Abstract
Plant WRKY transcriptional factors play an important role in response to biotic and abiotic stresses. In this study, a WRKY transcription factor was isolated from grapevine. This transcription factor showed 66% and 58% identity at the DNA and amino acid sequence levels, respectively, with Arabidopsis AtWRKY11 genes, and was therefore designated VvWRKY11. Phylogenetic analysis and structure comparison indicated that VvWRKY11 protein belongs to group IIc. The VvWRKY11 protein was shown to be located in the nucleus based on green fluorescent protein analysis. Yeast one-hybrid analysis further indicated that VvWRKY11 protein binds specifically to the W-box element. The expression profile of VvWRKY11 in response to treatment with phytohormone salicylic acid or pathogen Plasmopara viticola is rapid and transient. Transgenic Arabidopsis seedlings overexpressing VvWRKY11 showed higher tolerance to water stress induced by mannitol than wild-type plants. These results clearly demonstrated that the VvWRKY11 gene is involved in the response to dehydration stress. In addition, the role of VvWRKY11 protein in regulating the expression of two stress response genes, AtRD29A and AtRD29B, is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotic and abiotic stresses negatively influence plant growth and crop productivity. Plants have evolved diverse defense mechanisms that enable them to adapt to environmental stresses [1]. A common feature of plant defense responses is the transcriptional activation of numerous genes upon external stimuli, including biotic and abiotic stresses [2]. Genes induced under stress conditions can be divided into two groups [3]. The first code for proteins that protect plants against environmental stresses, and the second are involved in signal transduction. For example, antifreeze proteins, manganese superoxide dismutases and FtsH proteins can enhance plant tolerance to cold, salt and drought stresses, respectively [4–6]. Protein kinases and phosphatases possess converse functions in signal transduction pathways [7, 8]. It has been reported that several kinds of transcription factor (TF) such as DREB, ERF, ZFP, QM, and WRKY are involved in response to various environmental stresses [9–13]. Therefore, it is clear that transcriptional regulation of plant defense-related genes is a vital part of plant defense responses [3].
The WRKY family represents a major group of plant-specific transcriptional regulators, and encodes a large of group of transcription factors [9]. For example, there are more than 74 and 81 WRKY TFs in Arabidopsis and rice, respectively [14]. The WRKY genes are characterized by the presence of one or two highly conserved WRKY domains. The WRKY domain is about 60 amino acids in length and consists of the absolutely conserved sequence motif WRKYGQK and a Cys(2)His(2) or Cys(2)HisCys zinc-binding motif [9]. The two motifs are necessary for the high binding affinity of WRKY proteins to W-boxes, which contain an invariant DNA sequence (T)(T)TGAC(C/T) [15–18]. The W-boxes are a major class of cis-acting elements and are present in the promoters of many defense-related genes. For example, the Vitis vinifera WRKY1 protein binds specifically to W-box elements present in the promoter regions of two pathogen-defense genes, PR1 in parsley and NPR1 in Arabidopsis [17].
WRKY proteins can be divided into three groups according to the number and type of WRKY domains [9, 19]. Group I proteins possess two WRKY domains, whereas those in groups II and III have only one WRKY domain. WRKY transcription factors are key regulators of responses to microbial infection in plants. For example, gene expression profile analyses indicated that 49 of 72 WRKY genes tested in Arabidopsis responded to bacterial infection [20]. Activation of the transcription factors WRKY22/WRKY29 confers resistance to both bacterial and fungal pathogens in Arabidopsis [21]. The transcription factor WRKY33 regulates the antagonistic relationship between defense pathways mediating responses to Pseudomonas syringae and necrotrophic pathogens in Arabidopsis [22]. The importance of WRKY transcription factors in plant disease response is further demonstrated by the strategy of modulating gene expression. For example, overexpression of WRKY70 significantly increases resistance to virulent pathogens [23]. Moderate expression of WRKY18 significantly increases the expression levels of pathogenesis-related genes, leading to resistance to the bacterial pathogen P. syringae in Arabidopsis [24]. In addition to regulating the expression of defense-related genes, WRKY transcription factors are also involved in hormone responses. For example, Arabidopsis WRKY70 has been shown to regulate cross talk between jasmonate (JA)- and salicylate (SA)-regulated disease response pathways [23]. Overexpression of WRKY70 results in constitutive expression of SA-induced pathogenesis-related genes, whereas antisense suppression of WRKY70 activates JA-responsive/COI1-dependent genes. The transcription factor WRKY21 from Larrea tridentata encodes an activator of the abscisic acid (ABA) signaling pathway [25]. The rice WRKY71 gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells [26]. Moreover, WRKY genes are also involved in plant development such as leaf senescence [27, 28] and embryogenesis [29], and abiotic stress responses such as nutrient stress [30, 31], wounding [32], cold and drought [33].
To date, there are few reports about the characterization of WRKY genes from grapevine. In this study, we isolated a WRKY family transcription factor, designated VvWRKY11, from cv. Beifeng. The subcellular localization of VvWRKY11 was examined and its ability to bind specifically to W-box DNA elements was demonstrated. The expression of VvWRKY11 is inducible by SA treatment. The biological role of VvWRKY11 was assessed by its overexpression in Arabidopsis. Transgenic lines carrying the VvWRKY11 gene exhibited reduced susceptibility towards osmotic stress, suggesting that VvWRKY11 is involved in response to plant drought tolerance.
Materials and methods
Plant materials
Two-node cuttings of the grapevine cv. Beifeng derived from an intercross between V. thunbergii and V. vinifera were planted in pots and grown in a greenhouse at 23 ± 2°C, 70–80% relative humidity with a 14 h light/10 h dark photoperiod. The Arabidopsis seedlings used in this study belong to Columbia ecotype.
Recovery of full-length VvWRKY11 cDNA
To identify members of the grapevine WRKY family, the Arabidopsis transcription factor WRKY11 was used as a query sequence to BLAST against Vitis EST database. An EST showing a high degree of similarity in both amino acid and DNA sequences with the Arabidopsis WRKY11 gene was identified. Based on the EST sequence, a gene specific primer 5′-CTGCATCGCGACCTGTGAGTGAGAG-3′ was then designed to recover the 5′-end sequence using the rapid-amplification of cDNA end (RACE) method. The PCR program consisted of 30 cycles of 30 s at 95°C, 30 s at 58°C, and 2 min at 72°C. PCR products of the expected size were cloned into pGEM-T vector (Promega, Madison, WI) and then subjected to sequencing.
Analysis of VvWRKY11 binding to W-box using a yeast one-hybrid system
Yeast one-hybrid analysis was performed using a Clontech system (Clontech, Mountain View, CA). A W-box-related cis-acting element located between −995 and −976 bp upstream of the promoter region of the grapevine VST1 gene and its corresponding W-box mutant (mW-box) element were synthesized [34]. The nucleotide sequences of W-box and mW-box elements were 5′-GCCGTTGAAAAGTCAAATGA-3′ and 5′-GCCGTTGAAAATATTAATGA-3′, respectively. These two elements were individually inserted into the SmaI site of pHis3 vector and the XhoI site of pLacZi vector, respectively (Clontech). Four constructs designated pW-box-His3, pmW-box-His3, pW-box-LacZi, and pmW-box-LacZi were generated. The whole coding region of VvWRKY11 was cloned in-frame into the yeast expression vector pGAD424 to fuse with the GAL4 activation domain, generating the expression vector pGAD424-VvWRKY11. All the constructs were confirmed by direct sequencing, and then transferred into yeast (Saccharomyces cerevisiae) strain YM4271 (Clontech). The empty vector pGAD424 was also transformed as a negative control. The transformed yeast cells were grown on SD medium containing 40 mM 3-aminotrizole (3-AT; a competitive inhibitor of the His3 gene product), and β-galactosidase activity was assessed according to the manufacturer’s instruction (Clontech, USA).
Subcellular localization of the VvWRKY11 protein using green fluorescent protein (GFP) fusion proteins
The coding region of VvWRKY11 was amplified using a pair of gene-specific primers, 5′-GTCGACGACATCTTCCTTTGTCTGGTAGT-3′ and 5′-CGCGGATCCAGTTGACTCGAACACCAAGCC-3′, containing SalI and BamHI sites at the respective 5′-ends. The PCR product was digested using SalI and BamHI, and then inserted into the 5′-end of the green fluorescent protein (GFP) coding region of the vector pJIT163 (a gift from Prof. HongQing Ling from Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China) under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The construct and empty vector pJIT163 were then transformed into onion epidermal cells using the bombardment procedure [35]. The transformed onion cells were incubated at 25°C in darkness for 16–20 h. Images were captured using a laser scanning confocal microscope (Leica, Wetzlar, Germany) at the wavelength of 488 nm.
Construction of VvWRKY11 expression vector and Arabidopsis transformation
The coding region of VvWRKY11 was amplified using a pair of primers, 5′-CGGGGTACCGACATCTTCCTTTGTCTGGTAGT-3′/5′-TGCTCTAGAATTTACAGATCCAACCCATTCCC-3′, containing KpnI and XbaI sites at respective 5′-ends. The PCR products were cloned into pGEM-T Easy vector (Promega) and confirmed by direct sequencing. Subsequently, the plasmid DNA of the VvWRKY11 clone was digested with KpnI and XbaI. DNA fragments containing the full-length cDNA of VvWRKY11 were recovered and cloned into pCAMBIA2300. The expression vector of VvWRKY11 was introduced into Agrobacterium tumefaciens GV3101, and Arabidopsis transformation was performed using the floral dip method [36]. Transgenic seedlings were grown in a growth chamber at 22°C and 70% relative humidity with a 16 h photoperiod.
Osmotic stress treatment of Arabidopsis transgenic lines expressing VvWRKY11 gene
Wild-type and T2 seeds were surface-sterilized with 20% (v/v) commercial bleach for 10 min. The sterilized seeds were plated on 1/2 MS medium at 4°C for 2–4 days and then transferred to growth chambers. To examine the effect of osmotic stress on the growth of VvWRKY11 transgenic and wild-type plants, 4-day-old seedlings were transferred and vertically plated onto 1/2 MS media supplemented with different concentration of mannitol. The Chlorophyll contents of leaves were measured 7 days after mannitol treatment. A total of 0.05 grams of leaves from each sample were ground in liquid nitrogen. The ground leaves were extracted with 80% acetone, and the supernatant was incubated in the dark for 2 h at room temperature. Optical density (OD) was measured at 663 and 645 nm. The contents of chlorophyll a and b were calculated according to the method described previously [37].
Pathogen challenge of grapevine and transgenic Arabidopsis
For grapevine, two-node cuttings of cv. Beifeng were planted in pots and grown in greenhouse at 23 ± 2°C, 70–80% relative humidity with a 14-h photoperiod. P. viticola inoculum was collected from sporulated field leaves, resuspended in water and adjusted to 50,000 sporangia/ml using a Fuchs–Rosenthal hemocytometer (Thoma, Freiburg, Germany). The sporangia suspension was then sprayed onto the abaxial leaf surfaces.
For transgenic Arabidopsis, virulent P. syringae pv. tomato strain DC3000 (Pst DC3000) was cultured at 28°C on King’s B medium supplemented with 50 mg/ml rifampicin. Inoculation was performed using the injection method [38]. Briefly, 5-week-old plants were infiltrated with a bacterial suspension (OD600 = 0.0001 in 10 mM MgCl2) using a needless syringe. The inoculated plants were kept in a moist chamber at 23–25°C in darkness for 24 h and then under an 8-h photoperiod. The infected leaf tissues were collected on days 0 and 3 after inoculation. To assess pathogen challenge, the leaves were homogenized in 10 mM MgCl2 and tenfold dilutions were then plated onto King’s B medium agar containing 50 mg/ml rifampicin. The bacterial colony forming units were counted after overnight incubation. Three replicates of each experiment were conducted.
RNA extraction and gene expression analysis
Total RNA was extracted according to a method reported previously [39], and was then treated with 10 units of RNase-free DNase I (Takara Bio Inc., Kyoto, Japan) to remove genomic DNA contamination. The first-strand cDNA was synthesized in a total volume of 25 μl containing 2 μg of total RNA template, 500 ng of M13APN, and 200 units of Promega M-MLV Reverse Transcriptase according to the manufacturer’s instructions.
Expression of the VvWRKY11 gene in Arabidopsis T2 transgenic lines was analyzed by RT–PCR. Total RNA was extracted from young seedlings, and RT–PCR was carried out using a two-step procedure. Two primer pairs 5′-CGCATGCTGTCTCATCAGACCAATC-3′/5′-GAGCTGGAGTACTTTCCGGAGATATC-3′ and 5′-AAGCTTGCTGATAACTGTACTGGT-3′/5′-GGTTTGGAACTCAGTGACATCA-3′ were designed to amplify VvWRKY11 and tubulin genes, respectively. PCR was performed for 27 and 22 amplification cycles for VvWRKY11 and tubulin, respectively.
To examine the expression profiles of AtRD29A and AtRD29B genes under osmotic stress condition, 4-week-old wild-type and transgenic Arabidopsis seedlings were treated with 500 mM mannitol. Leaves were harvested at different stage after treatment, and subjected to RNA extraction. Expression profiles of two ABA-mediated cell signaling genes AtRD29A and AtRD29B and the actin gene were investigated by quantitative real-time PCR, and the following pairs of primers were used: 5′-CCAGAGATGATTTTGTGGAGACGAG-3′/5′-CACTTGAGTTTGATCTCCCACCG-3′, 5′-AAGTAGAGAGTGGATTGGGAAGAGAC-3′/5′-GAAGCTAACTGCTCTGTGTAGGTGC-3′, 5′-GGTAACATTGTGCTCAGTGGTGG-3′/5′-AACGACCTTAATCTTCATGCTGC-3′, respectively. PCR amplification was performed using the 7900 Sequence Detection System (Applied Biosystems, Foster City, CA). Each reaction well contained 2.5 μl of template cDNA, 12.5 μl of 2× Quantitect SYBRR® Green I Mix (Applied Biosystems), and 12.5 pmol of a gene-specific primer. The PCR program consisted of 95°C for 10 min, followed by 40 cycles at 94°C for 15 s and 60°C for 1 min. Three replicates of each reaction were performed, and data was analyzed according to the comparative CT method [40]. The two gene expression levels were normalized against the actin gene expression level.
Results
Isolation of the VvWRKY11 gene in grapevine
The coding DNA sequence of Arabidopsis transcription factor AtWRKY11 was BLAST against the Vitis EST database, and an EST sequence with a significant hit from V. vinifera (GenBank accession no. EC935078) was identified. The EST consists of 3′-untranslated region (UTR) and a poly(T) tail, and its deduced amino acid sequence showed 61% identity with the AtWRKY11 protein. Thus, the EST was deemed to be a fragment of the grapevine WRKY cDNA sequence, and the 5′-end sequence of the grapevine WRKY gene was sequentially recovered using 5′-RACE method. The coding sequence of the grapevine WRKY gene was compared with the Arabidopsis protein database using BLASTX program, and two top hits were found: AtWRKY11 (E values = 6e−104) and AtWRKY17 (E values = 5e−96), suggesting that the grapevine WRKY gene may be an orthologous gene of AtWRKY11. Moreover, the cDNA sequence of the grapevine WRKY gene was compared with the V. vinifera whole genome sequence database, and the result showed that it matches exactly with a contig genomic DNA sequence (GenBank accession no. AM462490). This result further indicated that the cDNA sequence was most likely derived from the V. vinifera genome. Therefore, the grapevine WRKY gene was designated VvWRKY11, and deposited in the NCBI database with accession no. AM886167.
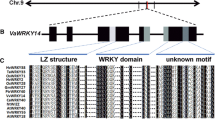
The cDNA sequence of VvWRKY11 gene is 1,255 bp in length, including 31 bp of the 5′-UTR and 207 bp of the 3′-UTR. The VvWRKY11 gene is composed of three exons and two introns, and its genomic structure is similar to that of AtWRKY11, with the last two exons almost identical in size (Fig. 1). The deduced polypeptide encoded by the VvWRKY11 gene consists of 338 amino acids with a molecular mass of 25 kDa and isoelectric point of 5.03. Like the Arabidopsis AtWRKY11 protein, the grapevine VvWRKY11 contains the following four domains: a conserved WRKY domain, a putative nuclear localization signal or sequence (NLS), a HARF domain and a N-terminal C domain which is unique to group II members of the WRKY family (Fig. 1). The WRKY domains of VvWRKY11 and AtWRKY11 show 88% identity at the amino acid sequence and are characterized by a single C–C–H–H zinc finger. Moreover, phylogenetic analysis clearly indicated that VvWRKY11 belongs to group IId (Supplementary Fig. 1).
VvWRKY11 binds to W-box elements
Yeast cells transformed with both pGAD-VvWRKY11 and pW-box-His3 vectors can grow on SD/His-/Leu-/Ura-media supplemented with 40 mM 3-AT (Fig. 2c) and show β-galactosidase activity as well (Fig. 2d), suggesting that the interaction between VvWRKY11 and the W-box element activates transcription of the two reporter genes HIS3 and LacZ genes. Conversely, yeast strains transformed with both pmW-box-His3 and pGAD-VvWRKY11 or pGAD424 vectors could not grow on SD/His-/Leu-/Ura-media supplemented with 40 mM 3-AT (Fig. 2c) and did not exhibit any β-galactosidase activity (Fig. 2d). This indicated there is no interaction between VvWRKY11 and the mW-box element, resulting in the failure of synthesis of both HIS and LacZ proteins. In short, the HIS3 expression analysis and the X-gal colony-lift filter assay strongly indicated that VvWRKY11 binds specifically to TGAC, the sequence of the W-box core motif.
Assay of VvWRKY11 binding to the W-box using a yeast one-hybrid system. a Schematic illustration of the positions of four kinds of yeast strains transformed with W-box-His3 or mW-box-His3 combined with pGAD-VvWRKY11 or pGAD-424; b Transformed yeast strains growing on SD/Leu-/His-/Ura-media; c Yeast strains growing on SD/Leu-/His-/Ura-media supplemented with 40 mM 3-aminotrizole (3-AT); d Analysis of β-galactosidase activity of different yeast strains using the colony-lift filter method
VvWRKY11 protein is localized in the nucleus
As a putative transcription factor, VvWRKY11 is likely to be localized in the nucleus because it consists of a putative nuclear localization signal or sequence (Fig. 1). To confirm the subcellular localization of VvWRKY11, the open reading frame of VvWRKY11 was fused in-frame to the hGFP coding sequence under the control of the CaMV35S promoter. The fused expression vector was then introduced into onion epidermal cells for transient expression. The VvWRKY11-hGFP fusion protein is located exclusively in the nucleus (Fig. 3b, c), whereas the control hGFP is uniformly distributed throughout the cell (Fig. 3e, f). This result clearly indicated that VvWRKY11 is localized in nucleus.
Nuclear localization of the VvWRKY11 protein. Onion epidermal cells were transformed with vectors expressing hGFP (d, e, and f) or fusion protein VvWRKY11-hGFP (a, b, and c) under the control of the CaMV 35S promoter. Photographs were taken under the following conditions: dark field (a and d), bright field (c and f), and a combination of both dark and bright fields (b and e)
Expression profile of grape VvWRKY11 in response to P. viticola infection and SA
Downy mildew (P. viticola) is one of the most important diseases in grape. Since WRKY gene family is related to plant defense response, the role of VvWRKY11 in response to biotic stress in grapevine was investigated after inoculation of P. viticola. The expression of VvWRKY11 was monitored at different time intervals after treatment with the pathogen in cv. Beifeng by real-time PCR, and the results are shown in Fig. 4. The levels of the VvWRKY11 transcript increased significantly at 2 h after pathogen inoculation (hpi) and reach a peak at 4 hpi. The transcript accumulation decreased significantly at 12 hpi, and rapidly reverted to the normal level at 24 hpi.
The plants of grapevine cv. Beifeng were also sprayed with 5 mM SA solution, and the expression of VvWRKY11 in leaves was monitored at different time intervals after treatment. The accumulation of VvWRKY11 transcripts increased significantly at 2 h after SA treatment and decreased rapidly 4 h after treatment (Fig. 4). This result clearly indicated that the response of VvWRKY11 to SA is both rapid and transient.
Transgenic Arabidopsis overexpressing VvWRKY11 shows tolerance to osmotic stress
The putative role of VvWRKY11 in response to abiotic stress was investigated by ectopic expression in Arabidopsis. More than ten Arabidopsis transgenic lines were generated, and two lines, TL-1 and TL-2, showed high-level expression of the grapevine gene VvWRKY11 based on the results of semiquantitative RT–PCR analysis (Fig. 5a). Thus, the two transgenic lines were selected for further analysis of resistance to osmotic stress. Four-day-old transgenic and wild-type Arabidopsis seedlings were transferred onto MS media plates containing different concentration of mannitol and placed in a vertical orientation for a further 7 days. Both the wide-type and transgenic seedlings grew well on MS plates without mannitol, and no obvious differences in seedling growth or root development were observed (Fig. 5d-I). However, the growth of both wild-type and transgenic seedlings was significantly inhibited under stress conditions in the presence of both 400 and 500 mM mannitol (Fig. 5d-II, III). The leaves of wild-type plants began to turn yellow on MS medium supplemented with 400 mM mannitol (Fig. 5d-II) and were completely yellow on MS medium supplemented with 500 mM mannitol (Fig. 5d-III). However, the leaves of transgenic lines were green on MS media supplemented with both 400 and 500 mM mannitol (Fig. 5d-II, III). Moreover, the contents of chlorophyll in transgenic lines were much higher than those in wild-type seedlings in the presence of 500 mM mannitol (Fig. 5c). These results unambiguously suggest that ectopic expression of VvWRKY11 in Arabidopsis contributes to tolerance to osmotic stress.
Comparison between wild type and transgenic Arabidopsis lines expressing VvWRKY11. a Expression of VvWRKY11 testified by RT–PCR analysis. b Resistance to Pseudomonas syringae. c Chlorophyll contents under mannitol stress. The values were measured 7 days after treatment, and the analysis was repeated three times for each sample. d Resistance to osmotic stress. Four-day-old seedlings were transferred from the growth chamber and placed vertically on MS plates supplemented with 0 mM mannitol (I), 400 mM mannitol (II), and 500 mM mannitol (III). Photographs were taken 11 days after treatment. e Expression profiles of AtRD29A and AtRD29B in 4-week-old seedlings under conditions of osmotic stress. WT and TL represented wild-type and transgenic line, respectively
The expression profiles of two stress response genes AtRD29A and AtRD29B in wild-type and transgenic Arabidopsis seedlings under osmotic stress condition were also assessed (Fig. 5e). The transcript accumulations of both AtRD29A and AtRD29B in Arabidopsis transgenic seedlings were significantly higher than those in wild-type seedlings at 5 h after treatment (Fig. 5e), suggesting that VvWRKY11 may be involved in the regulation of the two stress response genes.
Ectopic expression of VvWRKY11 in Arabidopsis contributes little to Pseudomonas resistance
The leaves of 4-week-old wild-type and transgenic Arabidopsis plants were syringe-infiltrated with a bacterial suspension of Pst DC3000. The bacterial densities were calculated 0 and 3 days after inoculation, and the results are shown in Fig. 5b. There was no significant difference in disease index between the transgenic and wild-type plants, suggesting that the expression of VvWRKY11 has no effect on the improvement of resistance to P. syringae in Arabidopsis.
Discussion
WRKY transcription factors have been isolated in a variety of plants [32, 33, 41, 42], and their functions have been widely investigated in Arabidopsis. However, there are few reports on biological roles of WRKY genes in grapevine. To date, only two WRKY genes, VvWRKY1 and VvWRKY2, have been identified in grapevine, and they have been shown to be involved in the response to biotic stress [17, 43]. Recently, an extensive EST collection was conducted in Vitis vinifera (357,849 sequences in the NCBI database as of Sep. 2009), which provides the opportunity to isolate WRKY genes in grapevine. Here, we reported the isolation and characterization of a new transcription factor VvWRKY11 from the grapevine genome.
WRKY proteins can be divided into three groups. Group I proteins contain two WRKY domains at N- and C-terminal regions, respectively, whereas the other two groups possess only one WRKY domain at C-terminal region [9]. It has been reported that the C-terminal domain of the two-WRKY-domain proteins appears to be ancestor to those with only a single WRKY domain [44]. Thus, the N-terminal WRKY domains of group I members were not selected and used for phylogenetic analysis in this study. The phylogenetic tree derived from the amino acid sequences of the C-terminal WRKY domains indicated that group I and group IIc are clustered in the same clade with a bootstrap value of 45 (supplementary Fig. 1). Recently, a total of 46 WRKY genes were isolated from canola, and phylogenetic analysis was conducted based on the amino acid sequences of WRKY domains [42]. Interestingly, the results also showed that groups I and IIc are clustered into the same clade. Thus, group I seems to have a higher degree of similarity with group IIc than with the other groups, suggesting that groups I and IIc are most likely derived from a common ancestor. In addition, the N-terminal WRKY domain of AtWRKY10 genes in Arabidopsis has been found to be lost after the divergence of dicots from monocots [9]. Thus, it is reasonable to speculate that the loss of the N-terminal domain may have occurred during the evolution of group IIc members.
It has been reported that WRKY genes appear to have originated in early eukaryotes, and have been duplicated many times during plant evolution, resulting in a large gene family for WRKY proteins in higher plants [44]. Gene duplication has played an important role in generating functional diversity within the plant WRKY gene family. For example, two closely related homologs exist in Arabidopsis, AtWRKY11 and AtWRKY17, both of which consist of three exons with the second exon identical in length (supplementary Table 1). This suggests that AtWRKY11 and AtWRKY17 must be derived from a common ancestor. Functional analysis further demonstrated that the Atwrky11 mutant rather than Atwrky17 mutant shows enhanced resistance to Pst, suggesting the duplicated genes have diverged in function [45]. Intriguingly, we compared the cDNA sequence of VvWRKY11 against the database of the whole-genome shotgun sequence of V. vinifera, and a homologous sequence, designated VvWRKY17, was identified. VvWRKY11 and VvWRKY17 have a similar genomic structure with the second exon identical in size (supplementary Table 1), and overall 69% amino acid sequence identity. Thus, VvWRKY11 and VvWRKY17 must also have a common ancestor. Likewise, rice OsWRKY51 and OsWRKY68 are homologous to Arabidopsis AtWRKY11 or AtWRKY17. OsWRKY51 and OsWRKY68 share an overall 53% identity in amino acid sequence and are identical in the size of the second exon (supplementary Table 1), indicating they are derived from a common ancestor. Moreover, VvWRKY11, VvWRKY17, OsWRKY51, and OsWRKY68 all share similar genomic structure and more than 50% of overall identity in amino acid sequence with both AtWRKY11 and AtWRKY17. Taken together, these results suggest that the three gene pairs AtWRKY11/AtWRKY17, VvWRKY11/VvWRKY17, and OsWRKY51/OsWRKY68 must be derived from a common ancestor, which is most likely duplicated before the divergence of monocots and dicots, resulting in homologous WRKY gene pairs in different higher plants.
WRKY transcription factors have been implicated in the regulation of various biological processes, including pathogen response and hormone signaling [46]. Our study presented here demonstrated that the expression of grapevine VvWRKY11 is strongly induced at 4 h after P. viticola challenge and then returns to normal at 24 h after inoculation. The grapevine gene VvWRKY11 in response to pathogen challenge is quite similar to its ortholog of Arabidopsis AtWRKY11, which shows rapid and transient induction with a peak at 2 h after Pst infection [45]. However, expression of the canola BnWRKY11 gene, an ortholog of Arabidopsis AtWRKY11, is unaffected by pathogen treatment [42]. This discrepancy may have been due to the difference in time points selected for monitoring gene expression. Both AtWRKY11 and VvWRKY11 expression levels were examined within 12 or 24 h after inoculation, whereas the measures of BnWRKY11 in response to challenge with the two pathogens Sclerotinia sclerotiorum and Alternaria brassicae were conducted more than 12 h post-inoculation. The induction of AtWRKY11 and VvWRKY11 is transient and their expression levels decreased significantly after 12 h postinoculation, suggesting that the response of WRKY11 genes to pathogen infection should be measured in the early stage. Therefore, the observation that BnWRKY11 is not induced by pathogens may have been due to missing the ideal stage for monitoring gene expression in response to pathogen stress. It is worth noting that the accumulation of JA occurs within the 1st hour after pathogen infection [47]. The pathogen-induced accumulation of JA may repress the expression of WRKY11 genes, leading to the transient induction of the VvWRKY11 gene in response to pathogen P. viticola infection. Moreover, AtWRKY11 does not respond to JA [47, 48], suggesting that it acts upstream in the JA signal transduction pathway [45]. Here, we further demonstrated that VvWRKY11 is involved in the response to SA, and shows rapid and transient induction with a peak at 2 h after SA treatment. More recently, it has also been found that the expression of VvWRKY1 is induced by SA treatment [17]. VvWRKY1 and VvWRKY11 belong to groups I and IIc, respectively. WRKY proteins belonging to different groups share common biological roles in the response to SA treatment, suggesting the functional redundancy of WRKY genes in plants.
Most defense-related genes contain W-box elements in their promoters that are specifically recognized by WRKY proteins [49–51]. Therefore, the rapid increases in the expression of WRKY genes is generally believed to play important roles in activating or attenuating expression of downstream defense-related genes, such as PR and NPR1 genes[45, 52–55]. As mentioned above, VvWRKY11 is inducible by pathogen. However, the transgenic Arabidopsis seedlings overexpressing VvWRKY11 did not show any enhanced resistance to Pst. The AtWRKY11 gene acts as negative regulator of basal resistance to Pst in Arabidopsis. The grapevine VvWRKY11 is the ortholog of AtWRKY11. It is thus appears that VvWRKY11 is also a negative regulator of basal resistance in grapevine. Therefore, ectopic expression of VvWRKY11 in Arabidopsis contributes little to Pseudomonas resistance.
WRKY transcription factors are well known to be involved in response to a wide range of abiotic stresses. For example, microarray analysis has revealed that the expressions of many WRKY transcription factors can be induced by abiotic stresses such as drought, salinity, and cold [56, 57]. Barley HvWRKY38 is involved in cold and drought stress responses [32]. Overexpression of TcWRKY53 in tobacco under drought stress strongly inhibited the expression of stress response genes such as ERF5 and EREBP-1 [58]. However, the functional roles of the grapevine WRKY genes in tolerance to abiotic stress have not been fully investigated. In this study, we investigated the biological role of VvWRKY11 in response to dehydration stress induced by mannitol, which has been widely used to evaluate plant tolerance to water stress [58, 59]. Transgenic Arabidopsis seedlings overexpressing VvWRKY11 show higher tolerance to dehydration stress than wild-type plants, suggesting that VvWRKY11 is a positive regulator of resistance to dehydration stress. Moreover, the Arabidopsis gene AtWRKY11 acts as a negative regulator of basal resistance [45]. In addition, Chlorophyll content is an important factor in determining plant growth capacity [60]. Our study indicated that Arabidopsis plants overexpressing VvWRKY11 have higher chlorophyll level than wild-type plants (Fig. 5c). Taken together, these results suggest that WRKY11 genes may be associated with both biotic and abiotic stress response signaling pathways and therefore play multiple roles in plants.
Stress response genes, such as RD29A and RD29B, contain at least two types of cis-acting element, the dehydration-responsive element (DRE) and the ABA-response element (ABRE), in their promoter regions [61, 62]. It is thus clear that higher internal ABA levels are closely related to plant tolerance to osmotic stress [63]. Here, we showed that the expression levels of AtRD29A and AtRD29B in VvWRKY11 transgenic Arabidopsis seedlings were higher than those in wild-type plants under dehydration stress, suggesting that VvWRKY11 gene product affects the expression of AtRD29A and AtRD29B in Arabidopsis. At least two possible explanations may explain this observation. First, interaction between DRE binding protein (DREB) and ABRE binding protein (ABEB) has been proposed to be involved in the regulation of AtRD29A expression [61]. Meanwhile, it has been reported that DREB2A gene requires posttranslational modification before it can activate downstream genes under normal growth conditions [62]. Thus, the VvWRKY11 protein may interact with DREB and/or ABEB, resulting in the increased expression of AtRD29A and AtRD29B. Second, we checked the genomic DNA sequence of AtRD29A and found several TGAC-like sequences in the promoter region. The TGAC-like sequences are the core of W-box. Thus, it seems that the VvWRKY11 protein may regulate the expression of AtRD29A and AtRD29B at transcriptional level. In short, the WRKY11 gene plays an important role in improving plant tolerance to water stress. This result will be helpful toward obtaining a comprehensive understanding regarding the roles of the WRKY11 protein in the regulation of biological responses to biotic and abiotic stresses.
References
Schmidt F, Marnef A, Cheung MK et al (2010) A proteomic analysis of oligo(dT)-bound mRNP containing oxidative stress-induced Arabidopsis thaliana RNA-binding proteins ATGRP7 and ATGRP8. Mol Biol Rep 37:839–845
Yang Y, Shah J, Klessig DF (1997) Signal perception and transduction in plant defense responses. Genes Dev 11:1621–1639
Singh KB, Foley RC, Onate-Sanchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5:430–436
Wang Y, Qu G, Li H et al (2010) Enhanced salt tolerance of transgenic poplar plants expressing a manganese superoxide dismutase from Tamarix androssowii. Mol Biol Rep 37:1119–1124
Zhu B, Xiong A, Peng R et al (2010) Over-expression of ThpI from Choristoneura fumiferana enhances tolerance to cold in Arabidopsis. Mol Biol Rep 37:961–966
Yue G, Hu X, He Y et al (2010) Identification and characterization of two members of the FtsH gene family in maize (Zea mays L.). Mol Biol Rep 37:855–863
Guo X, Deng K, Wang J et al (2010) Mutational analysis of Arabidopsis PP2CA2 involved in abscisic acid signal transduction. Mol Biol Rep 37:763–769
Wu T, Tian Z, Liu J et al (2009) A novel leucine-rich repeat receptor-like kinase gene in potato, StLRPK1, is involved in response to diverse stresses. Mol Biol Rep 36:2365–2374
Eulgem T, Rushton PJ, Robatzek S et al (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
Bhardwaj PK, Ahuja PS, Kumar S (2010) Characterization of gene expression of QM from Caragana jubata, a plant species that grows under extreme cold. Mol Biol Rep 37:1003–1010
Agarwal P, Agarwal PK, Joshi AJ (2010) Overexpression of PgDREB2A transcription factor enhances abiotic stress tolerance and activates downstream stress-responsive genes. Mol Biol Rep 37:1125–1135
Liu Q, Xu K, Ma N et al (2010) Isolation and functional characterization of DgZFP: a gene encoding a Cys2/His2-type zinc finger protein in chrysanthemum. Mol Biol Rep 37:1137–1142
Zhang G, Chen M, Chen X et al (2010) Isolation and characterization of a novel EAR-motif-containing gene GmERF4 from soybean (Glycine max L.). Mol Biol Rep 37:809–818
Xie Z, Zhang ZL, Zou X et al (2005) Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol 137:176–189
Rushton PJ, Macdonald H, Huttly AK et al (1995) Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of alpha-Amy2 genes. Plant Mol Biol 29:691–702
Du L, Chen Z (2000) Identification of genes encoding receptor-like protein kinases as possible targets of pathogen and salicylic acid induced WRKY DNA-binding proteins in Arabidopsis. Plant J 24:837–847
Chloe M, Rim M, Laurent D et al (2007) Isolation and characterization of a Vitis vinifera transcription factor, VvWRKY1, and its effect on responses to fungal pathogens in transgenic tobacco plants. J Exp Bot 58:1999–2010
Zheng Z, Mosher SL, Fan B et al (2007) Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae. BMC Plant Biol 7:2
Park CY, Lee JH, Yoo JH et al (2005) WRKY group IId transcription factors interact with calmodulin. FEBS Lett 579:1545–1550
Dong J, Chen C, Chen Z et al (2003) Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51:21–37
Asai T, Tena G, Plotnikova J et al (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415:977–983
Zheng Z, Qamar SA, Chen Z et al (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48:592–605
Li J, Brader G, Palva ET et al (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16:319–331
Chen C, Chen Z (2002) Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol 129:706–716
Zou X, Seemann JR, Neuman D (2004) A WRKY gene from Creosote Bush encodes an activator of the abscisic acid signaling pathway. J Biol Chem 279:55770–55779
Zhang Z, Xie Z, Zou X et al (2004) A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol 134:1500–1513
Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev 16:1139–1149
Miao Y, Laun T, Zimmermann P et al (2004) Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol 55:853–867
Lagace M, Matton DP (2004) Characterization of a WRKY transcription factor expressed in late torpedo-stage embryos of Solanum chacoense. Planta 219:185–189
Sun C, Palmqvist S, Olsson H et al (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to sugar-responsive elements of the iso1 promoter. Plant Cell 15:2076–2092
Devaiah BN, Karthikeyan AS, Raghothama KG (2007) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143:1789–1801
Hara K, Yagi M, Kusano T et al (2000) Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Mol Gen Genet 263:30–37
Mare C, Mazzueotelli E, Crosatti C (2004) Hv-WRKY38: a new transcription factor involved in cold- and drought-response in barley. Plant Mol Biol 55:399–416
Schubert R, Fischer R, Hain R et al (1997) An ozone-responsive region of the grapevine resveratrol synthase promoter differs from the basal pathogen-responsive sequence. Plant Mol Biol 34:417–426
Varagona MJ, Schmidt RJ, Raikhel NV (1992) Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein opaque-2. Plant Cell 4:1213–1227
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Wellburn AR (1994) The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Whalen MC, Innes RW, Bent AF (1991) Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 3:49–59
Zhang J, Wang Y, Wang X et al (2003) An improved method for rapidly extracting total RNA from Vitis. J Fruit Sci 20:178–181
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Dellagi A, Helibronn J, Avrova AO (2000) A potato gene encoding a WRKY-like transcription factor is induced in interactions with Erwinia carotovora subsp. atroseptica and Phytophthora infestans and is coregulated with class I endochitinase expression. Mol Plant-Microbe Interact 13:1092–1101
Yang B, Jiang Y, Rahman MH et al (2009) Identification and expression analysis of WRKY transcription factor genes in canola (Brassica napus L.) in response to fungal pathogens and hormone treatments. BMC Plant Biol 9:68
Mzid R, Marchive C, Blancard D et al (2007) Overexpression of VvWRKY2 in tobacco enhances broad resistance to necrotrophic fungal pathogens. Physiol Plant 131:434–447
Zhang Y, Wang L (2005) The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol 5:1
Journot-Catalino N, Somssich IE, Roby D et al (2006) The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell 18:3289–3302
Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10:366–371
Pauwels L, Morreel K, De Witte E et al (2008) Goossens, mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc Natl Acad Sci 105:1380–1385
Goda H, Sasaki E, Akiyama K et al (2008) The AtGen-express hormone and chemical treatment data set: experimental design, data evaluation, model data analysis and data access. Plant J 55:526–542
Rushton PJ, Torres JT, Parniske M et al (1996) Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J 15:5690–5700
Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13:1527–1540
Ulker B, Somssich IE (2004) WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 7:491–498
Lee SC, Kim YJ, Hwang BK (2001) A pathogen-induced chitin binding protein gene from pepper: its isolation and differential expression in pepper leaves treated with pathogens, ethephon, methyl jasmonate or wounding. Plant Cell Physiol 42:1321–1330
Turck F, Zhou A, Somssich IE (2004) Stimulus-dependent, promoter specific binding of transcription factor WRKY1 to its native promoter and the defense-related gene PcPR1-1 in Parsley. Plant Cell 16:2573–2585
Kim KC, Fan B, Chen Z (2006) Pathogen-induced Arabidopsis WRKY7 is a transcriptional repressor and enhances plant susceptibility to Pseudomonas syringae. Plant Physiol 142:1180–1192
Shen QH, Saijo Y, Mauch S et al (2007) Nuclear activity of MLA immune receptors links isolate-specific and basal disease resistance responses. Science 315:1098–1103
Seki M, Narusaka M, Ishida J et al (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA micro array. Plant J 31:279–292
Fowler S, Thomashow F (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14:1675–1690
Wei W, Zhang Y, Han L et al (2008) A novel WRKY transcriptional factor from Thlaspi caerulescens negatively regulates the osmotic stress tolerance of transgenic tobacco. Plant Cell Rep 27:795–803
Verslues PE, Agarwal M, Katiyar-Agarwal S et al (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45:523–539
Xu J, Tian Y, Peng R et al (2010) Cyanobacteria MT gene SmtA enhance zinc tolerance in Arabidopsis. Mol Biol Rep 37:1105–1110
Narusaka Y, Nakashima K, Shinwari ZK et al (2003) Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J 34:137–148
Sakuma Y, Maruyama K, Osakabe Y et al (2006) Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 18:1292–1309
Pandey GK, Grant JJ, Cheong YH et al (2005) ABR1, an APETALA2-domain transcription factor that functions as a repressor of ABA response in Arabidopsis. Plant Physiol 139:1185–1193
Acknowledgments
We thank Dr. Yucheng Guan for his advice on yeast one-hybrid analysis. This study was funded by the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-N-032) and the CAS/SAFEA International Partnership Program for Creative Research Teams.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Yang, W., Liu, D. et al. Ectopic expression of a grapevine transcription factor VvWRKY11 contributes to osmotic stress tolerance in Arabidopsis . Mol Biol Rep 38, 417–427 (2011). https://doi.org/10.1007/s11033-010-0124-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0124-0