Abstract
One risk factor of neovascular age-related macular degeneration is systemic hypertension; hypertension is mainly caused by extracellular hyperosmolarity after consumption of dietary salt. In retinal pigment epithelial (RPE) cells, high extracellular osmolarity induces vascular endothelial growth factor (VEGF)-A (Hollborn et al. in Mol Vis 21:360–377, 2015). The aim of the present study was to determine whether extracellular hyperosmolarity and chemical hypoxia trigger the expression of further VEGF family members including placental growth factor (PlGF) in human RPE cells. Hyperosmotic media were made up by addition of 100 mM NaCl or sucrose. Chemical hypoxia was induced by CoCl2. Gene expression was quantified by real-time RT-PCR, and secretion of PlGF-2 was investigated with ELISA. Nuclear factor of activated T cell 5 (NFAT5) was depleted using siRNA. Extracellular hyperosmolarity triggered expression of VEGF-A, VEGF-D, and PlGF genes, and secretion of PlGF-2. Hypoosmolarity decreased PlGF gene expression. Hypoxia induced expression of VEGF-A, VEGF-B, VEGF-D, and PlGF genes. Extracellular hyperosmolarity and hypoxia produced additive PlGF gene expression. Both hyperosmolarity and hypoxia induced expression of KDR and FLT-4 receptor genes, while hyperosmolarity caused neuropilin-2 and hypoxia neuropilin-1 gene expression. The hyperosmotic, but not the hypoxic, PlGF gene expression was in part mediated by NFAT5. The expression of PlGF in RPE cells depends on the extracellular osmolarity. The data suggest that high consumption of dietary salt may exacerbate the angiogenic response of RPE cells in the hypoxic retina via transcriptional activation of various VEGF family member genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is the primary cause of blindness in elder individuals [1]. Key characteristics of wet (neovascular) AMD are choroidal neovascularization, i.e., the development of abnormal vessels which grow from the choriocapillaris towards the retinal pigment epithelium (RPE) and the subretinal space, and subretinal edema which results from leakage of newly formed vessels and opening of the outer blood-retinal barrier that is normally created by tight junctions between RPE cells [2]. Hypoxia of the outer retina is an important pathogenic condition of choroidal neovascularization [3]. Malfunction of the RPE and edema formation cause photoreceptor degeneration resulting in reduction of the visual acuity [4]. Vascular endothelial growth factor-A (VEGF-A) is the major angiogenic factor that promotes choroidal neovascularization and edema [5]. RPE cells are a source of angiogenic factors like VEGF-A in the retina [6]. However, it has been shown that the action of VEGF-A alone is insufficient to produce choroidal neovascularization [7]. The action of further angiogenic factors is mandatory for the angiogenic effect of VEGF-A [8, 9]. These findings has led to the suggestion that the simultaneous inhibition of various angiogenic factors will have greater therapeutic benefit regarding antiangiogenesis in wet AMD than inhibition of VEGF-A alone [10]. In addition to VEGF-A, RPE cells produce further members of the human VEGF family, i.e., VEGF-B, -C, and -D, as well as placental growth factor (PlGF) [11–16].
It has been shown that, in the ischemic retina, the action of PlGF is indispensable for the angiogenic effect of VEGF [17]. PlGF promotes experimental choroidal neovascularization [18, 19]; the growth of choroidal vessels is inhibited in PlGF-deficient mice [20]. PlGF was shown to induce proliferation of vascular endothelial cells and vascular permeability via a mechanism that includes potentiation of the action of VEGF-A [21–23].
Various factors including age, race, genetic markers and lifestyle factors such as sun light exposure, cigarette smoking, and nutrition influence the risk of AMD. Furthermore, systemic hypertension is associated with a higher risk of wet AMD [24–26]. The primary condition which induces acute hypertension is a rise of the extracellular osmolarity after consumption of dietary salt [27]. There are age-dependent increases of the extracellular osmolarity and the hypertension-inducing effect of dietary salt [28, 29]. On the other hand, the use of antihypertensive medication was shown to be unassociated with the risk of early AMD and with an increased risk of wet AMD [24, 30]. Therefore, it was suggested that hypertension-related conditions such as high extracellular osmolarity and high extracellular salt (NaCl) rather than hypertension per se may aggravate neovascular AMD [31]. Indeed, it has been described that these conditions induce expression and secretion of VEGF-A in RPE cells [31]. However, it is unknown whether high extracellular osmolarity and NaCl, respectively, also induce expression of further VEGF family members like PlGF in RPE cells. Therefore, we determined the effects of high extracellular NaCl on the gene expression levels of VEGF family members in human RPE cells and found that this condition induces expression of VEGF-D and PlGF genes. We further examined the intra- and extracellular signaling mechanisms which contribute to the gene expression of PlGF in RPE cells under high NaCl- and hypoxic conditions, and investigated whether the activity of the osmosensitive nuclear factor of activated T cell 5 (NFAT5) [32] mediates the hyperosmotic expression of the PlGF gene in RPE cells.
Materials and methods
Materials
The culture components were obtained from Gibco BRL (Paisley, UK). Recombinant human transforming growth factor (TGF)-β1 was obtained from R&D Systems (Abingdon, UK). AG1478, HIF inhibitor, LY294002, PD98059, SP600125, and SU1498 were purchased from Calbiochem (Bad Soden, Germany). SB203580 and caffeic acid phenethyl ester (CAPE) were obtained from Tocris (Ellisville, MO). Stattic was from Enzo Life Science (Plymouth Meeting, PA), and PD173034 was kindly provided by Pfizer (Karlsruhe, Germany). Small interfering ribonucleic acid (siRNA) against human NFAT5 and nontargeted siRNA were from Qiagen (Hilden, Germany; catalog numbers SI03076906 and 1027280) and Santa Cruz Biotechnology (Heidelberg, Germany; catalog numbers sc-43968 and sc-37007), respectively. AG1296, SB431542, and the other substances used were purchased from Sigma-Aldrich (Taufkirchen, Germany).
Human material
The study was performed according to the Declaration of Helsinki for research involving human subjects; the use of human retinal cells was approved by the University of Leipzig Ethics Committee (approval #745, 07/25/2011). Eyes of post-mortem cornea donors without reported eye disease were obtained from 13 females and 11 males within 48 h of death; the written informed consent was obtained from the relatives. The age of the donors was 36–79 years (mean ± SD, 56.2 ± 16.0 years for females, and 55.5 ± 16.1 years for males). The donors suffered from following end-stage diseases: multiple organ failure (n = 8), subarachnoid hemorrhage (n = 4), cerebral hemorrhage (n = 3), cardiac infarction (n = 3), pulmonary embolism (n = 2), lung failure and hepatocerebral syndrome due to chronic liver cirrhosis (n = 1), traumatic brain injury (n = 1), cerebral edema (n = 1), and heparin-induced thrombocytopenia, renal failure, and bronchopneumonia (n = 1). Data received with cells of younger and aged donors, and with cells of females and males, were not significantly different (not shown).
Cell culture
Preparation and culture of RPE cells was described previously [33]. Cell lines of different donors were used in passage numbers 3–5. When a confluency of approximately 90 % was achieved, the cells were cultured in serum-free medium for 16 h. Thereafter, test substances were added to the serum-free medium. Extracellular hyperosmolarity was induced by addition of NaCl (100 mM) or sucrose (100 mM) to the culture medium. Extracellular hypoosmolarity (60 % osmolarity) was achieved by addition of distilled water. CoCl2 (150 µM) was used to induce chemical hypoxia. Pharmacological inhibitors were preincubated for 30 min.
Extraction of total RNA and cDNA synthesis
Extraction of the total RNA was performed with the InviTrap Spin Universal RNA Mini Kit (Stratec Molecular, Berlin, Germany). The A260/A280 ratio of the optical density of RNA samples in agarose gel electrophoresis blots (measured with NanoDrop1000; peQLab, Erlangen, Germany) was 1.95–2.03, suggesting adequate RNA quality. The RNA samples were treated with DNase I (Roche, Mannheim, Germany), and cDNA was synthesized from 1 µg RNA with a reverse transcription kit (Fermentas, St. Leon-Roth, Germany).
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Real-time RT-PCR was carried out using the Single-Color Real-Time PCR Detection System (BioRad, Munich, Germany). The primer sequences are given in Table 1. The amplification reaction mixture (15 μl) consisted of 7.5 μl of 2 × iQ SYBR Green Supermix (BioRad), specific primer set (0.2 µM), and 1 μl (1.25 ng) cDNA. Forty five amplification cycles were carried out for each sample (1 cycle of denaturation at 95 °C for 3 min, 45 cycles denaturation at 95 °C for 30 s, annealing at 58 °C for 20 s, extension at 72 °C for 45 s, and melting curve at 55 °C with the temperature gradually increased for 0.5 °C up to 95 °C). To prove the correct lengths of PCR products, the samples were analyzed with agarose gel electrophoresis. RT-PCR for ß-actin messenger (m)RNA was used as an internal control. The results were analyzed with the \(2^{{ - \Delta \Delta C_{\text{t}} }}\) method.
Enzyme-linked immunosorbent assay (ELISA)
Cells were seeded in 12-well plates (3 × 103 cells per well). After reaching a confluency of approximately 90 %, the medium was changed to serum-free medium for 16 h. Thereafter, NaCl (100 mM) or TGF-β1 (10 ng/ml) were added to the serum-free medium. After 24 h, the level of PlGF-2 protein in the cultured media (100 µl) was quantified with ELISA (R&D Systems; catalog number DPG00).
siRNA transfection
Cells were seeded in 12-well plates (7 × 104 cells per well). When a confluency of 60–80 % was achieved, NFAT5 siRNA or nontargeted siRNA were transfected into the cells with HiPerfect reagent (Qiagen) in fetal bovine serum (10 %)-containing F-10 medium. After 48 h, the cells were cultured 2 h in serum-free medium and then for 6 and 24 h in serum-free iso- or hyperosmotic medium (+100 mM NaCl), or isoosmotic medium that contained CoCl2 (150 µM). After RNA extraction, the level of PlGF mRNA was evaluated by real-time RT-PCR.
Statistics
Each experiment was carried out with a cell line derived from one donor. Each test involved at least three experiments using cell lines of different donors. Data are shown as mean ± SEM. Statistical analysis was carried out with Prism (Graphpad Software, San Diego, CA). Comparisons between groups were performed with one-way ANOVA followed by Bonferroni’s multiple comparison test and Mann–Whitney U test. A value of P < 0.05 was considered statistically significant.
Results
Osmotic regulation of VEGF isotype and PlGF genes
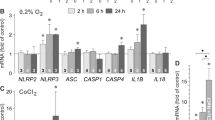
It has been shown that elevation of the extracellular osmolarity induces expression and secretion of VEGF-A in RPE cells [31]. To determine whether extracellular hyperosmolarity also induces expression of further members of the VEGF family, we performed real-time RT-PCR analysis of RNA extracted from cultured human RPE cells. Cells stimulated with a hyperosmotic medium (+100 mM NaCl) displayed increased expression of VEGF-A, VEGF-D, and PlGF genes, while the expression of VEGF-B and VEGF-C genes was not altered compared to cells cultured in isoosmotic medium (Fig. 1a). High extracellular NaCl induced a dose-dependent increase of the cellular PlGF mRNA level (Fig. 1b). The level of PlGF transcripts was also increased when 100 mM sucrose was added to the medium (Fig. 1c). A decrease of the extracellular osmolarity (60 % osmolarity) resulted in a time-dependent reduction of the cellular level of PlGF gene transcripts (Fig. 1d). The data indicate that the expression of the PlGF gene in RPE cells is dependent on the extracellular osmolarity. Addition of 100 mM NaCl to the culture medium also triggered secretion of PlGF-2 protein from the cells (Fig. 1e). In the presence of high NaCl, the mean PlGF-2 protein content of the cultured media was significantly (P < 0.05) increased to 8.7 ± 0.2 pg/ml when compared to unstimulated control (7.5 ± 1.4 pg/ml; mean ± SEM; n = 5). As positive control, TGF-β1 was used (Fig. 1e). TGF-β1 is a known inducer of PlGF-2 secretion from RPE cells [14]. In the presence of TGF-β1 (10 ng/ml), the mean PlGF-2 protein content of the cultured media increased to 9.6 ± 0.9 pg/ml (P < 0.05 vs. unstimulated control; n = 5).
Osmotic and hypoxic regulation of VEGF isotypes and PlGF genes in human RPE cells. mRNA levels in cells cultured 2, 6, and 24 h (as indicated by the panels of the bars), under different conditions were determined with real-time RT-PCR analysis, and are expressed as folds of unstimulated control. The following conditions were tested: hyperosmolarity induced by addition of NaCl (100 mM; a) or sucrose (100 mM; c) to the culture medium, hypoosmolarity (60 % osmolarity; d), and chemical hypoxia induced by addition of CoCl2 (150 µM; f). b Dose-dependent effect of high extracellular NaCl on the cellular level of PlGF mRNA. Extracellular hyperosmolarity was induced by addition of 10–100 mM NaCl to the culture medium. e Secretion of PlGF-2 protein. The cells were stimulated by addition of NaCl (100 mM) and with TGF-β1 (10 ng/ml), respectively, for 24 h. The protein level in the cultured media was measured by ELISA. Data are expressed as percentage of untreated control (100 %). g Effect of coadministration of CoCl2 (150 µM) and NaCl (+100 mM) on the level of PlGF mRNA. The numbers of independent experiments using cell lines derived from different donors are given in or above the bars. Significant difference versus unstimulated control: asterisks P < 0.05. Significant difference versus NaCl control: filled circle P < 0.05. Significant difference versus CoCl2 control: open circle P < 0.05
Hypoxic regulation of VEGF isotype and PlGF genes
In RPE cells, VEGF-A and PlGF are mainly induced by hypoxia [12, 31, 34, 35]. To compare the hypoxic regulation of VEGF isotype and PlGF genes, we performed real-time RT-PCR analysis of RNA from cells cultured in the presence of the hypoxia mimetic CoCl2 [36]. Chemical hypoxia induced expression of VEGF-A, VEGF-B, VEGF-D, and PlGF genes, while the expression of the VEGF-C gene remained unaltered (Fig. 1f). While increased VEGF-A gene expression was already observed after 2 h of hypoxic stimulation, the hypoxic induction of VEGF-B, VEGF-D, and PlGF gene expression was delayed and greatest after 24 h (Fig. 1f). The data show that hypoxia and high extracellular osmolarity induce expression of VEGF-A, VEGF-D, and PlGF genes in RPE cells.
Regulation of VEGF and PlGF receptor genes
Human RPE cells were shown to express different VEGF and PlGF receptor genes including VEGF receptor-1 (VEGFR1; the receptor of VEGF-A, VEGF-B, and PlGF), VEGFR2 (the receptor of VEGF-A, -C, and -D), VEGFR3 (the receptor of VEGF-C and -D), neuropilin-1 (the receptor of specific VEGF-A splice variants, VEGF-B, PlGF-2, and semaphorin-3A), and neuropilin-2 (the receptor of specific VEGF-A splice variants, VEGF-C, PlGF-2, and class 3 semaphorins) [11, 13, 14]. In order to compare the hyperosmotic regulation of VEGF and PlGF receptor genes in RPE cells, we performed real-time RT-PCR analysis of RNA from cells cultured in the presence of high (+ 100 mM) extracellular NaCl. As shown in Fig. 2a, hyperosmotic stimulation induced elevations in the expression of VEGFR2, VEGFR3, and neuropilin-2 genes. The expression of VEGFR1 and neuropilin-1 genes remained unaltered under hyperosmotic conditions (Fig. 2a). Chemical hypoxia induced upregulation of VEGFR2, VEGFR3, and neuropilin-1 genes, while the expression of VEGFR1 and neuropilin-2 genes were not altered (Fig. 2b). The data demonstrate that hypoxia and extracellular hyperosmolarity differentially induce upregulation of PlGF receptor genes in RPE cells, i.e., neuropilin-1 (hypoxia) and neuropilin-2 (hyperosmolarity), respectively.
Hyperosmotic and hypoxic regulation of VEGF and PlGF receptor genes in human RPE cells. mRNA levels in cells cultured 2, 6, and 24 h (as indicated by the panels of the bars) under hyperosmotic (a) and hypoxic conditions (b) were determined with real-time RT-PCR analysis, and are expressed as folds of unstimulated control. Hyperosmolarity and chemical hypoxia were induced by addition of NaCl (100 mM) and CoCl2 (150 µM) to the culture medium, respectively. The gene expression of following receptor proteins was investigated: VEGFR1 (FLT-1), VEGFR2 (KDR), VEGFR3 (FLT-4), neuropilin-1 (NRP1), and neuropilin-2 (NRP2). The numbers of independent experiments using cell lines derived from different donors are given in the bars. Significant difference versus unstimulated control: *P < 0.05
Intracellular signaling involved in PlGF expression
Chemical hypoxia and hyperosmolarity induced additive PlGF gene expression (Fig. 1g), suggesting that different intracellular signaling mechanisms are involved in mediating the gene expression under both conditions. To determine the intracellular signaling mechanisms which mediate the PlGF gene expression, we used inhibitors of key signal transduction pathways. We found that, in dependence on the time period of stimulation, different signal transduction pathways mediate the hyperosmotic PlGF gene expression. After 6 h of stimulation with high extracellular NaCl, the PlGF gene expression was significantly (P < 0.05) decreased by inhibitors of the p38 mitogen-activated protein kinase (p38 MAPK; SB203580) and the phosphatidylinositol-3 kinase (PI3K; LY294002), while after 24 h of stimulation, the PlGF mRNA level was reduced by inhibitors of p38 MAPK (SB203580) and extracellular signal-regulated kinases 1 and 2 (ERK1/2; PD98059) (Fig. 3a). Inhibition of c-Jun NH2-terminal kinase (JNK) by SP600125 did not alter the PlGF mRNA level under hyperosmotic conditions (Fig. 3a). The CoCl2-induced expression of the PlGF gene was significantly (P < 0.05) reduced by the p38 MAPK inhibitor SB203580 after 6 h of stimulation, and by the JNK inhibitor SP600125 after 24 h (Fig. 3b). The hypoxic expression of the PlGF gene was not decreased by inhibitors of ERK1/2 (PD98059) and PI3 K (LY294002) activation (Fig. 3b).
Intracellular signaling involved in osmotic (a) and hypoxic (b) expression of the PlGF gene in RPE cells. a The level of PlGF mRNA was determined with real-time RT-PCR analysis in cells cultured 6 (left) and 24 h (right) in iso- and hyperosmotic (+100 mM NaCl) media, respectively, and are expressed as folds of unstimulated (isoosmotic) control. b The level of PlGF mRNA was determined in cells cultured 6 (left) and 24 h (right) under control conditions and in the presence of CoCl2 (150 µM), respectively. The following pharmacological inhibitors were tested: the inhibitor of p38 MAPK activation, SB203580 (10 µM), the inhibitor of ERK1/2 activation, PD98059 (20 µM), the JNK inhibitor SP600125 (10 µM), and the inhibitor of PI3K-related kinases, LY294002 (5 µM). Vehicle control was made with dimethylsulfoxide (DMSO; 1:1000). The numbers of independent experiments using cell lines derived from different donors are given in the bars. Significant difference versus unstimulated control: asterisks P < 0.05. Significant difference versus NaCl control: filled circle P < 0.05. Significant difference versus CoCl2 control: open circle P < 0.05
Receptor signaling involved in PlGF expression
It has been shown that extracellular hyperosmolarity induces secretion of growth factors like VEGF and bFGF from RPE cells [31, 37]. To evaluate an involvement of autocrine/paracrine receptor activation in mediating the PlGF gene expression under hyperosmotic conditions, we used blockers of receptor kinases. The PlGF gene expression induced by stimulation with high NaCl for 6 h was significantly (P < 0.05) reduced by the blocker of the platelet-derived growth factor (PDGF) receptor tyrosine kinase, AG1296, and increased by the blocker of the fibroblast growth factor (FGF) receptor kinase, PD173074 (Fig. 4a). The NaCl-induced expression of the PlGF gene was not altered by blockers of VEGF receptor-2 (SU1498), the epidermal growth factor (EGF) receptor tyrosine kinase (AG1478), and TGF-β1 superfamily activin receptor-like kinase receptors (SB431542) (Fig. 4a). None of the blockers tested decreased the PlGF gene expression in cells stimulated 24 h with high extracellular NaCl (Fig. 4b). The data suggest that, within 6 h of stimulation with high extracellular NaCl, autocrine/paracrine PDGF signaling may be involved in mediating the PlGF gene expression while autocrine/paracrine FGF signaling inhibits the expression. The hypoxic expression of the PlGF gene was significantly (P < 0.05) decreased by the inhibitor of TGF-β1 superfamily activin receptor-like kinase receptors, SB431542, after 6 h of stimulation with CoCl2, and increased by the VEGF receptor-2 inhibitor SU1498 after 24 h of stimulation (Fig. 4b).
Extracellular signaling involved in the osmotic (a) and hypoxic (b) expression of the PlGF gene in RPE cells. a The level of PlGF mRNA was determined with real-time RT-PCR analysis in cells cultured 6 (left) and 24 h (right) in iso- (control) and hyperosmotic (+100 mM NaCl) media, respectively, and are expressed as folds of unstimulated (isoosmotic) control. b The level of PlGF mRNA was determined in cells cultured 6 (left) and 24 h (right) under control conditions and in the presence of CoCl2 (150 µM), respectively. The following blocking agents were tested: the inhibitor of VEGF receptor-2, SU1498 (10 µM), the blocker of the PDGF receptor tyrosine kinase, AG1296 (10 µM), the inhibitor of the EGF receptor tyrosine kinase, AG1478 (600 nM), the inhibitor of TGF-β1 superfamily activin receptor-like kinase receptors, SB431542 (10 µM), and the FGF receptor kinase inhibitor, PD173074 (500 nM). The numbers of independent experiments using cell lines derived from different donors are given in or above the bars. Significant difference versus unstimulated control: asterisk P < 0.05. Significant difference versus NaCl control: filled circle P < 0.05. Significant difference versus CoCl2 control: open circle P < 0.05
Role of transcription factors in PlGF expression
It was shown that elevated extracellular osmolarity induces the expression of different transcription factors in RPE cells like hypoxia-inducible transcription factor 1α (HIF-1α), nuclear factor (NF)-κB, and NFAT5 [31]. To determine which transcription factors mediate the hyperosmotic and hypoxic PlGF gene expression, we used pharmacological blockers. The hyperosmotic PlGF gene expression was not altered in the presence of a HIF inhibitor [38], the inhibitor of signal transducer and activator of transcription 3 (STAT3), Stattic [39], and the NF-κB inhibitor CAPE [40] (Fig. 5a). The PlGF gene expression induced by chemical hypoxia was significantly (P < 0.05) increased by HIF and NF-κB inhibitors after 6 h of stimulation with CoCl2, and decreased by the STAT3 inhibitor Stattic after 24 h (Fig. 5b). Inhibition of NF-κB activity with CAPE triggered a significant (P < 0.05) upregulation of the PlGF gene expression under unstimulated control conditions which was observed after 24 h but not after 6 h of stimulation (Fig. 5a).
Involvement of transcription factor activities in osmotic (a) and hypoxic (b) expression of the PlGF gene. a The level of PlGF mRNA was determined with real-time RT-PCR analysis in cells cultured 6 (left) and 24 h (right) in iso- (control) and hyperosmotic (+100 mM NaCl) media, respectively, and are expressed as folds of unstimulated (isoosmotic) control. b The level of PlGF mRNA was determined in cells cultured 6 (left) and 24 h (right) under control conditions and in the presence of CoCl2 (150 µM), respectively. The following blocking agents were tested: a HIF inhibitor (HIF-Inh; 5 µM), the STAT3 inhibitor Stattic (1 µM), and the NF-κB inhibitor caffeic acid phenethyl ester (CAPE; 5 µM). The numbers of independent experiments using cell lines derived from different donors are given in the bars. Significant difference versus unstimulated control: asterisk P < 0.05. Significant difference versus CoCl2 control: open circle P < 0.05
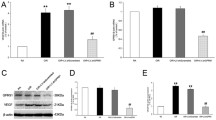
In different cell systems, the cell survival in hyperosmotic stress is supported by NFAT5 [32]. To determine whether hyperosmotic and hypoxic PlGF gene expression depends on NFAT5 activity, we depleted NFAT5 expression using siRNA. NFAT5 siRNA reduced significantly (P < 0.05) the NFAT5 mRNA level by approximately 50 % in cells which were cultured 48 h in control medium (Fig. 6a). Nontargeted siRNA was without effect (Fig. 6a). NFAT5 siRNA also significantly (P < 0.05) diminished the hyperosmotic PlGF gene expression by approximately 50 % while nontargeted siRNA had no effect (Fig. 6b). In contrast, NFAT5 siRNA did not alter the upregulation of the PlGF mRNA level induced by chemical hypoxia (Fig. 6c). Similar results were obtained using NFAT5 siRNA from two different commercial suppliers (not shown). The data indicate that the hyperosmotic upregulation of the PlGF gene expression is in part mediated by the activity of NFAT5.
Involvement of NFAT5 activity in osmotic and hypoxic expression of the PlGF gene. The mRNA levels were determined with real-time RT-PCR analysis in cells transfected with NFAT5 siRNA (siNFAT5; 10 nM) and nontargeted siRNA (siNon; 10 nM), respectively, and are expressed as folds of unstimulated (isoosmotic) control. a Transfection of RPE cells with NFAT5 siRNA resulted in a reduction of the NFAT5 mRNA level in cells cultured 48 h in isoosmotic control medium. Nontargeted siRNA had no effect. b The PlGF mRNA level was determined in cells cultured 6 (left) and 24 h (right) in iso- (control) and hyperosmotic (+100 mM NaCl) media, respectively. c The PlGF mRNA level was determined in cells cultured 6 (left) and 24 h (right) in the presence of CoCl2 (150 µM), respectively. siRNA transfection was done 48 h before osmotic (b) or hypoxic (c) stimulation. The numbers of independent experiments using cell lines derived from different donors are given in the bars. Significant difference versus unstimulated control: asterisk P < 0.05. filled circle P < 0.05
Discussion
Systemic hypertension is associated with a higher risk of wet AMD [24–26]. On the other hand, the use of antihypertensive medication was shown to be associated with an increased risk of wet AMD [24]. Therefore, hypertension-related conditions, in particular high extracellular osmolarity following the consumption of dietary salt [27], are assumed to aggravate the development of neovascular AMD [31]. It was described that elevation of the extracellular osmolarity induces production of VEGF-A in RPE cells [31], the most relevant angiogenic factor implicated in the development of choroidal neovascularization [5]. Here, we describe that increased extracellular osmolarity also promotes the expression of VEGF-D and PlGF genes in RPE cells (Fig. 1a), and that chemical hypoxia induces the expression of VEGF-A, VEGF-B, VEGF-D, and PlGF genes (Fig. 1f). Because PlGF gene expression induced by high NaCl and addition of sucrose had similar time dependencies (Fig. 1a and c), PlGF gene expression is rather caused by extracellular hyperosmolarity and not by variation of the transmembrane NaCl gradient. Apparently, the PlGF gene expression in RPE cells is osmolarity-dependent, with up- (Fig. 1a) and downregulation (Fig. 1d) under hyper- and hypoosmotic conditions, respectively. The expression of the PlGF gene triggered by chemical hypoxia and hyperosmolarity was additive (Fig. 1g), suggesting that extracellular hyperosmolarity induces expression of the PlGF gene in RPE cells independently from hypoxia and that different signaling mechanisms are involved in mediating the PlGF gene expression under these conditions.
NaCl-induced extracellular hyperosmolarity induced expression of VEGFR2, VEGFR3, and neuropilin-2 genes (Fig. 2a). On the other hand, hypoxia induced expression of VEGFR2, VEGFR3, and neuropilin-1 (Fig. 3B). Neuropilins are receptors of specific VEGF isotypes and PlGF and are thought to act in concert with VEGFRs to modulate angiogenic processes. By complex formation between neuropilins and VEGFRs, neuropilins enhance VEGF activity via VEGFRs. The different expression of both neuropilin genes under hyperosmotic and hypoxic conditions may suggest different functional roles of neuropilin-1 and neuropilin-2 in hypoxia and osmotic stress. Whether the different expression of neuropilins results in distinct regulation of the angiogenic action of VEGF and PlGF under hypoxic and hyperosmotic conditions remains to be investigated in future studies.
It was shown that hyperosmotic stress activates different protein kinases like p38 MAPK and ERK1/2 in RPE cells, and induces secretion of growth factors such as VEGF and bFGF [31, 37]. We found that, in dependence on the time period of stimulation, activation of different intracellular signal transduction contribute to the hyperosmotic expression of the PlGF gene (Fig. 3a). However, blockers of p38 MAPK, PI3K, and ERK1/2 activation did not completely inhibit the hyperosmotic PlGF gene expression (Fig. 3a), suggesting that further signaling molecules may be involved. We also found that autocrine/paracrine PDGF signaling is required for the full induction of the PlGF gene expression under hyperosmotic conditions whereas FGF receptor signaling inhibits the gene expression under these conditions (Fig. 4a).
There are conflicting data regarding the hypoxic expression of VEGF isotypes and PlGF in the RPE and retina. It was shown, for example, that hypoxia induces upregulation of VEGF-A and downregulation of VEGF-C and -D in RPE cells [12]. Other studies showed that retinal ischemia induces upregulation VEGF-A and VEGF-C [41], and expression of PlGF protein but not PlGF mRNA [42]. Here, we found that chemical hypoxia induces expression of VEGF-A, VEGF-B, VEGF-D, and PlGF genes in RPE cells (Fig. 1f). The results are in accordance with previous studies that showed that chemical hypoxia induces secretion of VEGF-A, but not VEGF-C, from RPE cells [43], and that hypoxia induces a delayed (15 h) upregulation of PlGF gene expression in RPE cells [35]. While the expression of the VEGF-A gene displayed an immediate upregulation in response to hypoxic stimulation, the expression of the further genes investigated was delayed and displayed a peak after 24 h of stimulation (Fig. 1f). This suggest that different cellular signaling mechanisms are involved in mediating the hypoxic expression of the various genes. We found that the hypoxic expression of the PlGF gene was increased when the VEGF receptor-2 inhibitor SU1498 was present in the culture medium (Fig. 4b), suggesting that autocrine/paracrine VEGF signaling may suppress the hypoxic expression of the PlGF gene. Because we found that a HIF inhibitor increased the hypoxic expression of the PlGF gene (Fig. 5b), it is conceivable that (at least in part) newly synthesized VEGF-A initially suppresses the PlGF gene expression under hypoxic conditions.
It was shown in RPE cells that hyperosmotic stress activates multiple transcription factors like HIF-1α, NF-κB, and NFAT5 [31]. The hyperosmotic expression of the PlGF gene is in part dependent on the activity of NFAT5 (Fig. 6b) while the hypoxic expression is partly dependent on the activity of STAT3 (Fig. 5b). The latter observation is consistent with a recent study which showed that STAT3 activity induces PlGF gene expression in synovial fibroblasts [44]. Inhibition of the NF-κB activity induced a delayed increase of the PlGF gene expression under control conditions (Fig. 5b). We found a similar CAPE-induced upregulation of distinct other growth factor genes in RPE cells, e.g., of the heparin-binding epidermal growth factor-like growth factor gene (M. Hollborn, unpublished data), but not of VEGF-A [31] and bFGF genes (M. Hollborn, unpublished data). The functional significance of these findings remains to be investigated in future studies. It was found in RPE cells that hyperosmotic stress induces expression of NFAT5, and NFAT5 DNA-binding activity, while hypoxia does not induce NFAT5 gene expression [31]. NFAT5 is the major transcription factor that regulates the cell survival in osmotic stress [32]. NFAT5 activity was shown to contribute to the hyperosmotic expression of the VEGF-A gene in RPE cells [31] and to the neuronal degeneration in the retina of diabetic rats [45]. Here, we found that the hyperosmotic (Fig. 6b), but not the hypoxic expression (Fig. 6c) of the PlGF gene in RPE cells is in part dependent on the activity of NFAT5. Together with previous data [31], the present results show that NFAT5 activity induces the expression of various angiogenic factors (VEGF-A and PlGF) in RPE cells under osmotic stress conditions; this suggests that NFAT5 may represent a target to treat choroidal neovascularization in wet AMD.
In summary, the present study shows that osmotic stress induces expression of various VEGF isotype and PlGF genes in RPE cells (Fig. 1a), as well as secretion of PlGF-2 from the cells (Fig. 1e). We observed stimulatory effects on the expression of the PlGF gene after addition of 10 mM NaCl to the culture medium (Fig. 1b). It was described that the blood osmolarity in human subjects can increase to values around 360 mosmol/kg under pathological conditions (which can be achieved by adding 40 mM NaCl to the culture medium) [46, 47]. Therefore, the present results may be relevant for the interpretation of in vivo conditions. The data support the assumption that high consumption of dietary salt may result in direct effects on the RPE. Because high extracellular NaCl induces PlGF expression independently from hypoxia (Fig. 1g), high intake of dietary salt may aggravate the angiogenic response of RPE cells in the hypoxic retina. The salt-induced production of PlGF may contribute to the development of choroidal neovascularization in situ, via promotion of the angiogenic effect of VEGF-A. PlGF produced by RPE cells may stimulate various steps in the development of subretinal neovascularization and edema including vascular tube formation [48], breakdown of the outer blood-retinal barrier [35, 49], and infiltration of microglia/macrophages into the neovascular tissue [18]. On the other hand, RPE-derived PlGF may protect retinal neurons and photoreceptors from degeneration [50, 51]. However, it remains to be investigated in future studies whether salt-induced production of PlGF aggravates the development of wet AMD in vivo, and whether a decrease of the salt consumption and an increase of the water intake (which reduces the extracellular osmolarity) may be helpful to protect the aged retina from the progression of AMD.
References
Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE (2007) Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology 114:253–262
Roth F, Bindewald A, Holz FG (2004) Keypathophysiologic pathways in age-related macular disease. Graefes Arch Clin Exp Ophthalmol 242:710–716
Kent DL (2014) Age-related macular degeneration: beyond anti-angiogenesis. Mol Vis 20:46–55
Green WR, Enger C (1993) Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 100:1519–1535
Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO (2003) Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22:1–29
Adamis AP, Shima DT, Yeo KT, Yeo TK, Brown LF, Berse B, D’Amore PA, Folkman J (1993) Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun 193:631–638
Oshima Y, Oshima S, Nambu H, Kachi S, Hackett SF, Melia M, Kaleko M, Connelly S, Esumi N, Zack DJ, Campochiaro PA (2004) Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J Cell Physiol 201:393–400
Schlingemann RO (2004) Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 242:91–101
De Oliveira Dias JR, Rodrigues EB, Maia M, Magalhães O Jr, Penha FM, Farah ME (2011) Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol 95:1631–1637
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY (2012) Age-related macular degeneration. Lancet 379:1728–1738
Martin G, Schlunck G, Hansen LL, Agostini HT (2004) Differential expression of angioregulatory factors in normal and CNV-derived human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol 242:321–326
Ikeda Y, Yonemitsu Y, Onimaru M, Nakano T, Miyazaki M, Kohno R, Nakagawa K, Ueno A, Sueishi K, Ishibashi T (2006) The regulation of vascular endothelial growth factors (VEGF-A, -C, and -D) expression in the retinal pigment epithelium. Exp Eye Res 83:1031–1040
Zhao B, Ma A, Cai J, Boulton M (2006) VEGF-A regulates the expression of VEGF-C in human retinal pigment epithelial cells. Br J Ophthalmol 90:1052–1059
Hollborn M, Tenckhoff S, Seifert M, Köhler S, Wiedemann P, Bringmann A, Kohen L (2006) Human retinal epithelium produces and responds to placenta growth factor. Graefes Arch Clin Exp Ophthalmol 244:732–741
Hollborn M, Petto C, Steffen A, Trettner S, Bendig A, Wiedemann P, Bringmann A, Kohen L (2009) Effects of thrombin on RPE cells are mediated by transactivation of growth factor receptors. Invest Ophthalmol Vis Sci 50:4452–4459
Puddu A, Sanguineti R, Durante A, Nicolò M, Viviani GL (2012) Vascular endothelial growth factor-C secretion is increased by advanced glycation end-products: possible implication in ocular neovascularization. Mol Vis 18:2509–2517
Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG (2001) Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7:575–583
Huang H, Parlier R, Shen JK, Lutty GA, Vinores SA (2013) VEGF receptor blockade markedly reduces retinal microglia/macrophage infiltration into laser-induced CNV. PLoS One 8:e71808
Tarallo V, Bogdanovich S, Hirano Y, Tudisco L, Zentilin L, Giacca M, Ambati J, De Falco S (2012) Inhibition of choroidal and corneal pathologic neovascularization by Plgf1-de gene transfer. Invest Ophthalmol Vis Sci 53:7989–7996
Rakic JM, Lambert V, Devy L, Luttun A, Carmeliet P, Claes C, Nguyen L, Foidart JM, Noël A, Munaut C (2003) Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci 44:3186–3193
Park JE, Chen HH, Winer J, Houck KA, Ferrara N (1994) Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269:25646–25654
Dull RO, Yuan J, Chang YS, Tarbell J, Jain RK, Munn LL (2001) Kinetics of placenta growth factor/vascular endothelial growth factor synergy in endothelial hydraulic conductivity and proliferation. Microvasc Res 61:203–210
Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Communi D, Shibuya M, Collen D, Conway EM, Carmeliet P (2003) Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors flt1 and flk1. Nat Med 9:936–943
Hyman L, Schachat AP, He Q, Leske MC (2000) Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 118:351–358
Fraser-Bell S, Wu J, Klein R, Azen SP, Hooper C, Foong AW, Varma R (2008) Cardiovascular risk factors and age-related macular degeneration: the Los Angeles Latino Eye Study. Am J Ophthalmol 145:308–316
Klein R, Cruickshanks KJ, Nash SD, Krantz EM, Nieto FJ, Huang GH, Pankow JS, Klein BE (2010) The prevalence of age-related macular degeneration and associated risk factors. Arch Ophthalmol 128:750–758
Lifton RP, Gharavi AG, Geller DS (2001) Molecular mechanisms of human hypertension. Cell 104:545–556
Khaw KT, Barrett-Connor E (1988) The association between blood pressure, age and dietary sodium and potassium: a population study. Circulation 77:53–61
Kenney WL, Chiu P (2001) Influence of age on thirst and fluid intake. Med Sci Sports Exerc 33:1524–1532
Van Leeuwen R, Tomany SC, Wang JJ, Klein R, Mitchell P, Hofman A, Klein BE, Vingerling JR, Cumming RG, de Jong PT (2004) Is medication use associated with the incidence of early age-related maculopathy? Pooled findings from 3 continents. Ophthalmology 111:1169–1175
Hollborn M, Vogler S, Reichenbach A, Wiedemann P, Bringmann A, Kohen L (2015) Regulation of the hyperosmotic induction of aquaporin 5 and VEGF in retinal pigment epithelial cells: involvement of NFAT5. Mol Vis 21:360–377
Cheung CY, Ko BC (2013) NFAT5 in cellular adaptation to hypertonic stress-regulations and functional significance. J Mol Signal 8:5
Chen R, Hollborn M, Grosche A, Reichenbach A, Wiedemann P, Bringmann A, Kohen L (2014) Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in retinal pigment epithelial cells. Mol Vis 20:242–258
Mousa SA, Lorelli W, Campochiaro PA (1999) Role of hypoxia and extracellular matrix-integrin binding in the modulation of angiogenic growth factors secretion by retinal pigmented epithelial cells. J Cell Biochem 74:135–143
Miyamoto N, de Kozak Y, Jeanny JC, Glotin A, Mascarelli F, Massin P, BenEzra D, Behar-Cohen F (2007) Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia 50:461–470
An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM (1998) Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 392:405–408
Veltmann M, Hollborn M, Reichenbach A, Wiedemann P, Kohen L, Bringmann A (2016) Osmotic induction of angiogenic growth factor expression in human retinal pigment epithelial cells. PLoS One 11:e0147312
Lee K, Lee JH, Boovanahalli SK, Jin Y, Lee M, Jin X, Kim JH, Hong YS, Lee JJ (2007) (Aryloxyacetylamino)benzoic acid analogues: a new class of hypoxia-inducible factor-1 inhibitors. J Med Chem 50:1675–1684
Schust J, Sperl B, Hollis A, Mayer TU, Berg T (2006) Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol 13:1235–1242
Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB (1996) Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA 93:9090–9095
Simpson DA, Murphy GM, Bhaduri T, Gardiner TA, Archer DB, Stitt AW (1999) Expression of the VEGF gene family during retinal vaso-obliteration and hypoxia. Biochem Biophys Res Commun 262:333–340
Yao YG, Yang HS, Cao Z, Danielsson J, Duh EJ (2005) Upregulation of placental growth factor by vascular endothelial growth factor via a post-transcriptional mechanism. FEBS Lett 579:1227–1234
Nagineni CN, Raju R, Nagineni KK, Kommineni VK, Cherukuri A, Kutty RK, Hooks JJ, Detrick B (2014) Resveratrol suppresses expression of VEGF by human retinal pigment epithelial cells: potential nutraceutical for age-related macular degeneration. Aging Dis 5:88–100
Tu HJ, Lin TH, Chiu YC, Tang CH, Yang RS, Fu WM (2013) Enhancement of placenta growth factor expression by oncostatin M in human rheumatoid arthritis synovial fibroblasts. J Cell Physiol 228:983–990
Park J, Kim H, Park SY, Lim SW, Kim YS, Lee DH, Roh GS, Kim HJ, Kang SS, Cho GJ, Jeong BY, Kwon HM, Choi WS (2014) Tonicity-responsive enhancer binding protein regulates the expression of aldose reductase and protein kinase Cδ in a mouse model of diabetic retinopathy. Exp Eye Res 122:13–19
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA (2013) Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496:518–522
Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK (2013) Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496:513–517
Akrami H, Soheili ZS, Sadeghizadeh M, Ahmadieh H, Rezaeikanavi M, Samiei S, Khalooghi K (2011) PlGF gene knockdown in human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol 249:537–546
Kowalczuk L, Touchard E, Omri S, Jonet L, Klein C, Valamanes F, Berdugo M, Bigey P, Massin P, Jeanny JC, Behar-Cohen F (2011) Placental growth factor contributes to micro-vascular abnormalization and blood-retinal barrier breakdown in diabetic retinopathy. PLoS One 6:e17462
Englund-Johansson U, Mohlin C, Liljekvist-Soltic I, Ekström P, Johansson K (2010) Human neural progenitor cells promote photoreceptor survival in retinal explants. Exp Eye Res 90:292–299
Inoue Y, Shimazawa M, Nakamura S, Imamura T, Sugitani S, Tsuruma K, Hara H (2014) Protective effects of placental growth factor on retinal neuronal cell damage. J Neurosci Res 92:329–337
Acknowledgments
The authors thank Ute Weinbrecht for excellent technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft (KO 1547/7-1 to L.K.) and the Geschwister Freter Stiftung (Hannover, Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Margrit Hollborn and Konrad Reichmuth have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Hollborn, M., Reichmuth, K., Prager, P. et al. Osmotic induction of placental growth factor in retinal pigment epithelial cells in vitro: contribution of NFAT5 activity. Mol Biol Rep 43, 803–814 (2016). https://doi.org/10.1007/s11033-016-4016-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-016-4016-9