Abstract
To determine whether the cardioprotection effect of fluvastatin mediates by toll-like receptor 4 (TLR4) signaling pathway, fifty Sprague–Dawley rats were randomly divided into five groups: sham operation group, ischemia/reperfusion (I/R) group, fluvastatin groups (high-dosage, medium-dosage, low-dosage, n = 10 in each group). Except sham operation group, the rest four groups of rats were artificially afflicted with coronary occlusion for 30 min, then reperfusion 2 h. Light microscope and transmission electronic microscope were used to observe structural changes of myocardium. RT–PCR was used to measure TLR4 mRNA expression level, TLR4 protein expression was detected by immunohistochemistry. Western blot was used to measure myocardial NF-κB protein level; ELISA was used to measure the level of TNF-α in myocardium. The results demonstrated that fluvastatin treatment markedly decreased ischemic injury caused by ischemia/reperfusion, and inhibited the expression levels of TLR4, TNF-α and NF-κB, all of which up-regulated by ischemia/reperfusion. Taken together, our results suggest that proper dosage of fluvastatin may have protective effect on the ischemic injury mediated by ischemia/reperfusion in the hearts, which might be associated with inhibition of TLR4 signaling pathway and inflammatory response during ischemia/reperfusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although early reperfusion of the ischemic myocardium in coronary artery infarction helps reduce necrosed myocardial area, it causes myocardial ischemia–reperfusion injury (MI/RI), which lowers the protective effect of reperfusion therapy. It is claimed by some scholars that the inflammatory response is a critical point in research of the MI/RI pathophysiological mechanisms. They also hold a belief that the increase of TNF-α, a specific markers of the inflammatory reaction, in the MI/RI [1, 2], and the activation of the innate immune pattern-recognition receptors (pattern-recognition receptors, PRRs) plus Toll-like receptor 4 (TLR4) in the MI/RI, promote the development of inflammatory response in the MI/RI [3–5].
Statins, as a traditional fat-lowering drug, can inhibit the formation of cholesterin and its precursor. It has been proved that the clinically curative effect of statins is more efficient than their cholesterol-lowering effect. Now the pleiotropic function of statins invites a wide concern. In 2006, Matsuki et al. [6] reported that pretreatment with fluvastatin on the ischemic and reperfused rats can reduce MI size and attenuate reperfusion injury, the underlying mechanism being inhibition of inflammation and endothelial dysfunction by fluvastatin by preventing the activation and extravasation of neutrophils. Inhibition of correlate inflammatory factor release and micromolecule activity compound formation reduces regional inflammation and oxidative damage [7, 8].
It remains unknown whether the statins anti-inflammatory effects are relevant with myocardial TLR4 expression in the MI/RI pathological process. This study attempts to establish a MI/RI model through fluvastatin pretreatment with rats to explore the relevance between anti-inflammatory effects of statins-based drugs and myocardial TLR4 expression and the drug’s mechanism of action.
Materials and methods
Materials
Fluvastatin was donated by Beijing Novartis Pharma Ltd (China). TLR4 and NF-κB antibodies were purchased from Wuhan Bo Shide Biological Engineering Company (China), Real-Time PCR kits were purchased from TaKaRa Biotechnology Company (Japan), TNF-α Kit was bought from Nanjing Jiancheng Biotechnology Institute (China).
Groups and pretreatment
The procedures for experiment and animal care were permitted by the animal care and use committee of Huazhong University of Science and Technology (HUST), and approved by the guide for the care and use of Laboratory Animals by the National Institutes of Health (NIH Publication No. 80-23). Fifty healthy male SD rats weighing 250–300 g (provided by Tongji Medical College of Huazhong University of Science and Technology) were randomly divided into five groups of 10 rats each: the sham-operated group, the I/R group and three groups of rats were pretreated with a given dosage of fluvastatin (the dosages are 20, 10 and 5 mg/kg respectively, the dosages were calculated in light of those dosages that suit 50 LD rats and adjusted with a reference to the data collected from pretest and relevant documents [9]). All five groups were given the same volume of normal saline for 10 days except that the normal saline used for the three drug groups was added with the respective dosages of fluvastatin mentioned before. Ten days later, when blood concentration of fluvastatin achieve stability, ischemia–reperfusion model were established among the three drug groups and the I/R group by occluding the anterior descending coronary artery for 30 min followed by 2 h of reperfusion.
Myocardial I/R surgical procedure
According to method reported by Stefano Chimenti et al. [10], each rat was anesthetized by an intraperitoneal injection of sodium pentobarbital (30 mg/kg), placed in the supine position. Four limbs were conjugated with electrocardiograph and performed a tracheotomy implemented with mechanical ventilation through an endotracheal cannula by using small animal breathing machine (50 breaths/min, tidal volume 20 ml/kg). Following a thoracotomy in the left 4th intercostal space, the pericardium was opened. The anterior descending left coronary artery was occluded temporarily with 6-0 silk thread for 30 min with a medical latex tube (socket, inner diameter, and 1.5 mm) placed in-between the ligature and the LAD. Myocardial ischemia was induced by compressing the LAD by tightening the ligature around the latex tube. The electrocardiogram was monitored for changes in the ST-T segment caused by tightening or loosing the ligature. After a 30-min ischemia, the latex tube was removed to reperfuse the myocardium for 120 min by restoring the coronary circulation. Two hours post-reperfusion, ten rats were killed, and part of the anterior wall of the left ventricular myocardium near the cardiac apex was removed for further analysis. Sham operate group rats underwent a similar operation without I/R.
Morphology
After myocardial HE staining, histomorphology changes were observed under 400× light microscope; the specimens were prefixed by 2.5% glutaraldehyde and rinsed in phosphate buffer saline (PBS), fixed in 1% osmium tetroxide and then dehydrated, soaked in epoxy resin (Epon 812) and subsequently sectioned into 70 nm ultra-thin slices for double-staining with uranyl acetate and lead citrate. The ultra-structural changes in the myocardial sections were observed with a transmission electronic microscope (Hitachi, Ltd, Japan).
Real-time RT–PCR detection of myocardial TLR4 mRNA expression
The total RNA from the cardiac muscle samples was extracted and purified using the Trizol reagent kit (Invitrogen, US). Total RNA was reversely transcribed into complementary DNA (cDNA) using the cDNA synthesis kit (TaKaRa, Japan) according to the manufacturer’s protocol. Real-time PCR: application of SYBRExScript RT–PCR Kit, and use two-step PCR reaction under conditions: 95°C 10 s, 95°C 5 s, 60°C 30 s 40 cycles. Using Opticon-2 fluorescence quantitative PCR reactor (MJ Research, US) for PCR amplification and quantitative analysis. This study was set up with GAPDH for the housekeeping gene, according to Gene Bank in TLR4 and GAPDH (NM_019178, NM_017008). TLR4 primer sequence: upstream primer: 5′-AGCCATTGCTGCCAACATCA-3′, downstream primer: 5′-ATGCAGGGGTT CTGG-3′, amplified length 148 bp; GADPH primer sequence: upstream primer: 5′-GACAACTTTGGCTCGTGGA-3′, downstream primer: 5′-ATGC AGGGGTTCTGG-3′, amplified length 133 bp. TLR4 mRNA levels were calculated based on the 2−ΔΔCt method [11].
TNF-α measurement
Different dosages of myocardial tissue were made and organized respectively in the UP-200H homogenate ultrasound machine (Germany DR.HEESCHER Company) Afterwards, homogenized liquid was centrifuged at 4,000 r/min for 15 min. Then detection was made in accordance with instruction for the rat TNF-α ELISA kit.
Myocardial TLR4 expression in rats
Paraffin sections were dewaxed, 10% rabbit serum was used to close non-specific sites of these sections. After being rinsed in PBS once, the sections were treated with goat anti-rat TLR4 antibody, and incubated in the 4°C wet box overnight. The sections were then rinsed in PBS three times, treated with rabbit anti-goat secondary antibody, and then incubated in the 37°C wet box for 1 h. After that the sections were again rinsed in PBS for three times, treated with NBT/BCIP solution, colorated for 30 min in a dark place. Finally, dyed with neutral red, the sections were dried and mounted by transparent resin. If the cytoplasm turned brown yellow that indicated TLR4 protein expression was positive. The treated sections were observed under light microscope by means of Leica microgram collection system. Five high power field map photo (×400) were randomly selected to identify the value of grey. Imagepro plus5.0 (an image analysis system) was used to calculated the IOD value of each image (IOD = the total size of positive area × average optical density of the total positive area). The average of 5 vision images of each slice represents the value of IOD of that slice (AIOD).
Myocardial NF-κB protein expression
After myocardial tissue was sampled, each gram of the sample was added in 5 ml cell lysis buffer, and then was homogenized in glass-homogenizer, centrifuged under 4°C, 3,000 r/min for 10 min to remove supernatant. The deposit was added with protein extract. The mixture was put into a glass-homogenizer and repeatedly homogenized so as to damage the nuclear membrane. Afterwards, the mix was centrifuged under 4°C, 12,000 r/min for 10 min to extract the supernatant (liquid protein). Coomassie brilliant blue method was used to detect the density of protein. 10 ug liquid-like protein samples taken from each group underwent denaturing polyacrylamide gel electrophoresis (SDS–PAGE), electro-transferred to the (PVDF) membrane. Next, confining liquid was added and the protein samples were fostered in the shaker under room temperature for 2 h to close non-specific protein binding sites. After being washed in TBST once, the protein samples were added with a 1:400 dilution of NF-κB antibody and kept at 4°C overnight. Then, the protein samples were washed in TBST for 5 times, added the anti-second (goat anti-rabbit HRP IgG), incubated at room temperature again, and shaken for 1 h. Then, the protein samples were washed in TBST in the same way. After being colored in light of ECL, the protein samples underwent image analysis.
Statistic analysis
Data were measured and analyzed as mean ± SD. Statistical comparison was carried out with three or more groups using one-way analysis of variance (ANOVA) and Student–Newman–Keuls (SNK)-q test. A P value of less than 0.05 was considered as statistically significant.
Results
Fluvastatin protects against ischemic injury mediated by ischemia/reperfusion
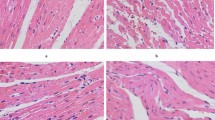
Myocardial cells sampled from sham group were examined under light microscope, myocardial cells lined up in order, cell membrane remained integrited, and no apomorphosis, necrosis, or other pathological changes were detected. The I/R group showed local swelling, myocardial necrosis, a disordered striated cardiac muscle, myocardium interstitium bleed. The low-dosage group also showed myocardial necrosis, myocardium interstitium bleed, whereas the medium-dosage group had well-arranged myocardial fibers, integrated structure, and a great quantity of leukomonocyte infiltration and the high-dosage group demonstrated a decreasing amount of myocardial necrosis, a tiny amount of monocytes or macrophages infiltration (Fig. 1).
Histopathological changes in the myocardium at different groups. a The sham group, with a well-arranged cardiac cells and integrated membrane. b The I/R group, showing swelling myocardial cells, a disordered striated cardiac muscle, a large number of red blood cells infiltration, and local myocardial necrosis. c The low-dosage fluvastatin-treated group, showing swelling myocardial cell, and partial necrotic focus formation. d The medium-dosage fluvastatin-treated group, showing a great quantity of neutrophil cell. e The high-dosage fluvastatin-treated group, with a well-arranged cardiac cells and a tiny amount of macrophages infiltration
Transmission electron microscope observation revealed that sham group had well-arrayed myocardial fiber, a clear inocomma zonation, a normal coloration in myocardial cell nuclear, no degeneration, necrosis, or other pathological changes, and a clear and integrated structure of mitochondria. I/R group had dissolved myocardial fibers, unclear inocomma, partial mitochondrial vacuolization and partially broken and dissolved cristae. The low-dosage fluvastatin-treated group displayed a vague myocardial fiber structure, unclear inocomma, and partially unclear mitochondria. The medium-dosage group had a stagger of normal myocardium with damaged one, with damaged blood vessels morphologically recuperating. The high-dosage group had roughly complete myocardial fiber structure, clear inocomma, and intensive cristae of mitochondria (Fig. 2).
Ultrastructural changes in the myocardium of different groups. a The sham group, showing well-arrayed myocardial fiber, clear sarcomere zonation, myocardial nuclear normal coloring, and a clear structure of mitochondrial integrity. b The I/R group, displaying myocardial fiber solution, a large number of mitochondria vacuoles, unclear sarcomere. c The low-dosage fluvastatin-treated group, showing fuzzy myocardial fiber structure, unclear mitochondrial structure, and unclear sarcomere. d The medium-dosage fluvastatin-treated group, showing clear fiber structure of myocardium, mitochondria cristae reduction, and partial morphological recovery of damaged blood vessels. e The high-dosage fluvastatin-treated group, showing clear sarcomere, a roughly integrated myocardial fibers and structure of mitochondria
Fluvastatin decreases myocardial TLR4 expression mediated by ischemia/reperfusion
TLR4 positive expression, which is manifested by a pervasive brown-yellow color in the myocardial cells, can be found in the myocardial tissue sampled from all the five groups (Fig. 3). In addition, the area of TLR4 protein positive expression of myocardial tissue sampled from I/R group and fluvastatin-treated groups is obviously larger than that from sham group. TLR4 protein expression of myocardial tissue from the low-dosage fluvastatin-treated group is not significant from that sampled from I/R group, whereas TLR4 expression of myocardial tissue from the medium or high-dosage fluvastatin-treated groups (13.72 ± 0.84, 12.68 ± 5.32 respectively) decreased remarkably, as compared with that from I/R group (17.44 ± 1.3, P < 0.01; Table 1).
TLR4 protein expression of myocardium were examined by immunohistochemistry (×400). a The sham group, TLR4 expression in the cytoplasm showing weak positive reaction. b The I/R group, TLR4 positive expression in the cytoplasm significantly higher than the corresponding expression sampled from sham group. c The low-dosage fluvastatin-treated group. The photo reveals that TLR4 positive expression in the cytoplasm is not significantly different from the corresponding expression occurred in the I/R group. d The medium-dosage fluvastatin-treated group, showing a remarkable decrease in TLR4 expression in the cytoplasm as compared with the corresponding expression found in I/R group and the low-dosage fluvastatin-treated group. e The high-dosage fluvastatin-treated group, showing a shrink of TLR4 expression in the cytoplasm, with positive index dropping down
Fluvastatin inhibits I/R-mediated myocardial TNF-α expression
Compared with the TNF-α expression found in sham group, TNF-α in the other four groups significantly increased. Among the three fluvastatin-treated groups, TNF-α expression found in the medium and high-dosage groups decreased notably (P < 0.01, Table 1) as compared with that in the I/R group. Whereas, the TNF-α expression in the low-dosage group did not change significantly (P > 0.05).
Fluvastatin suppresses TLR4 mRNA expression
TLR4 mRNA expression found in the I/R group and the three fluvastatin-treated groups significantly increased as compared with that in the sham group. TLR4 mRNA expression found in the I/R, low-dosage, medium-dosage and high-dosage group was respectively 11.28, 10.44, 2.72 and 2.45-fold higher than that found in the sham group. In addition, compared with I/R group, TLR4 mRNA expression found in the medium and high-dosage groups significantly decreased (Fig. 4).
The myocardial TLR4 mRNA expression in the five groups. 2−ΔΔCt Method, indicating the comparative expression level (in the form of percentage) between the myocardial TLR4 mRNA expression found in the four groups and that in the sham group, compared with the sham group * P < 0.01, and compared with the I/R group # P < 0.01
Fluvastatin inhibits NF-κB expression mediated by ischemia/reperfusion
As shown in Fig. 5, the results demonstrated that NF-κB protein expression observed in the medium or high-dosage fluvastatin-treated groups decreased markedly as compared with that in the control group. However, there was no obvious difference between the I/R and low-dosage groups.
Discussion
The study found that in medium-dosage and high-dosage group, as compared with I/R group, the expression of TLR4 in the rat myocardium decreased at mRNA and protein level after 2 h of ischemia–reperfusion that follows 30 min of ischemia. At the protein level, myocardial NF-κB and TNF-α expression significantly dropped. The inhibiting effect of fluvastatin was most obvious in the medium-dosage group, and not apparent in the low-dosage group. The low-dosage of fluvastatin may lessen this effect. The inhibiting effect of fluvastatin differed unremarkably in the high-dosage group and the medium-dosage one. Based on the above results, we speculate that pretreatment with an appropriate dose of fluvastatin can weaken myocardial ischemia–reperfusion injury, which may in part be attributed to fluvastatin’s role in diminishing the TLR4 expression in the myocardium, which, in turn, lessens the activity of the NF-κB and accordingly reduces inflammatory factor TNF-α expression.
TLR4, the first TLR found in mammal, manifests itself in all cell lines and is important innate immune pattern-recognition receptors (PRRs). It plays an important role in regulating the immune response and inflammatory reaction. The signal transduction pathway of TLR4 has been known clearly [12, 13], mainly through myeloid differentiation factor 88 (MyD88) dependent pathways to activate nuclear transcription factor (NF-κB). It induces the releasing of pre-inflammation factor TNF-α, IL-1, and IL-6. The secondary pathway is MyD88 independent pathway through interfering regulator 3(IRF3).
In our pre-study, we found a close relation between TLR4 activation plus the inflammatory responses stimulated by it and the MI/RI pathological process. When blood was reperfused after 30 min of myocardial ischemia, TLR4 mRNA expression increases rapidly, reaching peak after 1 h reperfusion, declines after 2 h. TLR4 up-regulation may help the ischemic myocardium synthesize and release pre-inflammation cytokines TNF-α et al., both of which may participate in the pathogenesis process of myocardial inflammatory reaction which is induced by the ischemia–reperfusion [14].
The studies regarding the relevance between the effect of statins-based drugs and TLR4 appear in several literatures. In 2005, Methe et al. [15] applied different dosages of atorvastatin and simvastatin to human CD14+ monocytes and found that both drugs can reduce the TLR4 expression emerging on the surface of CD14+ monocytes, and accordingly slow down the activation of IL-1 receptor-related kinase 1 (IRAK-1), reduce the expression of inflammation-related factors and B7-1. The following year, Niessner et al. [16] found that simvastatin can reduce the LPS-induced human monocyte surface TLR4 and TLR2 expression, and decrease TNF-α and MCP-1 creation. In 2008, Földes et al. [17] found that fluvastatin-based pretreatment can inhibit the over-expression of the LPS-stimulated TLR4 existed in mononuclear cells, and consequently shield myocardium from the autoimmune response injury. But the relevance between the inhibiting effect of statins-based drugs on the MI/RI and cardiac TLR4 expression remain unclear.
Generally, NF-κB dimers(P65/P50)are associated with an inhibitory IκB protein that acts to inhibit NF-κB function. The activated NF-κB shifts to the cell nucleus immediately and leads to alterations in synthesis and release of pro-inflammatory cytokines, such as TNF-α, IL-1, IL-6, which play an important role in immunity and inflammation reaction. At present, it is known that the activation of NF-κB can encourage the development of myocardial infarction after the MI/RI [18–20]. TNF-α, as an important marker of inflammatory response in myocardial ischemia reperfusion, has a high level of expression, may manifest its involvement in MI/RI. Studies have shown that the activation of cardiac NF-κB contributes to the increase of TNF-α, which, in turn, leads to reperfusion myocardial inflammatory tissue injury [21]. As a result, we hold that the statins-based drugs’ inhibition of TNF-α in the early stage of MI/RI explains why statins can improve the cardiac function after MI/RI. This study further reveals that inhibition of TNF-α by statins can lower their cardiac TLR4 expression.
In conclusion, proper dosage of fluvastatin could decrease myocardium TLR4 expression to protect myocardium from ischemic injury. With the wide clinical use of medical and operative thrombolysis technique, the exploration of the protective function of statins to MI/RI and its mechanism has great significance in the field of cardiovascular medicine.
References
Blancke F, Claeys MJ, Jorens P, Vermeiren G, Bosmans J, Wuyts FL, Vrints CJ (2005) Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediat Inflamm 2005:385–389
Gao X, Zhang H, Belmadani S, Wu J, Xu X, Elford H, Potter BJ, Zhang C (2008) Role of TNF-alpha-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am J Physiol Heart Circ Physiol 295:H2242–H2249
Oyama J, Blais C Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T (2004) Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109:784–789
Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED (2004) Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg 128:170–179
Cha J, Wang Z, Ao L, Zou N, Dinarello CA, Banerjee A, Fullerton DA, Meng X (2008) Cytokines link toll-like receptor 4 signaling to cardiac dysfunction after global myocardial ischemia. Ann Thorac Surg 85:1678–1685
Matsuki A, Igawa A, Nozawa T, Nakadate T, Igarashi N, Nonomura M, Inoue H (2006) Early administration of fluvastatin, but not at the onset of ischemia or reperfusion, attenuates myocardial ischemia–reperfusion injury through the nitric oxide pathway rather than its antioxidant property. Circ J 70:1643–1649
Tiefenbacher CP, Kapitza J, Dietz V, Lee CH, Niroomand F (2003) Reduction of myocardial infarct size by fluvastatin. Am J Physiol Heart Circ Physiol 285:H59–H64
Hodgkinson CP, Ye S (2008) Statins inhibit toll-like receptor 4-mediated lipopolysaccharide signaling and cytokine expression. Pharmacogenet Genomics 18:803–813
Zhao ZH, Shan J, Xiang MX, Xu G, Fu GS, Bao XF (2005) Influence of fluvastatin on left ventricular remodeling after myocardial infarction in rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 34:447–453, 464
Chimenti S, Carlo E, Masson S, Bai A, Latini R (2004) Myocardial infarction: animal models. Methods Mol Med 98:217–226
Marino JH, Cook P, Miller KS (2003) Accurate and statistically verified quantification of relative mRNA abundances using SYBR Green I and real-time RT-PCR. J Immunol Methods 283:291–306
Tanimura N, Saitoh S, Matsumoto F, Akashi-Takamura S, Miyake K (2008) Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys Res Commun 368:94–99
Gohda J, Matsumura T, Inoue J (2004) Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol 173:2913–2917
Yang J, Yang J, Ding JW, Chen LH, Wang YL, Li S, Wu H (2008) Sequential expression of TLR4 and its effects on the myocardium of rats with myocardial ischemia-reperfusion injury. Inflammation 31:304–312
Methe H, Kim JO, Kofler S, Nabauer M, Weis M (2005) Statins decrease toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arterioscler Thromb Vasc Biol 25:1439–1445
Niessner A, Steiner S, Speidl WS, Pleiner J, Seidinger D, Maurer G, Goronzy JJ, Weyand CM, Kopp CW, Huber K, Wolzt M, Wojta J (2006) Simvastatin suppresses endotoxin-induced upregulation of toll-like receptors 4 and 2 in vivo. Atherosclerosis 189:408–413
Földes G, von Haehling S, Okonko DO, Jankowska EA, Poole-Wilson PA, Anker SD (2008) Fluvastatin reduces increased blood monocyte toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int J Cardiol 124:80–85
Brown M, McGuinness M, Wright T, Ren X, Wang Y, Boivin GP, Hahn H, Feldman AM, Jones WK (2005) Cardiac-specific blockade of NF-kappaB in cardiac pathophysiology: differences between acute and chronic stimuli in vivo. Am J Physiol Heart Circ Physiol 289:H466–H476
Liao XX, Li X, Ma ZF, Wang LC, Du ZM, Dong YG, Ma H (2008) Role of nuclear factor-KappaB in endothelial injury in acute myocardial infarction. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 20:413–415
Moss NC, Tang RH, Willis M, Stansfield WE, Baldwin AS, Selzman CH (2008) Inhibitory kappa B kinase-beta is a target for specific nuclear factor kappa B-mediated delayed cardioprotection. J Thorac Cardiovasc Surg 136:1274–1279
Gu Q, Yang XP, Bonde P, DiPaula A, Fox-Talbot K, Becker LC (2006) Inhibition of TNF-alpha reduces myocardial injury and proinflammatory pathways following ischemia-reperfusion in the dog. J Cardiovasc Pharmacol 48:320–328
Author information
Authors and Affiliations
Corresponding author
Additional information
Jun Yang, Jian Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, J., Zhang, XD., Yang, J. et al. The cardioprotective effect of fluvastatin on ischemic injury via down-regulation of toll-like receptor 4. Mol Biol Rep 38, 3037–3044 (2011). https://doi.org/10.1007/s11033-010-9970-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-9970-z