Abstract
Acute myelod leukemia (AML), as a uncontrolled proliferation of cells, was arrested differentiation of progenitor cells. The present study aimed to explore Tanshinone IIA (TIIA) effects on OCI-AML3 and the involvement of the MAPK signaling pathway and miR-497 in TIIA-mediated effects. Cell growth percentage was detected using a cell counting kit. Expression of miR-497 was detected by qPCR. Phosphorylated ERK1/2, JNK and p38 were assessed using western blot. The growth percentage of OCI-AML3 decreased and the effected time increased with increasing TIIA concentration. The miR-497 was upregulated and the p-ERK1/2 was decreased when the TIIA added. TIIA cannot influence the p-ERK1/2. Hence, the proliferation of OCI-AML3 cells was raising. However, when the p-ERK1/2 was inhibited, there no influence on the miR-497 expression after TIIA added. TIIA upregulates miR-497, and decrease the p-ERK1/2 expression, when TIIA simulated OCI-AML3 cell in vitro. And in miR-497 might be involved in the regulation of proliferation in this process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acute myeloid leukemia (AML), as a uncontrolled proliferation of cells, was arrested differentiation of progenitor cells (Huang et al. 2016). The AML patients always do not obtain long-term survival. It was always used the chemotherapy to cue it (Trnovsky et al. 1993; Yu et al. 2010; Bachegowda et al. 2016). However, the complete remission was induced by chemotherapy. To improve the cure rates, the biology and novel targeted therapeutic methods was used. And other drug also was explored (Samanta et al. 2010; Huang et al. 2016; Wang et al. 2018; Han et al. 2019).
MicroRNAs, non-coding small RNAs, play a crucial role in regulating many genes (Zhang and Cohen 2013; Tay et al. 2014). MiRNAs target the 3-UTR of mRNAs with imperfect base-pairing, resulting in decreased translation or altered mRNA degradation (Beckman 2007). microRNAs also associated with AML. Abnormal expression of microRNAs was occurred in the AML patients. And it can help use to find the new treatment regimens. miR-497 can regulate the cell proliferation, apoptosis and other activity (Zhang et al. 2016; Yang et al. 2017; Yin et al. 2018). In the leukemic cells, miRNAs expression profiling revealed significant up-regulation of miR-497 in CD19+ cells compared with the normal B-cell population in chronic lymphocytic leukemia (Yang et al. 2016).
Tanshinone IIA (TIIA) is an important lipophilic diterpene extracted from Danshen a Chinese traditional herbal medicine (Danshen) for the treatment of many diseases, such as cardiovascular, cerebrovascular and postmenopausal syndromes (Jiao and Wen 2011; Kim et al. 2015). TIIA has many function such as anti-oxidative, anti-inflammatory, anti- proliferation. The effect of TIIA on the AML cell was explored. In addition, the involvement of MAPK and miR-497 was investigated.
Material and methods
The human AML cell line (OCI-AML3) (ScienCell, Carlsbad, ca., USA) was cultured under standard conditions (37 °C, 5% CO2, humidified air).
Cell growth
Cell viability and proliferation were evaluated using a Cell Counting Kit 8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan). Cells were added to 96-well plates (3000 cells/well, four replicates) and treated with LPS (0, 5, 10, and 20 ng/ml) for 12, 24, 36, and 48 h. After LPS treatment, cells were incubated with media containing CCK-8 (10 µl CCK-8 in 100 µl medium) for 2 h. The absorbance was determined at 450 nm (Bio-Tek Instruments, Winooski, VT, USA). Cell growth rate was calculated using the following equation: % growth rate = (mean experimental absorbance/mean control absorbance) × 100.
Determination of miRNA levels with qRT-PCR
Total miRNA, isolated with Tiangen reagent, was used as a template for complementary DNA (cDNA) synthesis using M-MLV reverse transcriptase. The Rotor-Gene 6000 system with SYBR Premix Ex Taq (Mansoori et al. 2016) was used for quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). The following primers were used in the qRT-PCR reactions: miR-497 (sense, 5′-CCT TCA GCA GCA CAC TGT GG-3′; antisense, 5′-CAG TGC AGG GTC CGA GGT AT -3′); for U6: (sense, 5′-CTC GCT TCG GCA GCACA-3′; antisense, 5′-TGG TGT CGT GGA GTCG-3′).
Western blotting
HSF lysates were separated using a 12% SDS-PAGE followed by transfer to a nitrocellulose membranes. After blocking, primary antibodies were added (overnight at 4 °C). The membranes were washed (3× with PBS) and species-compatible peroxidase-conjugated secondary antibodies were added. ECL (Pierce, Rockford, IL, USA) was used to detect membrane-bound antibodies.
Cell transfection
The miR-497 inhibitor were obtained from GenePharma (Shanghai, China). Lipofectamine 2000 was used to transfect plasmids or oligonucleotides into cells according to the protocols.
Statistical analysis
Comparisons between groups were made using GraphPad Prism version 5 software (GraphPad Software, La Jolla, ca., USA). One-way analysis of variance (ANOVA) with Newman–Keuls posthoc test was used to determine differences in gene expression levels. Replicates were included in the statistical model. A 95% confidence level (p < 0.05) was deemed statistically significant. Data are shown as mean ± S.D.
Results
The best concentration of TIIA effect on the proliferation of OCI-AML3 cells
To obtain the best concentration of TIIA effect on the proliferation of OCI-AML3 cells, the CCK-8 was used. The results showed that there is no significantly influence with the 5 ng/ml of TIIA. And when the TIIA concentration was reach 15 ng/ml at 36 h, the cell growth percentage was no significantly difference with the 20 ng/ml at 36, and no difference with 48 h. Therefore, the best concentration of TIIA is 15 ng/ml to inhibit the growth of OCI-AML3 cells (Fig. 1).
The influence of miR-497 expression by TIIA
To detect the expression of miR-497, the qPCR was used. The OCI-AML3 cells was effect by TIIA with 15 ng/ml at 0, 4, 8 and 12 h. The miR-497 was detected (Fig. 2). The results showed the expression of miR-497 at 8 and 12 h was higher than 0 and 4 h. However, there is no different between the 8 and 12 h. Hence, the expression of miR-497 was low at 8 h after the TIIA with 15 ng/ml added.
The influence of MAPK pathway by TIIA
The OCI-AML3 cells were effect by TIIA with 15 ng/ml at 0, 4, 8 and 12 h. The phosphorylation status of ERK1/2, JNK, p38 (main parts of MAPK) was detected (Fig. 3). The phosphorylation of ERK1/2 at 8 and 12 h was lower than 0 and 4 h. However, the phosphorylation status of JNK and p38 was no changes with the time increasing.
The influence of miR-497 inhibited
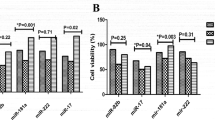
Further, to show the function of miR-497, the miR-497 was silenced by cas9 in OCI-AML3 cells. Then the OCI-AML3 cells were effect by TIIA with 15 ng/ml at 0, 4, 8 and 12 h (Fig. 4a). The phosphorylation of ERK1/2 was no changes with the time increasing (Fig. 4b). And other protein phosphorylation status was also no changes (Fig. 4c, d). The growth percentage of OCI-AML3 cells in miR-497 inhibited + TIIA with 15 ng/ml at 12, 24, 36, 48 h was higher than the TIIA only group. And there is no difference between the TIIA group and miR-497 inhibited group, but higher than blank group (Fig. 4e).
Influence of miR-497 inhibited in OCI-AML3 cells effected by TIIA with 15 ng/ml at 0, 4, 8 and 12 h. a Representative western blots showing ERK1/2, JNK, p38, p-ERK1/2, p-JNK, p-p38 and GAPDH protein detection. b–d Histograms summarizing results from a for ERK1/2, JNK, p38, respectively. e The growth percentage of OCI-AML3 cells effected by TIIA with15 ng/ml a12, 24, 36, 48 h after miR-497 inhibited
The influence of p-ERK1/2 inhibited
To show the function of p-ERK1/2, the p-ERK1/2 was inhibited. Then the OCI-AML3 cells were effect by TIIA with 15 ng/ml at 0, 4, 8 and 12 h. The phosphorylation status of ERK1/2, JNK, p38 was detected (Fig. 5a). The phosphorylation of ERK1/2 was very low in all time (Fig. 5b). However, the other protein phosphorylation status was no changes (Fig. 5c, d). And the miR-497 expression was showed (Fig. 5e). In TIIA group, ERK1/2 inhibited group and ERK1/2 inhibited with TIIA group, the miR-497 at 8 and 12 h was higher than 0 and 4 h. The miR-497 was also higher in these three groups than blank at 8 and 12 h (Fig. 5e). The growth percentage was also detected ( Fig. 5f). In TIIA group, ERK1/2 inhibited group and ERK1/2 inhibited with TIIA group, the OCI-AML3 cells growth percentage was higher than blank.
Influence of p-ERK1/2 inhibited in OCI-AML3 cells effected by TIIA with 15 ng/ml at 0, 4, 8 and 12 h. a Representative western blots showing ERK1/2, JNK, p38, p-ERK1/2, p-JNK, p-p38 and GAPDH protein detection. b–d Histograms summarizing results from a for ERK1/2, JNK, p38, respectively. e The expression of miR-497 in OCI-AML3 cells effected by TIIA with 15 ng/ml at 0, 4, 8 and 12 h. p < 0.05 vs. 0; #p < 0.05 vs. 4 h; &p < 0.05 vs. blank group at the same time piont. f The growth percentage of OCI-AML3 cells effected by TIIA with15 ng/ml a12, 24, 36, 48 h after miR-497 inhibited
Discussion
AML always have short survival. As a global disease, AML is a survival immature progenitors. In AML, many signal pathways were activated constitutive. In this study, we found the ERK1/2 pathway was activated. ERK1/2 pathway are involved in many important physiological processes, such as cell growth, apoptosis, differentiation et al (Hao and Fan 2017; Pedini et al. 2019).
When the TIIA effected on the OCI-AML3 cells, the p-ERK1/2 was down. TIIA a kind of traditional Chinese Medicine, is one of the main fat-soluble ingredients of Danshen, and a proverbial flavonoid that has demonstrated a valid antioxidant for protecting against atherosclerosis. Previous reports show that TIIA inhibits the atherosclerotic lesion in rat and rabbit through suppressing the oxidative stress and inflammation. In this study, the TIIA can inhibit the growth of OCI-AML3 cells.
Recently, the significance of miRNAs in cartilage function has been demonstrated. Multiple miRNAs are differentially expressed in normal skin versus infected skin. These miRNAs influence cellular processes, such as tumorigenesis and endoplasmic reticulum stress (Chen et al. 2019; Faranda et al. 2019). Several studies have reported that increasing miR-497 expression via pre-miR-497 transfection suppresses proliferation and increases apoptosis in adrenocortical carcinoma and pancreatic cancer and inhibits migration and invasion in bladder cancer and nasopharyngeal carcinoma. Additionally, miR-497 over-expression was found to block G0/G1 phase transition in MCF-7 breast cancer cells and induce G1/S arrest in SGC-7901 gastric cancer cells. miR-497 up-regulation by lentivirus LV-miR-497 transfection also significantly represses tumor angiogenesis in hepatocellular carcinoma and ovarian cancer. Conversely, the inhibition of miR-497 expression by an miR-497 inhibitor increased the growth and colony formation ability of breast cancer cells but reduced their apoptosis. miR-497 has also been proven to be relevant in many tumours (Liu et al. 2016; Yang et al. 2016; Chen et al. 2017). In this study, miR-497 can inhibit the growth of OCI-AML3 cells, through ERK1/2. When the miR-497 was inhibited, TIIA cannot influence the p-ERK1/2. Hence, the proliferation of OCI-AML3 cells was raising. However, when the p-ERK1/2 was inhibited, there no influence on the miR-497 expression after TIIA added.
Conclusionly, TIIA upregulates miR-497, and decrease the p-ERK1/2 expression, when TIIA simulated OCI-AML3 cell in vitro. And in miR-497 might be involved in the regulation of proliferation in this proccess. Suppression of miR-497 could active effect on MAPK/ERK1/2 signaling pathway activation. Summy, the TIIA inhibited the proliferation of OCI-AML3 cell by miR-497-p-ERK1/2 signaling pathway.
References
Bachegowda L, Morrone K et al (2016) Pexmetinib: a novel dual inhibitor of Tie2 and p38 MAPK with efficacy in preclinical models of myelodysplastic syndromes and acute myeloid leukemia. Cancer Res 76:4841–4849
Beckman M (2007) MicroRNAs found cavorting with p53. J Natl Cancer Inst 99:1664–1665
Chen X, Shi C et al (2017) The role of miR-497-5p in myofibroblast differentiation of LR-MSCs and pulmonary fibrogenesis. Sci Rep 7:40958
Chen Z, Pan X et al (2019) Baicalin suppresses the proliferation and migration of ox-LDL-VSMCs in atherosclerosis through upregulating miR-126-5p. Biol Pharm Bull 42:1517–1523
Faranda T, Grossi I et al (2019) Differential expression profiling of long non-coding RNA GAS5 and miR-126-3p in human cancer cells in response to sorafenib. Sci Rep 9:9118
Han L, Zhang Q et al (2019) Concomitant targeting of BCL2 with venetoclax and MAPK signaling with cobimetinib in acute myeloid leukemia models. Haematologica 105:697–707
Hao XZ, Fan HM (2017) Identification of miRNAs as atherosclerosis biomarkers and functional role of miR-126 in atherosclerosis progression through MAPK signalling pathway. Eur Rev Med Pharmacol Sci 21:2725–2733
Huang X, Schwind S et al (2016) Targeting the RAS/MAPK pathway with miR-181a in acute myeloid leukemia. Oncotarget 7:59273–59286
Jiao JW, Wen F (2011) Tanshinone IIA acts via p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and lung-resistance protein in cisplatin-resistant ovarian cancer cells. Oncol Rep 25:781–788
Kim JY, Song JJ et al (2015) Tanshinone IIA exerts antitumor activity against vestibular schwannoma cells by inhibiting the expression of hypoxia-inducible factor-1alpha. Mol Med Rep 12:4604–4609
Liu A, Huang C et al (2016) Twist promotes angiogenesis in pancreatic cancer by targeting miR-497/VEGFA axis. Oncotarget 7:25801–25814
Mansoori B, Mohammadi A et al (2016) HMGI-C suppressing induces P53/caspase9 axis to regulate apoptosis in breast adenocarcinoma cells. Cell Cycle 15:2585–2592
Pedini F, De Luca G et al (2019) Joint action of miR-126 and MAPK/PI3K inhibitors against metastatic melanoma. Mol Oncol 13:1836–1854
Samanta SK, Bhattacharya K et al (2010) Identification and quantification of the active component quercetin 3-O-rutinoside from Barringtonia racemosa, targets mitochondrial apoptotic pathway in acute lymphoblastic leukemia. J Asian Nat Prod Res 12:639–648
Tay Y, Tan SM et al (2014) Characterization of dual PTEN and p53-targeting microRNAs identifies microRNA-638/Dnm2 as a two-hit oncogenic locus. Cell Rep 8:714–722
Trnovsky J, Letourneau R et al (1993) Quercetin-induced expression of rat mast cell protease II and accumulation of secretory granules in rat basophilic leukemia cells. Biochem Pharmacol 46:2315–2326
Wang L, Dai HW et al (2018) Effect of quercetin on the cell cycle and adhesion molecules of NOD/SCID mice with acute B lymphocytic leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 26(6):1616–1620
Yang G, Xiong G et al (2016) miR-497 expression, function and clinical application in cancer. Oncotarget 7:55900–55911
Yang M, Li CJ et al (2017) MiR-497 approximately 195 cluster regulates angiogenesis during coupling with osteogenesis by maintaining endothelial Notch and HIF-1alpha activity. Nat Commun 8:16003
Yin Q, Han Y et al (2018) miR-145 and miR-497 suppress TGF-beta-induced epithelial-mesenchymal transition of non-small cell lung cancer by targeting MTDH. Cancer Cell Int 18:105
Yu CS, Lai KC et al (2010) Quercetin inhibited murine leukemia WEHI-3 cells in vivo and promoted immune response. Phytother Res 24:163–168
Zhang W, Cohen SM (2013) The Hippo pathway acts via p53 and microRNAs to control proliferation and proapoptotic gene expression during tissue growth. Biol Open 2:822–828
Zhang N, Shen Q et al (2016) miR-497 suppresses epithelial-mesenchymal transition and metastasis in colorectal cancer cells by targeting fos-related antigen-1. Onco Targets Ther 9:6597–6604
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, H., Tong, C., Li, C. et al. miR-497 plays a key role in Tanshinone IIA-attenuated proliferation in OCI-AML3 cells via the MAPK/ERK1/2 pathway. Cytotechnology 72, 427–432 (2020). https://doi.org/10.1007/s10616-020-00389-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-020-00389-5