Abstract
Objective
To determine the influence of genetic polymorphisms on warfarin maintenance dose and to explicate an algorithm using the pharmacogenetic and clinical factors to determine the maintenance and/or starting dose of warfarin in South Indian patients receiving warfarin therapy.
Methods
Patients receiving stabilized warfarin therapy (n = 257) were included in the study. Single nucleotide polymorphisms (SNPs) of CYP2C9 (rs1799853 and rs1057910), VKORC1 (rs9923231, rs7196161, rs2884737, rs9934438, rs8050894, rs2359612 and rs7294), CYP4F2 (rs2108622) and GGCX (rs11676382) were genotyped by the quantitative real time-PCR method.
Results
The mean daily maintenance dose of warfarin was found to be 4.7 ± 2.1 mg/day. Patients with the CYP2C9*1/*2, *1/*3 and *2/*3 variant genotypes required a 51.0 (2.8 mg), 60.9 (2.3 mg) and 62.2 % (2.2 mg) lower daily maintenance dose of warfarin, respectively, than those patients with the CYP2C9*1/*1 wild-type genotype (5.2 mg) (p < 0.0001). The genetic variants of CYP2C9, VKORC1 and GGCX were associated with decreased warfarin dose, except for rs7196161, rs7294 and rs2108622 which were associated with an increased warfarin dose. Genetic variations of CYP2C9 (*2 and *3), VKORC1 (rs9923231, rs7294, rs9934438 and rs2359612), CYP4F2, GGCX and non-genetic factors such as age, body weight, clinical status (post mechanical valve replacement) could explain up to 62.1 % of the overall variation (adjusted r 2 60.2 %, p < 0.0001) in warfarin maintenance dose.
Conclusion
Genetic polymorphisms of CYP2C9, VKORC1, CYP4F2 and GGCX are important predictive factors of warfarin maintenance dose, and the developed algorithm will be useful to predict the required maintenance and/or starting warfarin dose in South Indian populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Warfarin is an oral anticoagulant that is widely prescribed for the prevention of various thromboembolic events [1–5]. It is a drug with a narrow therapeutic index and, therefore, careful monitoring is needed together with accurate and frequent dose adjustment for the prevention of adverse drug reactions (ADRs) related to its use. The efficacy and safety of warfarin therapy are critically dependent on monitoring the prothrombin time (PT)—interpreted as the International Normalized Ratio (INR) [6]. Inter-individual variability to warfarin dose requirement is a major clinical problem and may occur due to the influence of many factors, such as age, sex, genetic variants, illness and drug interactions. Genetic variations in the genes encoding enzymes responsible for warfarin metabolism and its pharmacodynamic actions are major factors that influence the dose requirements.
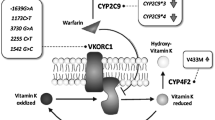
The key enzymes involved in the pharmacodynamics and pharmacokinetics of warfarin include cytochrome P450 (CYP) 2C9 (CYP2C9), vitamin K epoxide reductase complex 1 (VKORC1), CYP4F2 and gamma glutamyl carboxylase (GGCX). Recent studies have demonstrated that pharmacogenetic models using certain genetic polymorphisms of CYP2C9 (CYP2C9*2 and CYP2C9*3), VKORC1 [−1639 G > A, (rs9923231) and other SNPs] and clinical factors facilitate more accurate predictions of warfarin dose in various populations [7–10]. However, to date few studies have included the CYP4F2 (rs2108622) variant in their models. Other studies have shown that genetic polymorphism of the gene encoding GGCX leads to diminished carboxylation and is associated with a lower warfarin dose [11–13]. However, these models were limited to the specified study populations.
South Indians constitutes about 20.97 % of the total population of India and reside in four states [Andhra Pradesh (6.99 %), Tamil Nadu (5.96 %), Karnataka (5.17 %), Kerala (2.75 %)] and a union territory [Pondicherry (0.10 %)] (http://www.censusindia.gov.in/2011-prov-results/paper2-vol2/data_files/India2/Table_1_PR_Districts_TRU.pdf). Although this population shares a common ancestry (Dravidians), South Indians of today differ in terms of language, culture and dietary habits with limited admixture. David Reich et al. [14] found that the ancestral South Indians were distinct from the ancestral North Indians and East Indians. In our previous studies, we demonstrated that South Indians show significant differences from Caucasians, African Americans, Asians and other ethnic population in terms of genetic polymorphisms of drug-metabolizing enzymes and other genes [15–18].
The pharmacogenomics of warfarin therapy has been well studied in many ethnic populations. However, little information on the association of warfarin therapy and genetic polymorphisms is available on the South Indian population. Hence, the aim of this study was to ascertain the influence of genetic variability of the CYP2C9, VKORC1, CYP4F2 and GGCX genes on warfarin maintenance dose requirement in the South Indian patients and to develop a pharmacogenetic algorithm to predict the required warfarin dose based on these genetic and clinical factors.
Materials and methods
Study subjects
Patients aged 18–65 years of either gender who were attending the outpatient clinics of the department of cardiothoracic and vascular surgery and department of cardiology at Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) Hospital, Pondicherry were eligible for inclusion in the study. All patients were of South Indian ethnicity, which was assessed by a review of the family history of three generations living in four states (Tamil Nadu, Kerala, Karnataka and Andhra Pradesh) and a union territory Pondicherry. The study was approved by the Institute Ethics Committee and conducted according to declaration of Helsinki. The study was explained to all study participants and written informed consent was obtained.
All study participants were on stabilized anticoagulation with warfarin for prevention of thromboembolism due to various conditions (Table 1). The mean daily maintenance dose (mg/day) of warfarin was defined as “patients receiving the same dose of warfarin for a period of 3 months with three or more consecutive INR measurements in a 1-week interval within target range (2–3.5)”.
Dose modifications were made based on American College of Chest Physician (ACCP) guidelines (8th edition) for warfarin therapy. Data on each patient’s age, height, weight, body mass index, medication history, INR values and warfarin dose were obtained from the respective patient’s case records. Patients who were on concomitant therapy with drugs potentially interacting with warfarin, patients with liver or renal dysfunction, pregnant and lactating women, smokers and alcoholics were excluded from the study.
Genotyping of CYP2C9, VKORC1, CYP4F2 and GGCX
Samples of venous blood (5 mL) were collected from the study participants for estimation of PT and calculation of INR. Residual blood samples (cellular portion) after estimation of the INR were used for genotyping. DNA was extracted by the phenol–chloroform extraction procedure, and genotyping for polymorphisms of CYP2C9 (*2 and *3 alleles), VKORC1 (rs9923231, rs7196161, rs2884737, rs9934438, rs8050894, rs2359612 and rs7294), CYP4F2 (rs2108622) and GGCX (rs11676382) were performed in the Real-Time Thermo Cycler (model 7300; Applied Biosystems, Life Technologies Corp, Carlsbad, CA) using TaqMan SNP genotyping assays [Electronic Supplementary Material (ESM) Table 1]. The real time (RT)-PCR assays were carried out in triplicate in a 25-μL final volume that contained 12.5 μL of TaqMan universal PCR Master Mix (2×), 1.25 μL of 20× working stock of TaqMan SNP genotyping assay, 5.0 μL of genomic DNA diluted in DNAase-free water and 6.25 μL of MilliQ water (Millipore Corp, Billerica, MA). The thermocycling conditions consisted of one cycle at 95 °C for 10 min to activate the AmpliTaq gold DNA polymerase followed by 45 cycles of denaturation at 92 °C for 15 s and annealing/extension at 60 °C for 1 min. The genotype and allele calls were analyzed using Applied Biosystems PRISM 7300 Sequence Detection System (SDS) ver. 1.4.0 software.
Statistical analysis
GraphPad Instat® ver. 3.06 (GraphPad Software, San Diego, USA) and IBM® SPSS® Statistics ver. 19.0 (SPSS, Chicago, IL) software were used for statistical analysis. The genotype frequencies were analyzed for Hardy–Weinberg equilibrium using the chi-square test. The mean daily maintenance doses were compared between the genotype groups using the Kruskal–Wallis test (Dunn’s post hoc test) and Mann–Whitney U test.
Pairwise linkage disequilibrium (LD) pattern and haplotype frequencies were estimated using HAPLOVIEW ver. 4.1 (Daly Lab, Broad Institute, Cambridge, MA) [19]. All of the SNPs with minor allele frequencies of 0.01 % were excluded, and minimum haplotype frequency was set as 1 %. Haplotype blocks were defined using the solid spine rule incorporated by analysis in HAPLOVIEW software. Haplotypes were estimated by accelerated expectation–maximization (EM) algorithm in HAPLOVIEW. The confidence interval range for LD was set between 0.5 and 0.99. D′ values from 0.8–1.0 indicate strong LD between pair of SNPs, while a D′ value of < 0.8 indicates moderate LD and a D′ value of <0.2 indicates no LD.
The association between the genotype and drug dose was evaluated using linear regression analysis. Stepwise multivariate regression analysis was used to ascertain the influence of the independent variables (clinical and genetic) on the dependent variable (logarithmic transferred daily maintenance dose). All SNPs were included in univariate and multivariate analysis, the homozygote wild type, heterozygote and the homozygote variant genotypes were coded as 0, 1 and 2, respectively. Age, body weight, height and body mass index (BMI) were included as continuous variables. Clinical conditions were grouped and coded: (1) patients with various other conditions were coded as 1; those with mechanical heart valve replacement (required INR 3.0–3.5) were coded as 2. Green vegetable intake was categorized and coded as 0, no intake; 1, one time/week; 2 more than 1 time/week. A p value of <0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics of the subjects
A total of 257 patients were recruited for the study. Of these 17 patients were excluded due to either the lack of complete data or the lack of DNA samples as a result of technical problems, leaving 240 eligible patients ( 36.7 % male, 63.3 %, female). In approximately 74.2 % of patients, the indication for warfarin therapy was either rheumatic heart disease with mitral stenosis, mitral valve regurgitation or post atrial valve replacement. The demographic and clinical characteristics of the patients are presented in Table 1. All of the patients were under maintenance warfarin therapy for at least 3 months; the INR of these patients ranged from 2.0 to 3.5, and furosemide and phenoxymethyl penicillin were found to be co-prescribed with warfarin.
Genotype analysis
In our patient cohort, the genotype frequencies of CYP2C9*1/*1, CYP2C9*1/*2, CYP2C9*1/*3 and CYP2C9*2/*3 were 78.7, 6.7, 13.8 and 0.8 %, respectively, and homozygous CYP2C9*2/*2 and CYP2C9*3/*3 were not identified. The allele frequencies of CYP2C9*1, CYP2C9*2, and CYP2C9*3 were 88.9, 3.8 and 7.3 %, respectively. The genotype and allele frequencies of VKORC1, CYP4F2 and GGCX are given in Table 2. The genotype frequencies of all variants studied were found to be in Hardy–Weinberg equilibrium.
Haplotypes of VKORC1
All seven VKORC1 SNPs were in strong LD with each other. As a consequence, 26 haplotypes were inferred, with eight of these having a frequency of >1 %. The analysis revealed a very strong LD pattern (D′ > 0.8) between SNPs rs7294, rs2359612, rs8050894, rs9923231 and rs7196161 and a moderate LD pattern (0.5 < D′ < 0.8) between SNPs rs9934438 and rs2884737 (ESM Fig. 1). Among the eight major haplotypes, haplotype H1 (includes variant allele of rs7294) (ACGCTGT) was the most frequent (74.0 %) (ESM Table 2). The mean dose (5.46 ± 1.96 mg/day) in the H1 group was significantly different from that in the other haplotype groups, except for the H2 haplotypes. The reference haplotype H2 includes all of the wild-type alleles among the seven SNPs (GCGCTGT); the frequency of this haplotype was found to be 9.0 % and it was associated with a mean warfarin dose (5.83 ± 1.44 mg/day) that was significantly higher than the other combinations. The other haplotype groups consisted of variant alleles and were associated with significantly lower daily doses of warfarin.
The difference in daily maintenance dose of warfarin in the different genotype groups
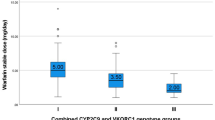
The mean daily warfarin maintenance dose was found to be 4.7 ± 2.1 mg/day. Patients with the CYP2C9*1/*2, CYP2C9*1/*3 or CYP2C9*2/*3 variant genotype required a 51.0, 60.9 or 62.2 %, respectively, lower daily maintenance dose of warfarin (2.8 ± 1.5, 2.3 ± 1.3 or 2.2 ± 0.4 mg, respectively) than the normal CYP2C9*1*1 genotype group (5.2 ± 2.1 mg). The influence of CYP2C9 genotypes on mean daily warfarin dose is shown in Fig. 1. The relative influence of the VKORC1, CYP4F2 and GGCX genotypes on warfarin maintenance dose is given in Table 2. The effect of additive and dominant models on warfarin dose was studied only in the VKORC1, CYP4F2 and GGCX genotype groups. There were no homozygous variants observed in the VKORC1 rs2884737 and GGCX rs11676382 groups. Hence, the additive and dominant models were not compared in these genotypes. In other genotype groups we observed significant differences in warfarin doses in terms of additive and dominant models (Table 2).
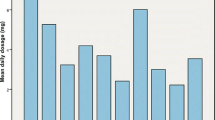
Effect of cytochrome P450 2C9 (CYP2C9) variants on daily warfarin dose in a South Indian population (n = 240). Box and whisker plot showing the distribution of warfarin daily maintenance dose for CYP2C9 genotypes. Box Median and interquartile range (2.5–97.5 percentile), vertical line above and below boxes Minimum and maximum values, respectively, number above the whisker mean value ± standard deviation. p < 0.001 for *1/*1 vs. *1/*2 and *1/*1 vs. *1/*3; p < 0.05 for *1*/1 vs. *2*/3. No significant difference was found between the other genotypes
Among the VKORC1 variants, the −1639 G > A (rs9923231) SNP and the intronic SNPs (rs2884737, rs9934438, rs80850894 and rs235912) were found to be associated with a significant reduction in daily dose. Patients having one defective allele in VKORC1 rs9923231, rs2884737, rs9934438, rs80850894 and rs235912 required a significantly lower daily maintenance dose of warfarin (47.97, 32.94, 48.41, 36.73 and 33.33 % mg/day, respectively) than patients carrying the wild-type allele. Patients having two defective alleles in rs9923231, rs9934438, rs80850894 and rs235912 required an 85.5, 88.9, 78.4 and 80.6 % lower daily maintenance dose of warfarin, respectively, than carriers of the wild-type allele. Among the study population no homozygous variant was identified in rs2884737. The VKORC1 rs7196161 and the untranslated region (UTR) 3′ variant rs7294 carriers required significantly higher doses of warfarin than the carriers of the wild-type genotype. Carriers of the variant allele in CYP4F2 (GA and AA) required significantly higher doses of warfarin (19.7 and 49.0 %, respectively) than carriers of the CYP4F2 GG genotype. In contrast, carriers of the GGCX mutant allele required significantly lower doses of warfarin (45.86 %) than carriers of the GGCX (CC) genotype.
Regression analyses
Univariate analysis was performed to determine the influence of 19 individual factors on daily warfarin dose requirement (Table 3). The results revealed that 17 of these variables were significantly associated with warfarin dose requirements. Vegetable intake and serum albumin levels did not significantly influence the daily maintenance dose of warfarin in the study patients, but only 19 (7.9 %) of the patients reported consuming green vegetables. Our analysis revealed that nongenetic factors (including age, sex, clinical condition, height, weight, BMI) and genetic [in CYP2C9 (*2 and *3), VKORC1 (rs9923231, rs2884737, rs9934438, rs80850894, rs235912, rs7196161 and rs7294), CYP4F2 (rs2108622) and GGCX (rs11676382) genes] factors significantly influenced the daily warfarin maintenance dose. The genetic variation of VKORC1 rs9923231 alone contributed up to 27.5 % of the variation in the required dose.
A multivariate regression analysis was performed by considering the significant variables identified in the univariate analysis. Only age, weight, clinical conditions and genetic variations in VKORC1 (rs9923231, rs2884737, rs9934438, rs80850894, rs235912, rs7196161 and rs7294), CYP2C9 (*2 and *3), CYP4F2 and GGCX were retained in the final model (Table 4). The multivariate regression analysis revealed that clinical and genetic factors together contribute 62.1 % (adjusted r 2 = 0.602, p < 0.0001) of the variation in daily maintenance dose of warfarin, with the genetic factors being the major predictors (46.6 %) of warfarin dose in our South Indian population. The stability of the multivariate model was confirmed by 1,000 bootstrap replications from the original data. The bootstrap validation was performed to determine the percentage variance in warfarin dose requirement, and the bootstrap estimate was found to be 100 %. There was a significant correlation between the actual dose and the predicted dose calculated by using the multivariate model (ESM Fig. 2). Our model predicted 63.5 % of the warfarin dose based on the linear regression curve estimation analysis.
To determine the clinical relevance, the performance of clinical and pharmacogenetic algorithms were compared in the low-dose (≤17.5 mg/week), intermediate-dose (≥17.5 to ≤49.0 mg/week) and high-dose (≥49.0 mg/week) groups (Table 5). For patients who required ≤17.5 mg/week (31.7 % of the total study patients), the pharmacogenetic algorithm yielded a better prediction than the clinical algorithm, with the pharmacogenetic algorithm showing only a 38.2 % overestimation in the low-dose group and the clinical algorithm showing a 86.8 % overestimation. The pharmacogenetic algorithm was found to predict the low and higher doses better than the clinical algorithm. However, there was no significant difference observed between the two algorithms in the intermediate-dose group. The performance of the pharmacogenetic algorithm of our study was compared then with the pharmacogenetic algorithm developed by the International Warfarin Pharmacogenetics Consortium (IWPC). The pharmacogenetic algorithm of our study was found to perform better than the IWPC algorithm in all dose groups, with underestimation being significantly higher in the IWPC algorithm compared with our new algorithm.
Discussion
The aim of our study was to develop a new dosing algorithm specifically designed for the South Indian population that considers clinical data and SNPs associated with warfarin sensitivity and resistance that have been largely unaccounted for in most of the previous studies. Our results in terms of genotype and allele frequencies of CYP2C9 and VKORC1 are in agreement with those of our previous studies. In the South Indian patients population of the present study, the warfarin dose requirement decreased with increasing age, and weight was a significant demographic factor that accounted for 9.2 % (p < 0.05) of the variability in warfarin dose. Among the genetic determinants of warfarin dose, CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants accounted for 12.4, 32.4, 1.9 and 0.6 % of the variability, respectively. The GGCX genetic variant was found to be rare in our population, however its effect on dose variation was significant (univariate r 2 = 0.019, p < 0.05).
Many studies have reported that polymorphisms of VKORC1 are a predictive factor of warfarin dose requirement [8, 20–38]. Our study demonstrated that multiple SNPs of VKORC1 can be used to predict the warfarin maintenance dose. Of the seven VKORC1 SNPS, rs7294 G > A was a more frequent variant and associated with higher warfarin dose (haplotype H1). The wild-type allele (haplotype H2) was also associated with a higher warfarin dose requirement. The haplotypes observed in our study were similar to those observed in our previous study in the Tamilian population [39]. Lal et al. [40] reported that haplotype TCGTCA (H7) is more common in Indians. In agreement with this result, in our study population we found that the TCGTCAG haplotype (H1 but categorized as H7 in previous studies) was associated with a higher warfarin dose requirement and that our study subjects required an intermediate warfarin dose. Several studies have demonstrated that SNPs in the 5′ UTR VKORC1 influences warfarin sensitivity. Rieder et al. demonstrated that nine haplotypes constructed from ten polymorphisms in the VKORC1 gene were significantly associated with warfarin dose requirement [41]. In the same study, the haplotype containing the 1173 C allele was found to be associated with the requirement for a higher warfarin dose than the 1173 T allele. Our results are in agreement with these findings. Previous studies have also shown that CYP2C9 and VKORC1 genetic polymorphisms are associated with variations in warfarin dose of ⋍5–20 % and ⋍13–34 %, respectively [42, 43]. In our study we have observed that CYP2C9 and VKORC1 contributed up to 12.4 and 32.4 % of the variability in warfarin dose, respectively.
In our study we observed that the effect of genetic polymorphisms of CYP2C9 was lower than that of VKORC1 in terms of influencing warfarin maintenance dose. Many studies have been conducted in various ethnic populations, but the predicting factors in the respective pharmacogenetic models differ [20–23, 25–28, 30–32, 35–38]. Although most of the clinical variables in these algorithms are similar, there are large inter-ethnic variations in the genes evaluated for the pharmacogenetic predictive models. The common loci in these algorithms are CYP2C9*2, CYP2C9*3 and VKORC1 -1639 G > A. Only a few studies have included CYP4F2, GGCX and EPHX1 [43–45].
Large inter-ethnic variations in terms of distribution of the variant alleles have been found in each population studied. Asians completely lack carriers of the CYP2C9*2 allele, with the latter being more prevalent in Caucasians. The prevalence of the CYP2C9*2 allele has been reported to be 4 % in South Indians [17]. The prevalence of CYP2C9*3 has been reported to be 5.8 % in Caucasians, 5.3 % in Chinese, 2.3 % in Japanese and 8 % in South Indian populations. Further, the allele frequency of VKORC1-1639A has been reported to be 10.8 % in African Americans, 67.1 % in Asians, 38.2 % in Caucasians and 12 % in the Tamilian population [42]. The distribution of the variant alleles of CYP4F2 and GGCX has also been reported to vary among the world’s populations [13]. Further, evidence that ethnicity is a limiting factor of warfarin maintenance dose has been steadily accumulating. A study conducted in Malays, Chinese and Indians revealed that Indian patients required significant higher doses of warfarin than Malays and Chinese [24]. In this same study, Indians were found to have a larger number of CYP2C9 variant genotypes than Chinese and Malays, and Chinese and Malays had a larger number of VKORC1 variant alleles than Indians. The authors also demonstrated that there was a significant difference in warfarin dose among these different ethnic populations, with Asian patients requiring a lower warfarin dose than Caucasians and Indians [24]. In our study we observed that our South Indian patients required an intermediate warfarin dose.
The IWPC has developed a pharmacogenetic algorithm based on a retrospective study in a relatively large sample size [derivation group n = 4,043; validation group n = 1,009). This algorithm predictes the required weekly warfarin dose more accurately than the clinical algorithm and fixed dose approach [25]. Large randomized clinical trials (RCTs) are currently underway to quantify how the pharmacogenomics-based dose initiation affects laboratory and clinical outcomes. Also, two multi-center RCTs, namely Clarification of Optimal Anticoagulation through Genetics (COAG; USA trial) and Pharmacogenomics Approach to Coumarin Therapy (EU-PACT; European trial) (ClinicalTrials.gov Identifiers NCT00839657 and NCT01119300, respectively) were conducted to compare the clinical algorithms with pharmacogenetic algorithms [46, 47]. The studies were conducted on Caucasians, African Americans and Asians (Chinese and Japanese), but they did not focus on ethnic diversity. However, in our study the population-specific algorithms were more useful than the multi-ethnic algorithms. The United States Food and Drug Administration (US FDA) updated the label of warfarin twice: in 2007, advising physicians to consider the use of “genetic tests to improve their initial estimate” of the initial dosage, and in 2010, adding a new table with the range of expected therapeutic warfarin doses based on CYP2C9 and VKORC1 genotypes (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108967.htm).
A study in a South Indian population by Pavani et al. [29] derived a pharmacogenetic algorithm which predicted up to 44.9 % of warfarin dose requirement. These authors reported that VKORC1 -1639 G > A, VKORC1*3, VKORC1*4, CYP2C9*3, CYP2C9*2, male gender, vitamin K intake, age and body BMI were the major predictive factors and that CYP2C9*3, VKORC1-1639 G > A and VKORC1*3 polymorphisms were associated with warfarin sensitivity. However, in our study we found that age, body weight and clinical conditions, such as post mechanical heart valve replacement, were the important clinical factors to predict warfarin dose. The important genetic factors to predict the warfarin dose in South Indian patients were the genetic polymorphisms of CYP2C9 (CYP2C9*2 and CYP2C9*3), VKORC1 (rs9923231, rs7294, rs9934438 and rs2359612), CYP4F2 (rs2108622) and GGCX (rs11676382). The clinical and genetic factors together contributed to 62.1 % of the variability in the required warfarin dose in our South Indian patients. Furthermore, many studies have explained the variation in warfarin dose requirement based on genetic polymorphisms, such as those in CYP2C18, MDR1, EPHX1, Factor II, Factor X, PROC, PROS and other genes in the warfarin metabolic pathway [43, 44, 48].
One limitation of our study is that it was conducted under strict exclusion criteria. Hence, the study result does not consider the impact of environmental factors, such as smoking and the concomitant consumption of alcohol and drugs. However, the patients attending the anticoagulant clinic of our hospital were advised by clinical pharmacologists on oral anticoagulation therapy and the complications of smoking and alcohol with oral anticoagulation. However, co-morbid conditions, such as liver and renal dysfunction and drug interactions, were not included in our analysis. Further prospective studies with the addition of other genetic factors, such as SNPs in the CYP2C18, MDR1 and EPHX1 genes, a larger sample size and consideration of concomitant drugs and illnesses may significantly contribute to the improvement of our dosing model.
In conclusion, the results of our study explain the effects of CYP2C9, VKORC1, CYP2C9 and GGCX genetic polymorphisms on warfarin daily maintenance dose in a South Indian population receiving warfarin maintenance therapy. The pharmacogenetic algorithm established will be useful for predicting the required starting dose of warfarin in South Indian patients.
References
Buller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE (2004) Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:401S–428S
Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW et al (2004) Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:338S–400S
Harrington RA, Becker RC, Ezekowitz M, Meade TW, O’Connor CM, Vorchheimer DA et al (2004) Antithrombotic therapy for coronary artery disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:513S–548S
Salem DN, Stein PD, Al-Ahmad A, Bussey HI, Horstkotte D, Miller N et al (2004) Antithrombotic therapy in valvular heart disease–native and prosthetic: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:457S–482S
Singer DE, Albers GW, Dalen JE, Go AS, Halperin JL, Manning WJ (2004) Antithrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:429S–456S
Horowitz B, Minor P, Morgenthaler JJ, Burnouf T, McIntosh R, Padilla A et al (2004) WHO Expert Committee on Biological Standardization. World Health Organ Tech Rep Ser 924:1–232
Schalekamp T, Brasse BP, Roijers JF, Chahid Y, van Geest-Daalderop JH, de Vries-Goldschmeding H et al (2006) VKORC1 and CYP2C9 genotypes and acenocoumarol anticoagulation status: interaction between both genotypes affects overanticoagulation. Clin Pharmacol Ther 80:13–22
Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP et al (2005) The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106:2329–2333
Wen MS, Lee M, Chen JJ, Chuang HP, Lu LS, Chen CH et al (2008) Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin Pharmacol Ther 84:83–89
Wu AH, Wang P, Smith A, Haller C, Drake K, Linder M et al (2008) Dosing algorithm for warfarin using CYP2C9 and VKORC1 genotyping from a multi-ethnic population: comparison with other equations. Pharmacogenomics 9:169–178
King CR, Deych E, Milligan P, Eby C, Lenzini P, Grice G et al (2010) Gamma-glutamyl carboxylase and its influence on warfarin dose. Thromb Haemost 104:750–754
Singh O, Sandanaraj E, Subramanian K, Lee LH, Chowbay B (2011) Influence of CYP4F2 rs2108622 (V433M) on warfarin dose requirement in Asian patients. Drug Metab Pharmacokinet 26:130–136
Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ (2010) Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics 11:781–791
Reich D, Thangaraj K, Patterson N, Price AL, Singh L (2009) Reconstructing Indian population history. Nature 461:489–494
Abraham BK, Adithan C, Mohanasundaram J, Shashindran CH, Koumaravelou K, Asad M (2001) Genetic polymorphism of CYP2D6 in Tamil population. Eur J Clin Pharmacol 56:849–850
Naveen AT, Adithan C, Soya SS, Gerard N, Krishnamoorthy R (2006) CYP2D6 genetic polymorphism in South Indian populations. Biol Pharm Bull 29:1655–1658
Jose R, Chandrasekaran A, Sam SS, Gerard N, Chanolean S, Abraham BK et al (2005) CYP2C9 and CYP2C19 genetic polymorphisms: frequencies in the south Indian population. Fundam Clin Pharmacol 19:101–105
Krishnakumar D, Gurusamy U, Dhandapani K, Surendiran A, Baghel R, Kukreti R et al (2012) Genetic polymorphisms of drug-metabolizing phase I enzymes CYP2E1, CYP2A6 and CYP3A5 in South Indian population. Fundam Clin Pharmacol 26:295–306
Barrett JC (2009) Haploview: visualization and analysis of SNP genotype data. Cold Spring Harb Protoc 2009(10). doi: 10.1101/pdb.ip71
Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP et al (2007) Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 116:2563–2570
Cavallari LH, Momary KM, Patel SR, Shapiro NL, Nutescu E, Viana MA (2011) Pharmacogenomics of warfarin dose requirements in Hispanics. Blood Cells Mol Dis 46:147–150
Cini M, Legnani C, Cosmi B, Guazzaloca G, Valdre L, Frascaro M et al (2012) A new warfarin dosing algorithm including VKORC1 3730 G > A polymorphism: comparison with results obtained by other published algorithms. Eur J Clin Pharmacol 68:1167–1174
Gage BF, Eby C, Milligan PE, Banet GA, Duncan JR, McLeod HL (2004) Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb Haemost 91:87–94
Gan GG, Phipps ME, Lee MM, Lu LS, Subramaniam RY, Bee PC et al (2011) Contribution of VKORC1 and CYP2C9 polymorphisms in the interethnic variability of warfarin dose in Malaysian populations. Ann Hematol 90:635–641
Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT et al (2009) Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 360:753–764
Miao L, Yang J, Huang C, Shen Z (2007) Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur J Clin Pharmacol 63:1135–1141
Ohno M, Yamamoto A, Ono A, Miura G, Funamoto M, Takemoto Y et al (2009) Influence of clinical and genetic factors on warfarin dose requirements among Japanese patients. Eur J Clin Pharmacol 65:1097–1103
Oner OG, Langaee TY, Feng H, Buyru N, Ulutin T, Hatemi AC et al (2008) VKORC1 and CYP2C9 polymorphisms are associated with warfarin dose requirements in Turkish patients. Eur J Clin Pharmacol 64:889–894
Pavani A, Naushad SM, Mishra RC, Malempati AR, Pinjala R, Kumar TR et al (2012) Retrospective evidence for clinical validity of expanded genetic model in warfarin dose optimization in a South Indian population. Pharmacogenomics 13:869–878
Shahin MH, Khalifa SI, Gong Y, Hammad LN, Sallam MT, El SM et al (2011) Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet Genomics 21:130–135
Shrif NE, Won HH, Lee ST, Park JH, Kim KK, Kim MJ et al (2011) Evaluation of the effects of VKORC1 polymorphisms and haplotypes, CYP2C9 genotypes, and clinical factors on warfarin response in Sudanese patients. Eur J Clin Pharmacol 67:1119–1130
Takeuchi F, Kashida M, Okazaki O, Tanaka Y, Fukuda S, Kashima T et al (2010) Evaluation of pharmacogenetic algorithm for warfarin dose requirements in Japanese patients. Circ J 74:977–982
Tham LS, Goh BC, Nafziger A, Guo JY, Wang LZ, Soong R et al (2006) A warfarin-dosing model in Asians that uses single-nucleotide polymorphisms in vitamin K epoxide reductase complex and cytochrome P450 2C9. Clin Pharmacol Ther 80:346–355
Verde Z, Ruiz JR, Santiago C, Valle B, Bandres F, Calvo E et al (2010) A novel, single algorithm approach to predict acenocoumarol dose based on CYP2C9 and VKORC1 allele variants. PLoS One 5:e11210
Wadelius M, Chen LY, Lindh JD, Eriksson N, Ghori MJ, Bumpstead S et al (2009) The largest prospective warfarin-treated cohort supports genetic forecasting. Blood 113:784–792
Wang TL, Li HL, Tjong WY, Chen QS, Wu GS, Zhu HT et al (2008) Genetic factors contribute to patient-specific warfarin dose for Han Chinese. Clin Chim Acta 396:76–79
Wells PS, Majeed H, Kassem S, Langlois N, Gin B, Clermont J et al (2010) A regression model to predict warfarin dose from clinical variables and polymorphisms in CYP2C9, CYP4F2, and VKORC1: Derivation in a sample with predominantly a history of venous thromboembolism. Thromb Res 125:e259–e264
Zhu Y, Shennan M, Reynolds KK, Johnson NA, Herrnberger MR, Valdes R Jr et al (2007) Estimation of warfarin maintenance dose based on VKORC1 (−1639 G > A) and CYP2C9 genotypes. Clin Chem 53:1199–1205
Kumar DK, Shewade DG, Surendiran A, Adithan C (2013) Genetic variation and haplotype structure of the gene Vitamin K epoxide reductase complex, subunit 1 in the Tamilian population. J Pharmacol Pharmacother 4:53–58
Lal S, Sandanaraj E, Jada SR, Kong MC, Lee LH, Goh BC et al (2008) Influence of APOE genotypes and VKORC1 haplotypes on warfarin dose requirements in Asian patients. Br J Clin Pharmacol 65:260–264
Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL et al (2005) Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 352:2285–2293
Cooper GM, Johnson JA, Langaee TY, Feng H, Stanaway IB, Schwarz UI et al (2008) A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood 112:1022–1027
Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C et al (2007) Association of warfarin dose with genes involved in its action and metabolism. Hum Genet 121:23–34
Pautas E, Moreau C, Gouin-Thibault I, Golmard JL, Mahe I, Legendre C et al (2010) Genetic factors (VKORC1, CYP2C9, EPHX1, and CYP4F2) are predictor variables for warfarin response in very elderly, frail inpatients. Clin Pharmacol Ther 87:57–64
Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N et al (2009) A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet 5:e1000433
Wang M, Lang X, Cui S, Fei K, Zou L, Cao J et al (2012) Clinical application of pharmacogenetic-based warfarin-dosing algorithm in patients of Han nationality after rheumatic valve replacement: a randomized and controlled trial. Int J Med Sci 9:472–479
Yamashita T, Koretsune Y, Yasaka M, Inoue H, Kawai Y, Yamaguchi T et al (2012) Randomized, multicenter, warfarin-controlled phase II study of edoxaban in Japanese patients with non-valvular atrial fibrillation. Circ J 76:1840–1847
Chan SL, Thalamuthu A, Goh BC, Chia KS, Chuah B, Wong A et al (2011) Exon sequencing and association analysis of EPHX1 genetic variants with maintenance warfarin dose in a multiethnic Asian population. Pharmacogenet Genomics 21:35–41
Acknowledgments
This study was a collaborative project between Institut national de la santé et de la recherche médicale (INSERM) UMR775–Bases Moléculaires de la réponse aux xénobiotiques located in the Université Paris-Descartes, Paris, France and the Indian council of Medical Research (ICMR), New Delhi, India (grant 2011-2012). It was supported by a research grant (ICMR Ref. No. 50/6/2010/BMS, dated 03/11/2010) from the ICMR, New Delhi. The technical staffs of the departments of pharmacology, cardiology and CTVS departments are gratefully acknowledged.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Table 1
Genotype condition for all the SNPs (DOCX 16 kb)
Suppl. Table 2
Assessment of the VKORC1 haplotypes influences on the daily warfarin dose requirement in South Indian patients (DOCX 15 kb)
Supplementary Figure 1
LD pattern of the haplotypes tagSNPs. The single nucleotide polymorphisms in chromosome 16 were positioned according to the order and orientation. Each of the variant is given with their specific chromosomal position and the rsID. LD pattern of the rs9923231, rs7196161, rs2884737, rs1770847, rs9934438, rs8050894, rs23596121and rs7294 in the study population. Red and pink colors represent a very strong LD pattern (D’ > 0.8) and white color represents moderate to low LD (D’ < 0.8 to >0.5). The D’ prime values given inside the color boxes respect to the LD between the SNPs (DOCX 152 kb)
Supplementary Figure 2
Relationship between observed warfarin maintenance dose and estimated maintenance dose determined by multivariate regression analysis (pharmacogenetic algorithm). There is a significant correlation between the predicted and observed warfarin maintenance doses (y = 1.7418 + 0.58914, r 2 = 0.635, p < 0.0001). (DOCX 145 kb)
Rights and permissions
About this article
Cite this article
Krishna Kumar, D., Shewade, D.G., Loriot, MA. et al. Effect of CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants on warfarin maintenance dose and explicating a new pharmacogenetic algorithm in South Indian population. Eur J Clin Pharmacol 70, 47–56 (2014). https://doi.org/10.1007/s00228-013-1581-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1581-x