compared to healthy controls. When it was clarified that miR-155 pathologically and significantly changed in patients, then we investigated whether sulforaphane could control the of AML cells by regulating miR-155 levels (in vitro study). Therefore, the obtained results can probably reveal another mechanism for the anticancer activity of sulforaphane. Very limited studies have been conducted on the effects of sulforaphane on miRNA expression so far. To the best of our knowledge. This study is pioneer has yet been conducted regarding the effect of sulforaphane on miR-155 expression.
In our study, the expression of miR-155 was compared between treatment and control samples. The obtained results indicated that expression of miR-155 significantly rose in the patients with AML, suggesting that it acts as an oncomir in AML. Interestingly, enhanced expression of miR-155 was correlated with increased subtype of AML (from M1 to M4). Our results are consistent with several previous studies. Peng and Core [8] indicated that changes in the expression of miRNAs and the process of cancer are associated with different stages of cancers. It has been reported that the increase in miR-155 expression in myeloid precursor cells is associated with poor prognosis of AML [19]. Xue et al. [20] found that miR-155 could affect some tumor suppressors in AML cells, such as SHIP1. They also reported that inhibition of miR-155 could induce apoptosis without interfering in the proliferation of AML cells. It was also observed that inhibition of miR-155 may be associated with activation of some tumor suppressors, which have an inhibitory effect on the AKT pathway [20].
Sulforaphane reduced the number of live cells and increased the mortality rate of AML cells particularly by induction of apoptosis. The results of flow cytometry demonstrated that apoptosis was induced in AML cell lines due to treatment with sulforaphane. In addition, it was observed that each dose of sulforaphane can show a different effect in the cell lines. It seems that the anti-cancer activity of sulforaphane against myeloid leukemia cells is mostly dose-dependent. Palma et al., [21] investigated the role of miR-155 in inducing apoptosis and cellular differentiation in AML subtypes. It has also been reported that expression of miR-155 decreased in response to the effect of sulforaphane isothiocyanate, where this miRNA was present across a wide range of cell cycles of cancers with increased expression [22]. Lewinska et al. [23] reported that sulforaphane is involved in inhibiting cellular cycle by DNA hypermethylation and changes of miRNA profiles in breast cancer cells. They observed that various doses of sulforaphane could inhibit the cell cycle in the G2/M and G0/G1 stages [23]. Furthermore, our study showed that the survival rate of AML cell lines was significantly augmented by decreasing the sulforaphane concentration (Fig. 3a). This result can be attributed to the vital factors that control the cell cycle and proliferation of cancer cells. Our results showed that most of the cells survived at the lowest dose of sulforaphane. According to the mentioned results, sulforaphane is expected to interfere with the process of the cell cycle of cancer cells. Although sulforaphane induced apoptosis and increased mortality rate in AML cancerous cell lines, it seems that it mostly acts as anti-proliferative agent.
In this project, we found that sulforaphane induced its anti-cancer activity by reducing the miR-155 level, where this impact was dose dependent. In other words, with heightening the sulforaphane concentration, the level of miR-155 was significantly reduced across all studied cell lines. We also observed that the lethal effect of sulforaphane on AML cells increases with elevating the concentrations of this compound, which is correlated with a decline in the expression of miR-155. Therefore, it could be concluded that sulforaphane might induce its anti-cancer effect against myeloid leukemia cells by reducing or even inhibiting primary differentiation of hematopoiesis. These findings conform to the study of O’Connell et al. [10], who reported that miR-155 is involved in the primary differentiation of myeloid progenitors to myeloid and erythroid cells [12]. By controlling the level of miR-155, sulforaphane may provide more time for myeloid progenitor cells to differentiate; hence, it would mitigate the progress of AML; however, further studies are required in this regard.
Conclusion
Briefly, examination of human samples indicated a higher expression of miR-155 in AML patients as compared with controls, which may function as an oncomir. Elevation of miR-155 was correlated with progression of AML from lower subtypes to higher. Thus, it can be considered as a potential biomarker for prognosis of cancer progress. The results suggested that increased expression of miR-155 seems to contribute to AML progression. Therefore, it can be deduced that a decline in the expression of this miRNA contributes to mitigation of the progress of AML in patients. Indeed, the mortality rate of leukemia cells increased by enhancing the dose of sulforaphane, confirming the positive role of this antioxidant in controlling AML. The simultaneous examination of the effect of sulforaphane on myeloid showed a significant induction of apoptosis in the myeloid leukemia cells. However, it seems that the anti-proliferative effects of this herbal isothiocyanate were more dominant and considerable. These findings suggested that sulforaphane exerts its anti-proliferative effects through controlling the miR-155 levels.
References
Döhner H, Weisdorf DJ, Bloomfield CD (2015) Acute myeloid leukemia. N Engl J Med 373(12):1136–1152
Estey E, Döhner H (2006) Acute myeloid leukaemia. Lancet 368(9550):1894–1907
O’Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, Attar E, Borate U, Coutre SE, Damon LE, Lancet J (2013) Acute myeloid leukemia, version 2.2013. J Natl Compr Cancer Netw 11(9):1047–1055
Fiegl M (2016) Epidemiology, pathogenesis, and etiology of acute leukemia. In: Hiddemann W (ed) Handbook of acute leukemia. New York, Springer International Publishing, pp 3–13
Tsuchiya S, Okuno Y, Tsujimoto G (2006) MicroRNA: biogenetic and functional mechanisms and involvements in cell differentiation and cancer. J Pharmacol Sci 101(4):267–270
Song G, Wang L (2008) MiR-433 and miR-127 arise from independent overlapping primary transcripts encoded by the miR-433-127 locus. PloS ONE 3(10):e3574
Zhang B, Pan X, Cobb GP, Anderson TA (2007) microRNAs as oncogenes and tumor suppressors. Dev Biol 302(1):1–2
Peng Y, Croce CM (2016) The role of MicroRNAs in human cancer. Signal Transduct Target Ther 1:15004
Deschler B, Lübbert M (2006) Acute myeloid leukemia: epidemiology and etiology. Cancer 107(9):2099–2107
O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D (2008) Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med 205(3):585–594
O’Connell RM, Zhao JL, Rao DS (2011) MicroRNA function in myeloid biology. Blood 118(11):2960–2969
Faraoni I, Antonetti FR, Cardone J, Bonmassar E (2009) miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta (BBA) Mol Basis Dis 1792(6):497–505
Amjad AI, Parikh RA, Appleman LJ, Hahm ER, Singh K, Singh SV (2015) Broccoli-derived sulforaphane and chemoprevention of prostate cancer: from bench to bedside. Curr Pharmacol Rep 1(6):382–390
Suppipat K, Park CS, Shen Y, Zhu X, Lacorazza HD (2012) Sulforaphane induces cell cycle arrest and apoptosis in acute lymphoblastic leukemia cells. PLoS ONE 7(12):e51251
Liu CM, Peng CY, Liao YW, Lu MY, Tsai ML, Yeh JC, Yu CH, Yu CC (2017) Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction. J Formos Med Assoc 116(1):41–48
Clarke JD, Dashwood RH, Ho E (2008) Multi-targeted prevention of cancer by sulforaphane. Cancer Lett 269(2):291–304
Narayan N, Morenos L, Phipson B, Willis SN, Brumatti G, Eggers S, Lalaoui N, Brown LM, Kosasih HJ, Bartolo RC, Zhou L (2017) Functionally distinct roles for different miR-155 expression levels through contrasting effects on gene expression, in acute myeloid leukaemia. Leukemia 31(4):808
Liang H, Dong Z, Liu JF, Chuang W, Gao LZ, Ren YG (2017) Targeting miR-155 suppresses proliferation and induces apoptosis of HL-60 cells by targeting Slug/PUMA signal. Histol Histopathol 32(9):899–907
Zhu YD, Wang L, Sun C, Fan L, Zhu DX, Fang C, Wang YH, Zou ZJ, Zhang SJ, Li JY, Xu W (2012) Distinctive microRNA signature is associated with the diagnosis and prognosis of acute leukemia. Med Oncol 29(4):2323–2331
Xue H, Hua LM, Guo M, Luo JM (2014) SHIP1 is targeted by miR-155 in acute myeloid leukemia. Oncol Rep 32(5):2253–2259
Palma CA, Al Sheikha D, Lim TK, Bryant A, Vu TT, Jayaswal V, Ma DD (2014) MicroRNA-155 as an inducer of apoptosis and cell differentiation in acute myeloid leukaemia. Mol Cancer 13(1):79
Slaby O, Sachlova M, Brezkova V, Hezova R, Kovarikova A, Bischofová S, Sevcikova S, Bienertova-Vasku J, Vasku A, Svoboda M, Vyzula R (2013) Identification of microRNAs regulated by isothiocyanates and association of polymorphisms inside their target sites with risk of sporadic colorectal cancer. Nutr Cancer 65(2):247–254
Lewinska A, Adamczyk-Grochala J, Deregowska A, Wnuk M (2017) Sulforaphane-induced cell cycle arrest and senescence are accompanied by DNA hypomethylation and changes in microRNA profile in breast cancer cells. Theranostics 7(14):3461
Acknowledgements
The authors highly appreciate the financial support of Research Vice Chancellor of Hormozgan University of Medical Sciences, Bandar Abbas, Iran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors of this article have no conflict of interest.
Rights and permissions
About this article
Cite this article
Koolivand, M., Ansari, M., Piroozian, F. et al. Alleviating the progression of acute myeloid leukemia (AML) by sulforaphane through controlling miR-155 levels. Mol Biol Rep 45, 2491–2499 (2018). https://doi.org/10.1007/s11033-018-4416-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4416-0







