Abstract
There is increasing evidence of a biochemical link between oxidative stress and bone metabolism. Oxidative stress has been shown to be involved in bone resorption as it causes loss of bone mineral density (BMD). Paraoxonase 1 (PON1), can prevent these effects of the oxidative stress on bone formation. It has been suggested that the PON1 gene as possibly implicated in reduced BMD in bone fragility cases. It has been hypothesized that PON1 gene polymorphisms may influence both the risk of osteoporosis and osteopenia occurrence and prognosis. The aim of our study is to evaluate the relationship between PON1 polymorphisms and bone fragility development. Seventy-four osteoporotic, 121 osteopenic and 79 nonosteoporotic postmenopausal women were recruited. For detection of the polymorphisms, polymerase chain reaction-restriction fragment length polymorphism techniques have been used. BMD was measured at the lumbar spine and hip by dual-energy X-ray absorptiometry. Distributions of PON1 (PON 192 and PON 55) polymorphisms in study groups were not significantly different. But, there was medium strength connection between in the osteopenic with control groups regarding PON1 55–PON1 192 haplotypes and we found a power strength connection between in the osteoporosis with control groups regarding PON1 55–PON1 192 haplotypes. Furthermore, subjects with PON1 192RR and PON1 55LL genotypes had lower PON activity values of osteoporotic subject compared to healthy control and this difference was statistically significant (p < 0.05). This result suggest that PON1 genotypes could be higher risk for osteoporosis, as determined by reduced BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal disorder defined as a diminished in bone mineral density (BMD) and a deterioration in the micro-architecture of bone, with a consequent susceptibility to fracture and enhance in bone fragility [1–3]. It has been reported that osteoporosis was related with a nearly four times higher fracture rate, while, osteopenia was associated with a 1.8-fold higher rate compared to that of normal BMD in women [4, 5]. It is widely accepted that risk factors for development of osteoporosis and osteopenia have been recognized such as diet, physical activity, increased oxidative stress and diminished antioxidant capacity [2]. Although the underlying causes of some changes in bone fragility in osteoporosis are still unclear, several possible mechanisms are known in bone growth modelling and remodelling [6–8]. Process of bone remodelling related with bone renewed to maintain bone strength and mineral homeostasis. BMD and other determinants of osteoporotic fracture are managed by strong genetic control and this relationship is well established [1, 6, 9, 10]. The oxidative stress is associated with osteoporosis or osteopenia, as it causes loss of BMD. Lipid peroxidation products, which cause oxidative stress, is one of the main factor.
Lipid peroxidation products, can inhibit differentiation of osteoblasts by changing mineral content, decreasing formation, and inhibiting mineralization and therefore this situation may cause osteoporosis [11–14]. Various antioxidant molecules such as PON1, can prevent these effects of oxidative stress on bone formation. PON1, confers antioxidant properties high density lipoprotein (HDL), with which it is associated by reducing the accumulation of lipid peroxidation products. PON1 activity has been suggested to be inversely related to oxidative stress in plasma. Serum PON1 activity is influenced by nutrition, several environmental factors, and PON1 polymorphisms [14, 17].
The human PON1 gene located on the long arm of chromosome 7 (q21.22) [13, 17]. PON1 coding region contains two functional polymorphic variants, which are characterized by the location of glutamine (Q genotype) and arginine (R genotype) at position 192, and leucine (L genotype) and methionine (M genotype) at position 55. Previous studies show modular activity of PON1, hence it has different kinetics and distinct activities in different physiological conditions. Furthermore, polymorphic variation may lead to change the enzyme activity. There are more than 160 defined polymorphisms, both in introns, exons and regulatory regions of the gene. Some studies reveal that some polymorphisms may cause high, intermediate or low paraoxonase activity [14–20]. Paraoxone hydrolysis activity varies among individuals due to the these polymorphisms of the PON1 gene. A major risk factor for osteoporotic or osteopenic fractures is low BMD, which is under strong genetic control. Therefore, this polymorphisms may be a crucial role for PON1 in bone metabolism [13]. The purpose of this study was to determine any relationship between serum PON1 activity and 584A>G(Gln192Arg) and 172T>A(Leu55Met) polymorphisms of the PON1 gene in osteoporosis.
Materials and methods
Patients selection and clinical investigation
PON1 activity, PONI 55/192 gene polymorphism has been studied among 74 osteoporotic, 121 osteopenic and 79 nonosteoporotic women. Patients were selected from Üsküdar State Hospital, Physical Therapy and Rehabilitation Clinic, İstanbul.
Quantification of paraoxonase activity
Paraoxonase activities was measured according to Furlong et al. [20]. The assay buffer contains 0.132 M Tris HCl (pH 8.5), 1.32 mM CaCl2 and 2.63 M NaCl. Addition of 200 μl of 6 mM freshly prepared paraoxon (0,0-diethyl-0-p-nitrophenylphosphate; Sigma, Poole, UK) and 40 μl of serum initiated the assay. The rate of generation of p-nitrophenol was determined at 37 °C, with the use of a continuously recording spectrophotometer at 405 nm. A molar extinction coefficient of 18.05 × 103 was used for calculation using paraoxon as substrate. Pararaoxonase activity is expressed as units/liter (unit: μmol paraoxon hydrolyzed/min).
Isolation of DNA
Blood samples from all subjects were collected into tubes containing EDTA. DNA was prepared from leukocyte pellet by SDS lysis ammonium acetate extraction and ethanol precipitation [21].
Genotyping methods of the paraoxonase 55/192 polymorphism
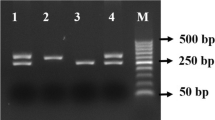
For the 192 polymorphism, sense primer 5′ TAT TGT TGC TGT GGG ACC TGA G 3′ and antisense primer 5′ CAC GCT AAA CCC AAA TAC ATC TC 3′ which encompass the 192 polymorphic region of the human PON1 gene, were used. For the 55 polymorphism, sense primer 5′ GAA GAG TGA TGT ATA GCC CCA G 3′ and antisense primer 5′ TTT AAT CCA GAG CTA ATG AAA GCC 3′ were used. The PCR reaction mixture contained 100 ng DNA template, 0.5 M of each primer, 1.5 mM MgCl2, 200 μM dNTPs and 1 U Taq DNA polymerase (MBI Fermentas). After denaturing the DNA for 5 min at 94 °C, the reaction mixture was subject to 35 cycles of denaturation for 1 min at 95 °C, 1 min annealing at 60 °C and 1 min extension at 72 °C for the 192 genotype. The 99 bp PCR product was digested with 8 U Bspl restriction endonuclease (MBI Fermentas, Lithuania) overnight at 55 °C and the digested products separated by electrophoresis on a 4 % metaphore agarose gel and visualized using ethidium bromide. The R-genotype (arginine) contains a unique Bspl restriction site which results in 66- and 33-bp products and the Q-genotype (glutamine) can not be cut, allowing the 192 genotype to be determined. For the PON1 55 polymorphism, the PCR reaction and the cycling conditions were the same as above. The PCR product (170 bp) was digested with Hsp19211 (Promega, USA) in the presence of BSA (0.1 μg/μl final concentration; 37 °C, overnight) and the digested products were separated and identified as above. Allele L (leucine) did not contain the Hsp19211 site whereas M (methionine) contained the Hsp19211 site giving rise to 126- and 44-bp products [18, 22, 23].
Statistical analyses
Statistical analyses were performed using the SPSS software package, revision 10.0. Clinical laboratory data are expressed as mean ± SD. Mean values were compared between patients and control subjects by the unpaired Student’s t test. Differences in the distribution of PON genotypes or alleles between cases and controls were tested using the Chi square statistic, respectively. PON allele frequencies were estimated by gene counting methods. p < 0.05 were considered statistically significant.
Results
Characteristics of patients with osteoporotic and osteopenic cases and healthy controls are shown in Table 1. The patient and control groups had similar distributions for age. The PON 192 and PON 55 genotypes and allele frequencies for osteoporotic and osteopenic patients and control subjects are shown in Table 2. Frequencies of PON 192 QQ, RR and QR genotypes among the patients with osteoporosis were 43.2, 9.5 and 47.3 %, respectively; among the osteopenia, they were 53.7, 10.7 and 35.5 % respectively; among the control, they were 43.0, 13.9 and 43.0 % respectively. The gene frequency for the PON1 192 and PON1 55 polymorphisms in study groups were not significantly different. The frequencies of PON 55LL, MM, and LM genotypes among the patients with osteoporosis were 40.5, 6.8 and 52.7 % respectively; among the osteopenic subjects, they were 52.9, 5.0 and 42.1 %, respectively: among the control, they were 50.6, 8.9 and 40.5 % respectively. However, we observed a medium strength connection between in the osteopenic with control groups regarding PON1 55–PON1 192 haplotypes and we found a power strength connection between in the osteoporosis with control groups regarding PON1 55–PON1 192 haplotypes.
Plasma PON1 activity of osteoporotic and osteopenic patients were significantly lower than in control subjects (32.31 ± 11.78 U/ml, 88.95 ± 44.24 U/ml and 243.30 ± 43.86 U/ml, p = 0.004). The association between respectively serum total PON concentration and PON 55/192 polymorphisms are shown in Table 3. In the osteoporotic women with PON1 192RR genotype in serum PON activity levels were observed between 7.2 ± 2.2 U/ml, while these levels in healthy women with the same genotype were between 342.09 ± 112.83 U/ml and this result were statistically significant (p < 0.05). Furthermore, in the osteoporotic women with PON1 55LL genotype, in serum PON activity levels were observed 11.71 ± 4.01 U/ml, while these levels in healthy women with the same genotype were between 286.67 ± 71.50 U/ml and this difference were statistically significant (p < 0.05). Plasma PON activity according to genotypes are shown in Fig. 1.
Discussion
In this study, we demonstrated the distribution of PON1 192 and PON1 55 polymorphisms and paraoxonase activity in Turkish patients with osteoporotic and in healthy control individuals, which has not been studied before.
PON1 activity was measured by using paraoxone substrate [23]. Human PON1 protein is a 43 kDa molecular mass and containing 354 amino acids. PON1 gene is located on the long arm of chromosome 7 between q21.3 and q22 [23]. Previously it was shown that the PON1 gene has important two single nucleotide polymorphisms (SNPs) which result in amino acid substitutions, that can change its activity. At position 55, leucine (Leu) is replaced by methionine and the other causing GlnArg at the 192nd position. The genotypes 55LL and 192RR demonstrate high PON1 activity, as determined by the ability to hydrolyze paraoxone [15, 16, 19, 20, 25–28]. It has been demonstrated that PON1 192Q genotype is six times more active than PON1 192R genotype and PON1 55 M genotype causes low serum activity and also decreases serum levels than PON1 55L genotype [15, 16, 19, 25, 27, 28]. PON1 55 genotypes are associated with high serum PON1 activity than PON1 192 genotypes [16]. In parallel with these studies, we found similar results.
PON1 activity changes in some other diseases such as oxidative stress, diabetes and cardiovascular diseases [29]. BMD is associated with the morphology, architecture, remodeling and quality of bone. The antioxidant capacity of PON1 may prevent these effects of oxidative stress on bone development. Therefore, PON1 may be relationship in bone metabolism and as a result of osteoporosis [30]. Yamada et al. [2] studied that two polymorphisms in the coding region-L55 M and Q192R-. They found that these polymorphisms were associated with BMD in Japanese elderly women [2]. For PON 55 genotypes, in study groups with the LL genotype had significantly lower BMD than carriers of the MM genotype. For PON 192, participants with the R allele carriers had significantly lower BMD than carriers of the Q allele carriers. As a result of these studies, they implicated that, PON 55 and 192 genotypes associated with lower BMD (55LL and 192RR) are the least effective in protecting LDL against oxidation [2, 31]. This observation suggests a potential mechanism by which PON1 affects risk of osteoporosis [2]. Kim et al. [30] reported that, the L55 M polymorphism was not prevalent, and the Q192R polymorphism had no association with BMD at in postmenopausal Korean populations. This result may be associated with genetic differences. But they suggest that other polymorphisms in the PON1 gene polymorphisms may be associated with osteoporosis in postmenopausal women [30]. However some previous studies have reported that there is no association between PON1 concentrations and osteoporosis [2, 32]. Yamada et al. found that those with the PON 55LL genotype were affected favorably by high serum PON activity in healthy women, with lower bone resorption in osteoporosis patients. Our result was similar to the these studies [2]. Our findings suggest a role of PON1 genotypes which confer a higher risk for osteoporosis, as determined by reduced BMD. One of the potential limitations of this study is the relatively small sample size. A larger sample size would strengthen the present study findings and provide further verification.
References
Huang QY, Kung AW (2006) Genetics of osteoporosis. Mol Genet Metab 88:295–306
Yamada Y, Ando F, Niino N, Miki T, Shimokata H (2003) Association of polymorphisms of paraoxonase 1 and 2 genes, alone or in combination, with bone mineral density in community- dwelling Japanese. J Hum Genet 48:469–475
Lorenc RS, Misiorowski W, Karczmarewicz E (2009) Critical points in strategies for the diagnosis and treatment of osteoporosis. Endokrynol Pol 60:124–133
Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM (2001) Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286:2815–2822
Shan LP, Bee OF, Suniza SS, Adeeb N (2011) Developing a Malaysian Osteoporosis Screening Tool (MOST) for early osteoporosis detection in Malaysian women. Sex Reprod Healthc 2:77–82
Andrew T, Antioniades L, Scurrah KJ, MacGregor AJ, Spector TD (2005) The risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing bone mineral density. J Bone Miner Res 20:67–74
Deng HW, Mahaney MC, Williams JT, Li J, Conway T, Davies KM, Li JL, Deng H, Recker RR (2002) Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Gene Epidemiol 22:12–25
Massart F, Reginster JY, Brandi ML (2001) Genetics of menopause-associated diseases. Maturitas 40:103–116
Huang QY, Recker RR, Deng HW (2003) Searching for osteoporosis genes in the post-genome era: progress and challenges. Osteoporos Int 14:701–715
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3:131–139
Costa LG, Vitalone A, Cole TB, Furlong CE (2005) Modulation of paraoxonase (PON1) activity. Biochem Pharmacol 69:541–550
Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL (2009) Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem 284:27438–27448
Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, Demer LL (1999) Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res 14:2067–2078
Mackinnon ES, El-Sohemy A, Rao AV, Rao LG (2010) Paraoxonase 1 polymorphisms 172T/A and 584A/G modify the association between serum concentrations of the antioxidant lycopene and bone turnover markers and oxidative stress parameters in women 25-70 years of age. J Nutrigenet Nutrigenomics 3:1–8
Deakin SP, James RW (2004) Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci (Lond) 107:435–447
Eren Z, Kantarci G, Biyikli N, Arikan H, Tuglular S, Ergen A, Isbir T, Akoglu E (2012) Paraoxonase 1 polymorphisms in patients with primary glomerulonephritis: a single-center study in Turkey. Iran J Kidney Dis 6:181–185
Sztanek F, Seres I, Harangi M, Locsey L, Padra J, Paragh GJ, Asztalos L, Paragh G (2012) Decreased paraoxonase 1 (PON1) lactonase activity in hemodialyzed and renal transplanted patients. A novel cardiovascular biomarker in end-stage renal disease. Nephrol Dial Transplant 27:2866–2872
Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, Dvir H, Ravelli RB, McCarthy A, Toker L, Silman I, Sussman JL, Tawfik DS (2004) Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol 11:412–419
Billecke S, Draganov D, Counsell R, Stetson P, Watson C, Hsu C, La Du BN (2000) Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab Dispos 28:1335–1342
Furlong CE, Richter RJ, Seidel SL, Costa LG, Motulsky AG (1989) Spectrophotometric assay for the enzymatic hydrolysis of the active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Anal Biochem 180:242–247
Miller SA, Dykes DD, Polesky HS (1988) Simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acid Res 16:1215
Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE (1993) The molecular basis of the human serum paraoxonase activity polymorphism. Nature Genet 3:73–76
Adkins S, Gan KN, Mody M, La Du BN (1993) Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase glutamine or arginine at position 191, for the respective A or B allozymes. Am J Hum Genet 52:598–608
Mackness B, Mackness MI, Arrol S, Turkie W, Durrington PN (1998) Effect of the human serum paraoxonase 55 and 192 genetic polymorphisms on the protection by high density lipoprotein against low density lipoprotein oxidative modification. FEBS Lett 423:57–60
Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE (1996) The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 14:334–336
Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, Lusis AJ, Furlong CE (2000) Catalytic efficiency determines the in vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenetics 10:767–779
Garin MC, James RW, Dussoix P, Blanche H, Passa P, Froguel P, Ruiz J (1997) Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J Clin Invest 99:62–66
Aviram M, Hardak E, Vaya J, Mahmood S, Milo S, Hoffman A, Billicke S, Draganov D, Rosenblat M (2000) Human serum paraoxonase (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid arteriosclerotic lesions. Circulation 101:2510–2517
McGrowder D, Riley C, Morrison EY, Gordon L (2011) The role of high-density lipoproteins in reducing the risk of vascular diseases, neurogenerative disorders, and cancer. Cholesterol 2011:496925
Kim BJ, Kim SY, Cho YS, Kim BJ, Han BG, Park EK, Lee SH, Kim HY, Kim GS, Lee JY, Koh JM (2011) Association of Paraoxonase 1 (PON1) polymorphisms with osteoporotic fracture risk in postmenopausal Korean women. Exp Mol Med 28(43):71–81
Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H (2001) Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 288:275–279
Verit FF, Celik H, Yazgan P, Erel O, Geyikli I (2007) Paraoxonase-1 activity as a marker of atherosclerosis is not associated with low bone mineral density in healthy postmenopausal women. Arch Gynecol Obstet 275:35–353
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toptaş, B., Kurt, Ö., Aydoğan, H.Y. et al. Investigation of the common paraoxonase 1 variants with paraoxonase activity on bone fragility in Turkish patients. Mol Biol Rep 40, 6519–6524 (2013). https://doi.org/10.1007/s11033-013-2770-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2770-5