Abstract
Interleukin (IL)-17 (also known as IL-17A), as the signature cytokine of the newly described T helper 17 (Th17) cell population, is the founding member of a new subclass of cytokines that have highly proinflammatory properties. Recently there is accumulating evidence that stipulates the involvement of IL-17 in the pathogenesis of cardiovascular diseases via amplifying the inflammation induced by other cytokines in synergistic interactions. The present review provides a summary of the potential roles of IL-17 in the context derived from both animal models and clinical settings in cardiovascular diseases, and perspectives for IL-17-targeted cytokine therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
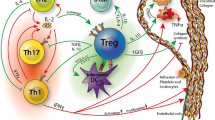
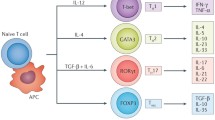
CD4+ T cells which can be induced to differentiate into various T helper (Th) subsets after activation are pivotal in regulating the immune and inflammation response in that they coordinate the functions of other immune cell types [1]. Traditionally, Th cells are thought to develop into Th1 and Th2 cell subsets with distinct cytokine profiles and functions. Th1 cells are involved in autoimmune diseases and the eradication of intracellular pathogens (by producing interferon-γ, IFN-γ) while the Th2 cell lineage is important for participating the elimination of extracellular organisms (by producing interleukin (IL)-4, IL-5, and IL-13) [2–4]. Recent findings have demonstrated that a new subset of preferential IL-17-producing cells (named Th17 cells) which is distinct from the Th1 or Th2 cells plays a critical role in inducing inflammatory tissue injury [5–12]. IL-17 is implicated in numerous immune and inflammatory responses primarily as a pro-inflammatory regulator by inducing the expression of various inflammatory mediators, such as cytokines, chemokines, adhesion molecules, and growth factors [6, 13–16]. There is emerging evidence that an increase in IL-17 level is closely associated with a range of inflammatory diseases including inflammatory bowel diseases, systemic lupus erythematosus, osteoporosis [17–19]. Consistently, recent studies have demonstrated that IL-17 has a pivotal role in cardiovascular diseases and has been considered as a crucial regulator in cardiovascular diseases. In this review, we highlight our current knowledge on IL-17 and its role in cardiovascular diseases and hope that this information may aid the development of novel therapeutic strategies for cardiovascular diseases.
IL-17: structure, sources and function
This cytokine was first described as a rodent complementary DNA transcript in 1993 and was originally termed cytotoxic T-lymphocyte-associated antigen 8 (CTLA-8), and later renamed IL-17(also called IL-17A) [20–22]. Subsequent genomic sequencing resulted in the discovery of other five additional family members called IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F and they all exerted proinflammatory activities [23, 24]. Among the IL-17 family members, IL-17 as a disulfide-linked homodimeric glycoprotein, is the most intensively investigated cytokine and has 155 amino acids with a molecular weight of 35 kDa [21, 25]. IL-17 gene is located on chromosome 6p12 and the cellular source of IL-17 is produced predominantly by a specific subset of Th cells, namely Th17 cells, but has more recently been expanded to include γδ T cells, CD8+ memory T cells, natural killer T cells, neutrophils eosinophils, macrophages and monocytes [18, 25–28]. After binding to its receptors, the key biological function of IL-17 is it involvement in inducing and mediating immune and proinflammatory responses via other cytokines in synergistic interactions. Many studies demonstrated that IL-17 as a proinflammatory cytokine is involved in inflammatory bowel diseases, systemic lupus erythematosus, osteoporosis [17–19].
IL-17: signaling pathway
Similar to the IL-17 cytokine family, IL-17 receptors form a unique family contained five members refer as to IL-17RA, IL-17RB, IL-17RC, IL-17RD, and IL-17RE [24]. The IL-17 receptors are expressed ubiquitously particularly in non-immune origin, particularly epithelial and mesenchymal cells within diseased or inflamed tissues [29, 30]. Among these IL-17 receptors, IL-17 binding to both the IL-17RA and IL-17RC subunits mediates signalling pathway [31]. The biological activity of IL-17 is dependent on a formation of receptor complex composed of IL-17RA and IL-17RC [32]. IL-17 signaling through its receptors is distinct compared to typical adaptive Th cell cytokines. Rather than activating JAK-STAT pathways, IL-17 stimulates proinflammatory pathways more typical of innate, proinflammatory cytokines such as IL-1 or TLR agonists [24]. It was demonstrated that IL-17 signaling can induce various downstream pathways which include nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK) pathways and the C/EBP transcription factors which can induce many production of various proinflammatory cytokines [33–35]. Recent studies have shown that Act-1(the adaptor protein NF-κB activator 1) plays an important role in IL-17-dependent signaling [36–38]. Additionally, the research by Schwander et al. showed that tumor necrosis factor receptor-associated factor 6 was essential for IL-17-induced NF-κB activation and the expression of IL-6 or intercellular adhesion molecule (ICAM)-1 [39]. Hence, the IL-17 signaling cascade is far from being completely understood.
IL-17 and cardiovascular diseases
Although significant advances in treatments, cardiovascular diseases remain the leading cause of morbidity and mortality around the world. There is accumulating evidence that inflammatory cytokines (such as IL-6, TLR4, NF-κB) play a key role in the occurrence and development of cardiovascular diseases including atherosclerosis, cardiac ischemia/reperfusion injury, heart failure, and myocarditis [40–43]. As its wide and intense proinflammation activity, the contribution of IL-17 in cardiovascular diseases has become investigative focus (Tabel 1 ).
IL-17 and Atherosclerosis
Atherosclerosis, defined as lipid-driven and chronic inflammation of the artery wall, involves a complicated interplay between many different cell types and cytokine networks. Both innate and adaptive immune responses have been demonstrated to regulate local and systemic inflammation during all stages of atherogenesis [44, 45]. Macrophages, T lymphocytes and, to a lesser extent, mast cells contribute to the smoldering inflammatory response in the vessel wall [46, 47]. Recent research by Erbel and colleagues showed evidence for the proatherogenic role of IL-17 via proinflammatory changes at multiple levels such as cell adhesion, extravasation, cell activation, T cell (co) stimulation/proliferation, and antigen presentation [48]. In line with this result, several studies by various investigators have also demonstrated that IL-17 level is increased and IL-17 plays an essential proatherogenesis role in animal models and patients with coronary artery syndrome [49–55]. Interestingly, in contrast to these studies, Taleb et al. revealed that loss of suppressor of cytokine signaling-3 (SOCS-3) in mouse T cells increased IL-17A production and induced an anti-inflammatory macrophage phenotype which can lead to a reduction in lesion development and vascular inflammation [56]. They concluded that IL-17 may have a protective role in atherogenesis despite it was unclear as to whether IL-10 or IL-17 was leading to the suppressor phenotype, as IL-10 can function as a very potent regulatory cytokine which is atheroprotective [56]. Additionally, these investigators also found that in vivo administration of IL-17 to LDLR –/– mice resulted in reduced endothelial VCAM-1 expression, reduced vascular T cell infiltration and atherosclerotic lesion development and then they concluded that endogenous expression of SOCS3 in T cells can interrupt a major regulatory pathway in atherosclerosis through inhibition of IL-17 production and that IL-17 may function as an atheroprotective cytokine. Taken together, these findings indicate that IL-17 plays dual role in the development of atherosclerosis and these potential mechanisms await more direct studies to address.
IL-17 and myocardial ischemia/reperfusion injury
Myocardial ischemia reperfusion injury (IRI) refers to the tissue damage which occurs when blood supply returns to tissue after a period of ischemia and is predominantly associated with myocardial infarction but can also be seen in trauma, stroke, solid organ transplantation and coronary artery bypass graft (CABG) surgery [57, 58]. The shorter the ischemic period, the better the clinical outcome. Both T and B cells constituting the primary arms of the adaptive immune response, conventionally thought of as innocent bystanders, play a variety of roles during all phases of IRI [59, 60]. In brain, lung, liver, heart, intestine, and kidney models of IRI, T cells particularly CD4+ T cells mediate tissue injury and possibly repair as well [59–62]. The expression of IL-17 was detected as early as 1 h after reperfusion, lasted for 24 h, and showed no peak in this period [63]. The study also found that CD4+ T lymphocyte was a major source of IL-17 in myocardial tissue after reperfusion and administration of anti-IL-17 leaded to a dramatical decrease in serum troponin T and myocardial infarct size, suggesting that IL-17 might be involved in the pathogenesis of myocardial IRI [63].
IL-17 and heart failure
Chronic heart failure,mainly caused by cardiac fibrosis and ventricle remodeling, is a progressive syndrome and the outcome of a variety of cardiovascular diseases. The pathologic changes in heart failure occur in two steps: one is cardiomyocyte hypertrophy, necrosis, and apoptosis and the other is cardiac fibroblasts hyperplasia and cardiac fibrosis defined as a progressive accumulation of fibrillar extracellular matrix (ECM). IL-17 can upregulate and/or function synergically with local inflammatory mediators such as IL-6, IL-1β, and TNF-α, and enhance ECM injury by activating the production of matrix metalloproteinases(MMPs) and inhibiting the synthesis of matrix repair components, such as proteoglycans and collagens [64]. Previous research found that the regulation of Th1/Th2/Th17 balance may be one of the underlying mechanisms of inflammation-mediated cardiac remodeling [65]. Recent study by Feng et al. directly demonstrated that IL-17 can contribute to myocardial fibrosis in isoproterenol-induced heart failure and the receptor activator of NF-κB ligand/osteoprotegerin (RANKL/OPG) system may serve as a link between IL-17 and MMP-1 in cardiac fibroblasts [66].
IL-17 and Myocarditis
IL-17 is closely correlated with myocarditis. For instance, IL-17 mRNA and/or protein obviously elevated in the model of viral myocarditis (VMC) mice [67–69]. In addition, IL-17 inhibition ameliorated the myocardium inflammation in IL-17 monoclonal antibody (IL-17mAb)-treated VMC mice, indicating IL-17 is crucially involved in the pathogenesis of murine VMC [70]. Recently, Baldeviano GC et al. demonstrated a critical role for IL-17A in postmyocarditis cardiac remodeling and the progression to dilated cardiomyopathy (DCM) [71]. Hence, Targeting IL-17 may be an attractive therapy for patients with myocarditis via ameliorating the myocardium inflammation.
Conclusions and perspectives
The effect of the revision of the Th1/Th2 paradigm to add Th17 cells cannot be overstated as it becomes a watershed in our understanding of the T cell-mediated pathogenesis of cardiovascular diseases. As the predominant product of Th17 cells, IL-17 owns an imperative proinflammatory role and clinical value in the occurence and development of cardiovascular diseases. Current cardiovascular researches suggest that the use of some drugs affecting the receptors and signal transduction pathway of IL-17 will become a hot area of clinical research and that the application of neutralizing antibodies or receptor antagonists of IL-17 to block its biological activity expression may provide a new therapeutic approach in cardiovascular disease research. With the mechanisms of IL-17 and its relevant cytokines to be further explored, IL-17 will have widespread application prospects in the prevention and clinical treatment of cardiovascular diseases.
References
Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136:2348–2357
Abbas AK, Murphy KM, Sher A (1996) Functional diversity of helper T lymphocytes. Nature 383:787–793
Murphy KM, Reiner SL (2002) The lineage decisions of helper T cells. Nat Rev Immunol 2:933–944
Romagnani S (1997) The Th1/Th2 paradigm. Immunol Today 18:263–266
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141
Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8:639–646
Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F (2007) Interleukins 1 beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 8:942–949
Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S (2007) Phenotypic and functional features of human Th17 cells. J Exp Med 204:1849–1861
Bettelli E, Oukka M, Kuchroo VK (2007) TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol 8:345–350
Dong C (2008) TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol 8:337–348
Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM (2006) Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24:677–688
Witowski J, Ksiazek K, Jörres A (2004) Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci 61:567–579
Kawaguchi M, Kokubu F, Kuga H, Matsukura S, Hoshino H, Ieki K, Imai T, Adachi M, Huang SK (2001) Modulation of bronchial epithelial cells by IL-17. J Allergy Clin Immunol 108:804–809
Kim SR, Lee KS, Park SJ, Min KH, Lee KY, Choe YH, Lee YR, Kim JS, Hong SJ, Lee YC (2007) PTEN down-regulates IL-17 expression in a murine model of toluene di isocyanate-induced airway disease. J Immunol 179:6820–6829
Von Vietinghoff S, Ley K (2010) Interleukin 17 in vascular inflammation. Cytokine Growth Factor Rev 21:463–469
Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y (2003) Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52:65–70
Zhao XF, Pan HF, Yuan H, Zhang WH, Li XP, Wang GH, Wu GC, Su H, Pan FM, Li WX, Li LH, Chen GP, Ye DQ (2010) Increased serum interleukin 17 in patients with systemic lupus erythematosus. Mol Biol Rep 37:81–85
Yuan FL, Li X, Lu WG, Zhao YQ, Li CW, Li JP, Sun JM, Xu RS (2012) Type 17 T-helper cells might be a promising therapeutic target for osteoporosis. Mol Biol Rep 39:771–774
Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P (1993) CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus Saimiri gene. J Immunol 150:5445–5456
Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK (1995) Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3:811–821
Yao Z, Timour M, Painter S, Fanslow W, Spriggs M (1996) Complete nucleotide sequence of the mouse CTLA 8 gene. Gene 168:223–225
Pappu BP, Angkasekwinai P, Dong C (2008) Regulatory mechanisms of helper T cell differentiation: new lessons learned from interleukin 17 family cytokines. Pharmacol Ther 117:374–384
Gaffen SL (2009) Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9:556–567
Kolls JK, Lindén A (2004) Interleukin-17 family members and inflammation. Immunity 21:467–476
Michel ML, Mendes-da-Cruz D, Keller AC, Lochner M, Schneider E, Dy M, Eberl G, Leite-de-Moraes MC (2008) Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A 105:19845–19850
Lockhart E, Green AM, Flynn JL (2006) IL-17 production is dominated by gamma delta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177:4662–4669
Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J (2001) IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 108:430–438
Lubberts E (2008) IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine 41:84–91
Garrett-Sinha LA, John S, Gaffen SL (2008) IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol 20:519–525
Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD (2007) Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol 179:5462–5473
Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J (2006) Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol 177:36–39
Gaffen SL (2008) An overview of IL-17 function and signaling. Cytokine 43:402–407
Shalom-Barak T, Quach J, Lotz M (1998) Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem 273:27467–27473
Shen F, Gaffen SL (2008) Structure–function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine 41:92–104
Chang SH, Park H, Dong C (2006) Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem 281:35603–35607
Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li XX (2007) The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 8:247–256
Li X (2008) Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling. Cytokine 41:105–113
Schwandner R, Yamaguchi K, Cao Z (2000) Requirement of tumor necrosis factor receptor-associated factor (TRAF) 6 in interleukin 17 signal transduction. J Exp Med 191:1233–1240
Fang NX, Yao YT, Shi CX, Li LH (2010) Attenuation of ischemia-reperfusion injury by Sevoflurane postconditioning involves protein kinase B and glycogen synthase kinase 3 beta activation in isolated rat hearts. Mol Biol Rep 37:3763–3769
Yang J, Zhang X-D, Yang J, Ding J-W, Liu Z-Q, Li S-G, Yang R (2010) The cardioprotective effect of fluvastatin on ischemic injury via down-regulation of toll-like receptor 4. Mol Biol Rep 38:3037–3044
Ke JJ, Yu FX, Rao Y, Wang YL (2011) Adenosine postconditioning protects against myocardial ischemia-reperfusion injury though modulate production of TNF-alpha, prevents activation of transcription factor NF-kappaB. Mol Biol Rep 38:531–553
Wang LS, Yan JJ, Tang NP, Zhu J, Wang YS, Wang QM, Tang JJ, Wang MW, Jia EZ, Yang ZJ, Huang J (2011) A polymorphism in the visfatin gene promoter is related to decreased plasma levels of inflammatory markers in patients with coronary artery diseases. Mol Biol Rep 38:819–825
Hansson GK, Libby P, Schonbeck U, Yan ZQ (2002) Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res 91:281–291
Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6:508–519
van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE (1989) Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest 61:166–170
Kovanen PT, Kaartinen M, Paavonen T (1995) Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 92:1084–1088
Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ (2009) Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol 183:8167–8175
Song L, Schindler C (2004) IL-6 and the acute phase response in murine atherosclerosis. Atherosclerosis 177:43–51
Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G (2009) Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 119:1424–1432
Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, Yao R, Chen Y, Liao YH (2008) The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol 127:89–97
Xie JJ, Wang J, Tang TT, Chen J, Gao XL, Yuan J, Zhou ZH, Liao MY, Yao R, Yu X, Wang D, Cheng Y, Liao YH, Cheng X (2010) The Th17/Treg functional imbalance during atherogenesis in ApoE (–/–) mice. Cytokine 49:185–193
de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, van Maldegem F, Aten J, van der Wal AC (2010) Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol 220:499–508
Smith E, Prasad KMR, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E (2010) Blockade of IL-17A results in reduced atherosclerosis in apoE-deficient mice. Circulation 121:1746–1755
Chen S, Shimada K, Crother T, Zhang W, Huang G, Arditi M (2010) IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol 185:5619–5627
Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Snick JV, Yoshimura A, Tedgui A, Mallat Z (2009) Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med 206:2067–2077
Ribichini F, Wijns W (2002) Acute myocardial infarction: reperfusion treatment. Heart 88:298–305
Frangogiannis NG, Smith CW, Entman ML (2002) The inflammatory response in myocardial infarction. Cardiovasc Res 53:31–47
Huang Y, Rabb H, Womer Kl (2007) Ischemia-reperfusion and immediate T cell responses. Cell Immunol 248:4–11
Linfert D, Chowdhry T, Rabb H (2009) Lymphocytes and ischemia-reperfusion injury. Transplant Rev 23:1–10
Edgerton C, Crispín JC, Moratz CM, Bettelli E, Oukka M, Simovic M, Zacharia A, Egan R, Chen J, Dalle Lucca JJ, Juang YT, Tsokos GC (2009) IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol 130:313–321
Lu L, Li G, Rao J, Pu L, Yu Y, Wang X, Zhang F (2009) In vitro induced CD4(+)CD25(+)Foxp3(+) Tregs attenuate hepatic ischemia-reperfusion injury. Int Immunopharmacol 9:549–552
Xia N, Tang TT, Liu Y, Zhou SF, Yan XX, Zhu ZF, Nie SF, Liu J, Zhang WC, Yang Y, Liao YH, Cheng X (2010) The role of IL- 17 in ischemia reperfusion injury. J Clin Cardiol 26:130–132
Afzali B, Lombardi G, Lechler RI, Lord GM (2007) The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol 148:32–46
Feng W, Li W, Liu W, Wang F, Li Y, Yan W (2009) IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp Mol Pathol 87:212–218
Liu W, Feng W, Wang F, Li W, Zhou B, Gao C, Li Y, Kong Y, Ma M, Fu S (2008) Adenovirus-mediated ICOSIg gene transfer alleviates cardiac remodeling in experimental autoimmune myocarditis. Immunol Cell Biol 86:659–665
Yuan J, Yu M, Lin QW, Cao AL, Yu X, Dong JH, Wang JP, Zhang JH, Wang M, Guo HP, Cheng X, Liao YH (2010) Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol 185:4004–4010
Qing K, Weifeng W, Fan Y, Yuluan Y, Yu P, Yanlan H (2011) Distinct different expression of Th17 and Th9 cells in coxsackie virus B3-induced mice viral myocarditis. Virol J 8:267
Yang F, Wu WF, Yan YL, Pang Y, Kong Q, Huang YL (2011) Expression of IL-23/Th17 pathway in a murine model of coxsackie virus B3-induced viral myocarditis. Virol J 8:301
Fan Y, Yuluan Y, Qing K, Yu P, Yanlan H (2011) Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of coxsackievirus b3-induced viral myocarditis reduces myocardium inflammation. Virol J 8:17
Baldeviano GC, Talor MV, Srinivasan S, Bedja D, Zhang D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D (2010) Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res 106:1646–1655
Acknowledgment
This work was supported in part by the National Natural Science Foundation of China (Grant No. 81170133) and the Natural Science Foundation of Hubei Province, China (Grant No. 2011CDB179).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, HS., Yang, J., Yang, J. et al. Interleukin-17 contributes to cardiovascular diseases. Mol Biol Rep 39, 7473–7478 (2012). https://doi.org/10.1007/s11033-012-1580-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1580-5