Abstract
Warfarin is an anticoagulant that is difficult to administer because of the wide variation in dose requirements to achieve a therapeutic effect. CYP2C9, VKROC1, and CYP4F2 play important roles in warfarin metabolism, and their genetic polymorphisms are related to the variability in dose determination. In this study we describe a new multiplex pyrosequencing method to identify CYP2C9*3 (rs1057910), VKORC1*2 (rs9923231), and CYP4F2*3 (rs2108661) simultaneously. A multiplex pyrosequencing method to simultaneously detect CYP2C9*3, VKORC1*2, and CYP4F2*3 alleles was designed. We assessed the allele frequencies of the polymorphisms in 250 Korean subjects using the multiplex pyrosequencing method. The results showed 100 % concordance between single and multiplex pyrosequencing methods, and the polymorphisms identified by pyrosequencing were also validated with the direct sequencing method. The allele frequencies of these polymorphisms in this population were as follows: 0.040 for CYP2C9*3, 0.918 for VKORC1*2, and 0.416 for CYP4F2*3. Although the allele frequencies of the CYP2C9*3 and VKROC1*2 were comparable to those in Japanese and Chinese populations, their frequencies in this Korean population differed from those in other ethnic groups; the CYP4F2*3 frequency was the highest among other ethnic populations including Chinese and Japanese populations. The pyrosequencing methods developed were rapid and reliable for detecting CYP2C9*3, VKORC1*2, and CYP4F2*3. Large ethnic differences in the frequency of these genetic polymorphisms were noted among ethnic groups. CYP4F2*3 exhibited its highest allele frequency among other ethnic populations compared to that in a Korean population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Warfarin is the most widely used anticoagulant drug for preventing cardiovascular diseases after ischemic stroke and thromboembolism related to atrial fibrillation, deep vein thrombosis, and pulmonary embolism [1].
However, determining the warfarin dosage is a challenge to clinicians because of its narrow therapeutic range and intersubject variability in the internationalized normal ratios obtained [2, 3].
An increasing number of genetic variations affecting warfarin pharmacokinetics and/or pharmacodynamics have a major impact on dosage requirements such as polymorphisms in the CYP2C9, VKORC1, and CYP4F2 genes [3, 4].
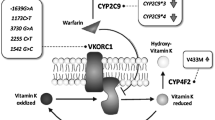
Warfarin is metabolized by CYP2C9, a drug metabolizing enzyme, and the warfarin response is related to CYP2C9 genetic polymorphisms. Several variants in the CYP2C9 gene have been reported, but the most prevalent and most studied are the CYP2C9*2 and CYP2C9*3 polymorphisms. The CYP2C9*2 allele is the result of a C>T transition at position 430 of the CYP2C9 gene, leading to an Arg-to-Cys substitution at residue 144 in the CYP2C9 molecule. The CYP2C9*3 allele is the result of an A>T transition at position 1075 in the CYP2C9 gene, leading to an Ile-to-Leu substitution at residue 359 in the CYP2C9 gene. Both alleles lead to a significant reduction in CYP2C9 enzymatic activity, representing the major cause of decreased CYP2C9 enzymatic activity, but CYP2C9*2 is not found in the Asian population including Koreans [1, 5–8].
Vitamin K epoxide reductase complex unit 1 (VKORC1) is an enzyme that recycles vitamin K 2,3-epoxide, reducing the amount of vitamin K-dependent clotting factors (factor II, VII, IX and X). VKORC1 is one of the most important genetic determinants for warfarin dosing [9–11], and polymorphisms in this gene may explain 20–30 % of the variation in warfarin dosing [1, 12, 13]. Among its polymorphisms, the functional promoter polymorphism G>A at position –1639 (in complete disequilibrium linkage with the intronic polymorphism 1173C>T) influences the warfarin dose requirement [9, 10].
CYP4F2 is primarily responsible for metabolizing arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE). Because 20-HETE is a potent cerebral artery vasoconstrictor, many studies have revealed that CYP4F2 gene polymorphisms are associated with ischemic stroke [14, 15]. CYP4F2 is involved in the metabolism of vitamin K in addition to vitamin K reductase [16]. Additionally, a V433 M polymorphism (CYP4F2*3, rs2108622) is associated with variations in vitamin K metabolic activity in vitro.
The distribution of the CYP2C9*3, VKORC*3, and CYP4F2*3 polymorphisms has been extensively assessed in many populations alone and together. Recent studies have revealed that pharmacogenetic models using these polymorphisms and clinical factors facilitate more accurate predictions of warfarin dose in various populations [1, 12, 17–19]. However, literature reviews showed that previous genetic analyses of these polymorphisms were conducted either by polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP), real-time PCR, or direct sequencing analyses. However, these methods are time-consuming or cost-ineffective [20, 21]. Therefore, we developed a method to identify CYP2C9*3, VKORC1*2, and CYP4F2*3 using a pyrosequencing method. The relatively low cost and rapid results of a pyrosequencing analysis are an advantage when genotyping population data.
Pyrosequencing is a non-electrophoretic, real-time DNA sequencing technology. It involves hybridization of a primer to a single-stranded PCR template and initiating the sequencing analysis by adding nucleotides [22]. It is consistent, easy to use, economically viable, and generates a high throughput analysis with a very high success rate [22]. Generally, one PCR fragment is produced for each sequencing reaction in the genotyping assay. Recently, a new single-tube multiplex pyrosequencing method for several polymorphisms in a single pyrosequencing reaction was developed [21, 23].
In this study, we developed a multiplex pyrosequencing method that can clinically detect the CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms simultaneously. To validate this method and to compare these allele frequencies among ethnic populations, we investigated the allelic frequencies of CYP2C9*3, VKORC1*2, and CYP4F2*3 in a Korean population, and compared them to those in other ethnic groups.
Materials and methods
Subjects and methods
Genomic DNA samples were obtained from 250 unrelated Korean subjects, and written and informed consent was obtained. This study protocol was approved by the ethics committee of Anam Hospital, Seoul, Korea.
Pyrosequencing method for detecting the CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms
Genomic DNA was isolated from peripheral leukocytes, as described previously [21]. We developed a pyrosequencing method to identify the following single nucleotide polymorphisms (SNPs): CYP2C9*3 c.1075A>T (rs1057910), VKORC1*2 (i-1639G>A) (rs9923231), and CYP4F2*3 c.1347C>T (rs2108622). The primers used for the PCR reaction and pyrosequencing are described in Table 1. PCR reactions were carried out to amplify sequences and identify each SNP using newly developed primer sets after attaching biotin to the 5′ end of each forward (or reverse) primer using PSQ Assay Design software (Pyrosequencing AB, Uppsala, Sweden). The DNA fragments containing polymorphic sites were amplified using newly developed primer sets after attaching biotin to the 5′ end of each forward (or reverse) primer using PSQ Assay Design software (Pyrosequencing AB). PCR was performed in a reaction volume of 30 μl containing genomic DNA (30 ng), 10 × PCR buffer, dNTPs (2.5 mM), 10 pmol primers (1 μl each) and 5 U Taq polymerase (iNtRON, Seongnam, Korea). PCR reactions were carried out with an initial denaturation step of 94 °C for 3 min, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s. A final termination step was performed at 72 °C for 5 min.
For the pyrosequencing reactions, 60 μl of the PCR template in a single well was immobilized by incubation (with shaking at 1400 rpm, 10 min, room temperature) with a mixture of 5 μl streptavidin beads (Streptavidin Sepharose High Performance, GE Healthcare Bio-Science AB, Uppsala, Sweden) and 70 μl binding buffer. A 50 μl aliquot of annealing buffer containing each 0.1 μM sequencing primer was incorporated into each well for primer annealing. All liquid was removed by a Vacuum Prep Workstation (Pyrosequencing AB) for strand separation. The beads captured on probes were incubated in 70 % ethanol, and the solution was flushed through filters for 5 s. The beads were then treated with a denaturing solution (0.2 M NaOH) that was flushed through filters for 5 s. A wash buffer (10 mM Tris–acetate, pH 7.6) was used to rinse the beads for 5 s. All liquid was completely drained from the probes, and the beads were released into a PSQ 96 Plate Low (Pyrosequencing AB) containing the sequencing primer. The PSQ 96 Plate Low was heated at 85 °C for 2 min, and the reactions were allowed to cool to room temperature. The resulting mixture was analyzed on a PSQ 96MA Pyrosequencer (Pyrosequencing AB). The accuracy of pyrosequencing was validated by direct DNA sequencing for the randomly selected samples using the same genomic DNA.
Statistical analysis
Genetic equilibrium and linkage disequilibrium were assessed according to the Hardy–Weinberg equilibrium using SNPalyzer ver 7.0.1 (Dynacom Co., Ltd, Yokohama, Japan).
Results
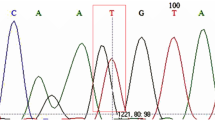
We developed a multiplex pyrosequencing method to identify each SNP for CYP2C9*3, VKORC1*2, and CYP4F2*3 simultaneously. Representative predicted histogram patterns for each genotype are presented in Fig. 1. The assay was designed to generate a specific sequence for each SNP by setting a suitable nucleotide addition order. Nucleotide sequences and pyrograms obtained to identify respective SNPs were consistent with the predicted histograms (Fig. 2). The SNPs and their sequencing data obtained from the pyrosequencing method were validated by comparisons with the direct DNA sequencing of SNPs for randomly selected samples, and the results showed 100 % concordance with the present pyrosequencing results, indicating 100 % specificity and sensitivity for the method.
When we analyzed the SNPs for CYP2C9*3, VKORC1*2, and CYP4F2*3 with the newly developed method in 250 unrelated Korean subjects, the observed allele frequencies of CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms were as follows: 0.040 for CYP2C9*3, 0.918 for VKORC1*2, and 0.416 for CYP4F2*3 (Table 2). All allele frequencies of CYP2C9*3, VKORC1*2, and CYP4F2*3 met Hardy–Weinberg equilibrium (χ2 = 0.0271, P = 0.870 for CYP2C9*3; χ2 = 0.0231, P = 0.879 for VKORC1*2; χ2 = 0.1035, P = 0.748 for CYP4F2*3).
When we compared the allele frequencies of these polymorphisms against data reported previously in other ethnic groups, the frequencies of the CYP2C9*3, VKORC1*2, and CYP4F2*3 showed large differences between the various ethnic groups (Table 3).
Discussion
We developed a rapid and robust pyrosequencing method to detect the CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms simultaneously and applied this method to identify these SNPs in a Korean population. The results showed substantial differences in allele frequencies of the CYP2C9*3, VKORC1*2, and CYP4F2*3 genotypes between our Korean sample and other ethnic groups.
A literature review revealed that CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms have been detected individually using PCR–RFLP, real-time PCR, or direct sequencing. However, this is the first study to identify these polymorphisms simultaneously using a pyrosequencing method.
S-Warfarin, the more potent enantiomer of racemic warfarin, is almost exclusively metabolized to 7-hydroxywarfarin by CYP2C9 [2]. Most warfarin studies have focused on CYP2C9*2 and CYP2C9*3 polymorphisms. Compared with subjects homozygous for CYP2C9*1, homozygous CYP2C9*2 reduces CYP2C9 enzyme activity to 12 %, whereas homozygous CYP2C9*3 reduces enzyme activity to 5 % [2, 24]. In accordance with this, a systematic review established that CYP2C9*2 and CYP2C9*3 alleles lead to 17 and 37 % reduction in the daily warfarin dose, respectively [25]. In the present study, the allele frequency of CYP2C9*3 was 0.040. This finding was comparable to that of other Asian populations including Japanese and Chinese [6–8]. However, we did not assess the CYP2C9*2 polymorphism because it is not found in Asian populations including Koreans [6–8]. Unlike East Asian populations, a higher frequency of CYP2C9*3 is found in Romanian, Turkish, and British populations [26, 27].
The VKORC1 genotype predicts 20–30 % of inter-patient variability in warfarin dose in white and Asian populations [1, 28]. In particular, a functional promoter polymorphism G>A at position -1639 is in complete linkage disequilibrium with the intronic i-1173C>T and influences warfarin dose requirement [9, 10]. The allele frequency of VKORC1*2 was 0.918 in this population, suggesting that the variant for the VKORC1*2 genotype is a dominant allele in Koreans. This finding was similar to East Asian populations including Chinese and Japanese [29]. However, we also found that it is a far higher frequency than those observed in British, Canadian, French, and Indonesian populations [19, 30–32]. Many studies have shown that patients with the VKORC1*2 polymorphism have lower dose requirements for warfarin gene-dose dependently [8, 29].
CYP4F2 is a vitamin K1 oxidase involved in the metabolism of vitamin K1 to vitamin K1 dihydroquinone [16]. An in vitro study showed that the CYP4F2*3 polymorphism, encoding a V433 M amino acid change, causes reduced CYP4F2 protein content and enzyme activity [16]. Interestingly, CYP4F2 is the enzyme responsible for most 20-HETE in the kidney, and the CYP4F2*3 polymorphism is associated with the development of hypertension and other cardiovascular outcomes in different studies [33–35]. In this study, we found that the CYP4F2*3 allele frequency was 0.416. When we reviewed the allele frequency in other ethnic groups, we observed that the allele frequency of most ethnic groups was < 0.300. Intriguingly, although the frequencies of CYP2C9*3 and VKORC1*2 in our study were similar to the East Asian populations, the Japanese (0.277) and Chinese (0.236) data are rather more comparable to French (0.294), British (0.0.288), or Canadian (0.297) populations [19, 31, 32, 36]. It suggests that the minor allele frequency (MAF) of the Korean population was relatively higher in CYP4F2*3 than those of other populations. Consistently, other studies done with other Korean populations also exhibited similar allele frequencies with the present findings (MAF = 0.34 ~ 0.35; P > 0.05) [37, 38]. It would appear that the contribution of this polymorphism to the stable warfarin doses could be greater in the Korean population compared with other ethnic populations [37].
In this study, we developed a new pyrosequencing method to analyze three genetic polymorphisms, which play a crucial role in warfarin dosing determination. These polymorphisms are also associated with the dosing of other anticoagulants, such as phenprocoumon and acenocoumarol [4, 39, 40]. The main advantages of a pyrosequencing assay are the relatively lower cost and time consumption [22, 23]. Usually, only one PCR product is used to analyze the genotype in these polymorphisms. However, we developed a multiplex pyrosequencing method to detect these polymorphisms to reduce laboratory steps and to make it more cost-effective [22, 23]. Furthermore, we reduced the time to analyze these polymorphisms simultaneously using the multiplex tool. Thus, we believe this method should be easier to apply to large population studies and routine clinical use [21]. One limitation of present study is that we analyzed CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms only in a Korean population. We could compare their frequencies with other populations based on the literature but it should be better to extend the analyses in other ethnic groups to make the method we developed more convincing and gain a better comparison with other previous methods.
Conclusion
The multiplex pyrosequencing method developed is a rapid and reliable genotyping method to identify CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms simultaneously. A large difference in CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms was noted when comparing this Korean population with other ethnic groups. In particular, CYP4F2*3 in this Korean population showed a higher frequency than that in the other ethnic populations.
References
Wen MS, Lee M, Chen JJ, Chuang HP, Lu LS, Chen CH, Lee TH, Kuo CT, Sun FM, Chang YJ, Kuan PL, Chen YF, Charng MJ, Ray CY, Wu JY, Chen YT (2008) Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin Pharmacol Ther 84(1):83–89. doi:10.1038/sj.clpt.6100453
Wadelius M, Pirmohamed M (2007) Pharmacogenetics of warfarin: current status and future challenges. Pharmacogenomics J 7(2):99–111. doi:10.1038/sj.tpj.6500417
Wang TL, Li HL, Tjong WY, Chen QS, Wu GS, Zhu HT, Hou ZS, Xu S, Ma SJ, Wu M, Tai S (2008) Genetic factors contribute to patient-specific warfarin dose for Han Chinese. Clin Chim Acta 396(1–2):76–79. doi:10.1016/j.cca.2008.07.005
Stehle S, Kirchheiner J, Lazar A, Fuhr U (2008) Pharmacogenetics of oral anticoagulants: a basis for dose individualization. Clin Pharmacokinet 47(9):565–594
Lee HW, Lim MS, Lee J, Jegal MY, Kim DW, Lee WK, Jang IJ, Shin JG, Yoon YR (2012) Frequency of CYP2C9 variant alleles, including CYP2C9*13 in a Korean population and effect on glimepiride pharmacokinetics. J Clin Pharm Ther 37(1):105–111. doi:10.1111/j.1365-2710.2010.01238.x
Nasu K, Kubota T, Ishizaki T (1997) Genetic analysis of CYP2C9 polymorphism in a Japanese population. Pharmacogenetics 7(5):405–409
Chern HD, Ueng TH, Fu YP, Cheng CW (2006) CYP2C9 polymorphism and warfarin sensitivity in Taiwan Chinese. Clin Chim Acta 367(1–2):108–113. doi:10.1016/j.cca.2005.11.026
Lee MT, Chen CH, Chou CH, Lu LS, Chuang HP, Chen YT, Saleem AN, Wen MS, Chen JJ, Wu JY, Chen YT (2009) Genetic determinants of warfarin dosing in the Han-Chinese population. Pharmacogenomics 10(12):1905–1913. doi:10.2217/pgs.09.106
D’Andrea G, D’Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, Grandone E, Margaglione M (2005) A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood 105(2):645–649. doi:10.1182/blood-2004-06-2111
Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE (2005) Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 352(22):2285–2293. doi:10.1056/NEJMoa044503
Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, Lu MJ, Hung CR, Wei CY, Chen CH, Wu JY, Chen YT (2005) A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet 14(13):1745–1751. doi:10.1093/hmg/ddi180
Wu AH, Wang P, Smith A, Haller C, Drake K, Linder M, Valdes R Jr (2008) Dosing algorithm for warfarin using CYP2C9 and VKORC1 genotyping from a multi-ethnic population: comparison with other equations. Pharmacogenomics 9(2):169–178. doi:10.2217/14622416.9.2.169
Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, Milligan PE, Grice G, Lenzini P, Rettie AE, Aquilante CL, Grosso L, Marsh S, Langaee T, Farnett LE, Voora D, Veenstra DL, Glynn RJ, Barrett A, McLeod HL (2008) Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther 84(3):326–331. doi:10.1038/clpt.2008.10
Miyata N, Roman RJ (2005) Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41(4):175–193
Roman RJ (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82(1):131–185. doi:10.1152/physrev.00021.2001
McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE (2009) CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433 M variant. Mol Pharmacol 75(6):1337–1346. doi:10.1124/mol.109.054833
Schalekamp T, Brasse BP, Roijers JF, van Meegen E, van der Meer FJ, van Wijk EM, Egberts AC, de Boer A (2007) VKORC1 and CYP2C9 genotypes and phenprocoumon anticoagulation status: interaction between both genotypes affects dose requirement. Clin Pharmacol Ther 81(2):185–193. doi:10.1038/sj.clpt.6100036
Krishna Kumar D, Shewade DG, Loriot MA, Beaune P, Balachander J, Sai Chandran BV, Adithan C (2014) Effect of CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants on warfarin maintenance dose and explicating a new pharmacogenetic algorithm in South Indian population. Eur J Clin Pharmacol 70(1):47–56. doi:10.1007/s00228-013-1581-x
Pautas E, Moreau C, Gouin-Thibault I, Golmard JL, Mahe I, Legendre C, Taillandier-Heriche E, Durand-Gasselin B, Houllier AM, Verrier P, Beaune P, Loriot MA, Siguret V (2010) Genetic factors (VKORC1, CYP2C9, EPHX1, and CYP4F2) are predictor variables for warfarin response in very elderly, frail inpatients. Clin Pharmacol Ther 87(1):57–64. doi:10.1038/clpt.2009.178
Mizugaki M, Hiratsuka M, Agatsuma Y, Matsubara Y, Fujii K, Kure S, Narisawa K (2000) Rapid detection of CYP2C18 genotypes by real-time fluorescence polymerase chain reaction. J Pharm Pharmacol 52(2):199–205
Kim KA, Song WK, Kim KR, Park JY (2010) Assessment of CYP2C19 genetic polymorphisms in a Korean population using a simultaneous multiplex pyrosequencing method to simultaneously detect the CYP2C19*2, CYP2C19*3, and CYP2C19*17 alleles. J Clin Pharm Ther 35(6):697–703. doi:10.1111/j.1365-2710.2009.01069.x
Pati N, Schowinsky V, Kokanovic O, Magnuson V, Ghosh S (2004) A comparison between SNaPshot, pyrosequencing, and biplex invader SNP genotyping methods: accuracy, cost, and throughput. J Biochem Biophys Methods 60(1):1–12. doi:10.1016/j.jbbm.2003.11.005
Pourmand N, Elahi E, Davis RW, Ronaghi M (2002) Multiplex Pyrosequencing. Nucleic Acids Res 30(7):e31
Takahashi H, Echizen H (2001) Pharmacogenetics of warfarin elimination and its clinical implications. Clin Pharmacokinet 40(8):587–603
Sanderson S, Emery J, Higgins J (2005) CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med 7(2):97–104
Aynacioglu AS, Brockmoller J, Bauer S, Sachse C, Guzelbey P, Ongen Z, Nacak M, Roots I (1999) Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol 48(3):409–415
Stubbins MJ, Harries LW, Smith G, Tarbit MH, Wolf CR (1996) Genetic analysis of the human cytochrome P450 CYP2C9 locus. Pharmacogenetics 6(5):429–439
Rettie AE, Farin FM, Beri NG, Srinouanprachanh SL, Rieder MJ, Thijssen HH (2006) A case study of acenocoumarol sensitivity and genotype-phenotype discordancy explained by combinations of polymorphisms in VKORC1 and CYP2C9. Br J Clin Pharmacol 62(5):617–620. doi:10.1111/j.1365-2125.2006.02688.x
Yoshizawa M, Hayashi H, Tashiro Y, Sakawa S, Moriwaki H, Akimoto T, Doi O, Kimura M, Kawarasaki Y, Inoue K, Itoh K (2009) Effect of VKORC1-1639 G > A polymorphism, body weight, age and serum albumin alterations on warfarin response in Japanese patients. Thromb Res 124(2):161–166. doi:10.1016/j.thromres.2008.11.011
Biss TT, Avery PJ, Williams MD, Brandao LR, Grainger JD, Kamali F (2013) The VKORC1 and CYP2C9 genotypes are associated with over-anticoagulation during initiation of warfarin therapy in children. J Thromb Haemost 11(2):373–375. doi:10.1111/jth.12072
Suriapranata IM, Tjong WY, Wang T, Utama A, Raharjo SB, Yuniadi Y, Tai SS (2011) Genetic factors associated with patient-specific warfarin dose in ethnic Indonesians. BMC Med Genet 12:80. doi:10.1186/1471-2350-12-80
Wells PS, Majeed H, Kassem S, Langlois N, Gin B, Clermont J, Taljaard M (2010) A regression model to predict warfarin dose from clinical variables and polymorphisms in CYP2C9, CYP4F2, and VKORC1: derivation in a sample with predominantly a history of venous thromboembolism. Thromb Res 125(6):e259–e264. doi:10.1016/j.thromres.2009.11.020
Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD (2008) A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension 51(5):1393–1398. doi:10.1161/HYPERTENSIONAHA.107.104463
Fava C, Montagnana M, Almgren P, Rosberg L, Lippi G, Hedblad B, Engstrom G, Berglund G, Minuz P, Melander O (2008) The V433 M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension 52(2):373–380. doi:10.1161/HYPERTENSIONAHA.108.114199
Stec DE, Roman RJ, Flasch A, Rieder MJ (2007) Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics 30(1):74–81. doi:10.1152/physiolgenomics.00003.2007
Biss TT, Kamali F (2012) Warfarin anticoagulation in children: is there a role for a personalized approach to dosing? Pharmacogenomics 13(11):1211–1214. doi:10.2217/pgs.12.92
Lee KE, Chang BC, Kim HO, Yoon IK, Lee NR, Park HY, Gwak HS (2012) Effects of CYP4F2 gene polymorphisms on warfarin clearance and sensitivity in Korean patients with mechanical cardiac valves. Ther Drug Monit 34(3):275–282. doi:10.1097/FTD.0b013e318256a77c
Choi JR, Kim JO, Kang DR, Yoon SA, Shin JY, Zhang X, Roh MO, Hong HJ, Wang YP, Jo KH, Lee KS, Yun HJ, Oh YS, Yoo KD, Jeon HG, Lee YS, Kang TS, Park HJ, Chung MW, Kang JH (2011) Proposal of pharmacogenetics-based warfarin dosing algorithm in Korean patients. J Hum Genet 56(4):290–295. doi:10.1038/jhg.2011.4
Cerezo-Manchado JJ, Rosafalco M, Anton AI, Perez-Andreu V, Garcia-Barbera N, Martinez AB, Corral J, Vicente V, Gonzalez-Conejero R, Roldan V (2013) Creating a genotype-based dosing algorithm for acenocoumarol steady dose. Thromb Haemost 109(1):146–153. doi:10.1160/TH12-08-0631
Teichert M, Eijgelsheim M, Uitterlinden AG, Buhre PN, Hofman A, De Smet PA, Visser LE, Stricker BH (2011) Dependency of phenprocoumon dosage on polymorphisms in the VKORC1, CYP2C9, and CYP4F2 genes. Pharmacogenet Genomics 21(1):26–34. doi:10.1097/FPC.0b013e32834154fb
Shalia KK, Doshi SM, Parikh S, Pawar PP, Divekar SS, Varma SP, Mehta R, Doctor T, Shah VK, Saranath D (2012) Prevalence of VKORC1 and CYP2C9 gene polymorphisms in Indian population and its effect on warfarin response. J Assoc Physicians India 60:34–38
Sullivan-Klose TH, Ghanayem BI, Bell DA, Zhang ZY, Kaminsky LS, Shenfield GM, Miners JO, Birkett DJ, Goldstein JA (1996) The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 6(4):341–349
Biss TT, Avery PJ, Brandao LR, Chalmers EA, Williams MD, Grainger JD, Leathart JB, Hanley JP, Daly AK, Kamali F (2012) VKORC1 and CYP2C9 genotype and patient characteristics explain a large proportion of the variability in warfarin dose requirement among children. Blood 119(3):868–873. doi:10.1182/blood-2011-08-372722
Kumar DK, Shewade DG, Manjunath S, Ushakiran P, Reneega G, Adithan C (2013) Inter and intra ethnic variation of vitamin K epoxide reductase complex and cytochrome P450 4F2 genetic polymorphisms and their prevalence in South Indian population. Indian J Hum Genet 19(3):301–310. doi:10.4103/0971-6866.120817
Fu Z, Nakayama T, Sato N, Izumi Y, Kasamaki Y, Shindo A, Ohta M, Soma M, Aoi N, Sato M, Matsumoto K, Ozawa Y, Ma Y (2008) Haplotype-based case-control study of the human CYP4F2 gene and essential hypertension in Japanese subjects. Hypertens Res 31(9):1719–1726. doi:10.1291/hypres.31.1719
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (No. 2009-0074126).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KA., Song, WG., Lee, HM. et al. Multiplex pyrosequencing method to determine CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms simultaneously: its application to a Korean population and comparisons with other ethnic groups. Mol Biol Rep 41, 7305–7312 (2014). https://doi.org/10.1007/s11033-014-3617-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3617-4