Abstract
Waterlogging stress disturbs plant metabolism through increased ion (manganese and iron) toxicity resulting from the changes in the soil redox potential under hypoxic conditions. Our previous study found a significant correlation between the tolerance to Mn2+ toxicity and waterlogging stress tolerance in barley, suggesting that waterlogging tolerance could be increased by improving the tolerance to Mn2+ toxicity. In this study, a doubled-haploid (DH) population from the cross between barley varieties Yerong and Franklin (waterlogging-tolerant and -sensitive, respectively) was used to identify QTL controlling tolerance to Mn2+ toxicity based on chlorophyll content and plant survival as selection criteria. Four significant QTL for plant survival under Mn2+ stress (QSur.yf.1H, QSur.yf.3H, QSur.yf.4H, and QSur.yf.6H) were identified in this population at the seedling stage. Two significant QTL (QLC.yf.3H and QLC.yf.6H) controlling leaf chlorosis under Mn2+ stress were identified on chromosomes 3H and 6H close to QSur.yf.3H and QSur.yf.6H. The major QTL QSur.yf.3H, located near the marker Bmag0013, explained 21% of the phenotypic variation. The major QTL for plant survival on 3H was validated in a different DH population (TX9425/Naso Nijo). This major QTL could potentially be used in breeding programmes to enhance tolerance to both manganese toxicity and waterlogging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Waterlogging is one of the most hazardous natural constraints affecting agricultural crop production. Based on soil moisture or water levels, waterlogging may refer to flood, submergence, soil saturation, anoxia, or hypoxia (Ahmed et al. 2012). Soil waterlogging is generally caused by the prolonged rain or flooding in combination with poor soil drainage. The yield loss can vary with duration of the stress, soil types, and the tolerance of different species and genotypes (Bailey-Serres and Voesenek 2008; Shabala 2011).
Waterlogging stress affects plant metabolism in multiple ways, with plants showing a broad range of morphological and physiological responses to waterlogging. The phenotypic features associated directly with waterlogging tolerance remain unclear and controversial (Pezeshki et al. 1999; Smethurst et al. 2005; Teakle et al. 2010), even though the severity of leaf chlorosis after waterlogging has been proven to be one of the reliable indicators of waterlogging tolerance (Li et al. 2008; Zhou 2011). Other symptoms caused by ion toxicity or accumulation of toxic organic metabolites in roots under waterlogging conditions (Pang et al. 2007) may also occur (Khabaz-Saberi et al. 2006; Bailey-Serres and Voesenek, 2008; Khabaz-Saberi and Rengel 2010; Shabala 2011). The availability of chemical elements in soils is affected by multiple interacting factors, including soil pH, redox potential, cation exchange capacity, and microbial activity. With the redox potential declining after waterlogging onset, metals such as Mn4+ and Fe3+ are utilised as alternative electron acceptors when oxygen is depleted, resulting in increased concentrations of soluble Mn2+ and Fe2+ (Kirk et al. 2003) that may exceed plant requirements and cause toxicity. Genotypes with tolerance to Mn2+ or Fe2+ toxicity generally perform better than the sensitive ones in waterlogged soils (Pang et al. 2007; Khabaz-Saberi and Rengel 2010; Khabaz-Saberi et al. 2012). Enhanced tolerance to multiple ion toxicities was reported to improve wheat performance in waterlogging-affected acid soils (Khabaz-Saberi and Rengel 2010). The significant correlation between tolerance to Mn2+ toxicity and tolerance to waterlogging in barley (Huang et al. 2015) suggests that plant breeding for tolerance to waterlogging traits may be advanced by targeting tolerance to Mn2+ toxicity.
Similar to other waterlogging-related traits, various Mn2+ tolerance mechanisms (e.g. avoidance versus tissue tolerance) operate in different tolerant genotypes (Huang et al. 2015). Direct selection for tolerance to Mn2+ toxicity is affected by environmental factors and is thus largely ineffective. Marker-assisted selection (MAS) is based on distinct genetic differences that can easily be scored and mapped in most segregating populations (Kearsey and Farguhar 1998). During past decades, many QTL have been reported for tolerance to waterlogging or submergence based on various agronomic and morphological characteristics in different doubled-haploid (DH) populations (Qiu et al. 2007; Li et al. 2008; Zhou 2011; Zhang et al. 2013). However, to the best of our knowledge, no QTL for tolerance to Mn2+ toxicity has been reported so far.
In the present study, one DH population was used to identify QTL controlling tolerance to Mn2+ toxicity, and the QTL were validated in another DH population. Chlorophyll content and plant survival under Mn2+ toxicity were used as the indicators of Mn2+ tolerance.
Materials and methods
Plant materials and growth condition
A total of 177 lines from a DH population originated from the cross between Yerong (waterlogging tolerant) and Franklin (waterlogging sensitive) were used to identify QTL conferring Mn2+ tolerance. Another 188 DH lines from the cross between TX9425 (waterlogging tolerant) and Naso Nijo (waterlogging sensitive) were used to validate the QTL identified in the Yerong/Franklin population.
The DH lines and parents were grown as reported previously (Huang et al. 2015). Seeds were surface-sterilised using 0.5% v/v sodium hypochlorite for 15 min and then thoroughly washed in running water for 30 min. After 1-day germination in a Petri dish, seeds were transferred to a floating mesh in 60-L (60 × 40 × 25) plastic containers with nutrient solution. The solution was bubbled with air for the duration of the experiment. The nutrient solution contained 1.5-mM KNO3, 1.0-mM Ca(NO3)2, 0.25-mM MgSO4, 0.25-mM (NH4)2HPO4, 0.25-mM NH4H2PO4, and 4-mM MES hydrate buffer (modified Rygol-Johnson solution) (Shabala et al. 2010). Aliquots of freshly prepared 1-M manganese (MnSO4) stock solution were added to the nutrient solution to attain 1-mM final concentration. Nutrient solutions were replaced every 2 days. The pH of nutrient solutions was adjusted to 4.8 daily using 1-M HCl or 1-M NaOH. Distilled water was added to maintain the volume of the solution to compensate for the loss by transpiration.
Evaluating severity of Mn2+ toxicity by SPAD chlorophyll measurements
A combined visual scoring system including plant survival (dead leaf percentage) and leaf chlorosis was used in the trial. The scoring (0 = not affected and 10 = all dead) was conducted after 10 days of Mn2+ treatment. Chlorophyll content of the oldest fully expanded leaves was determined using a SPAD-520 chlorophyll meter (Konica Minolta Sensing Inc., Sakai, Japan) as a measure of Mn2+ tolerance.
Two independent trials were conducted for each of the two DH populations. Both trials had three replications, with each replicate having three plants. Each replication was placed in single plastic containers, and the genotypes were randomly arranged.
Map construction
The genetic linkage map of the Yerong/Franklin population has been published earlier by Li et al. (2008), comprising 496 DArT markers and 28 microsatellite (SSR) markers. For the TX9425/Naso Nijo population, the DH lines and the two parental varieties were genotyped with DArTSeq (http://www.diversityarrays.com/dart-application-dartseq). Due to the large number of DNA markers (~ 30,000 SNP and DArTSeq markers), markers with the same positions or with greater distortion and missing data were removed from the map construction. These markers were combined with previous genotypic data (DArT and SSR markers) (Xu et al. 2012; Wang et al. 2014). A total of around 2500 markers were selected to construct the genetic map.
QTL mapping and statistical analysis
The average values from each experiment were used for the identification of QTL associated with Mn2+ tolerance. The software package MapQTL6.0 (Van Ooijen 2006, 2009) was used to detect QTL that were first analysed by interval mapping (IM). The closest marker at each putative QTL identified using IM was selected as a cofactor and the selected markers were used as genetic background controls in the approximate multiple QTL model (MQM). The logarithm of the odds (LOD) value thresholds, used to declare the presence of a QTL, were estimated by performing the genome-wide permutation tests using at least 1000 permutations of the original data set for each trait, resulting in a 95% significance at LOD threshold 3.0. Two LOD support intervals around each QTL were established by taking the two positions around the peak that had the LOD values two less than the maximum (Van Ooijen 2009) after performing restricted MQM mapping. The percentage of variance explained by each QTL (R2) was obtained using restricted MQM mapping implemented with MapQTL 6.0. Graphical representation of linkage groups and QTL was generated by using MapChart 2.2 (Voorrips 2002). All other statistical analyses, including calculation of mean values, standard errors, and analysis of variance (ANOVA), were performed using IBM SPSS Statistics 20 (Chicago, IL, USA).
Results
Response of parents and DH lines to Mn2+ toxicity
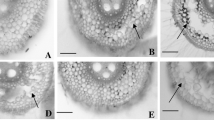
The presence of high concentration (1 mM) of Mn2+ in the growth solution had a significant effect on plant growth. Occurrence of tiny brown spots was the common symptom on older leaf tips at the seedling stage (Zadoks score of 11). The symptoms were later spread over the whole leaf and resulted in pronounced leaf chlorosis. The symptom development was faster on susceptible than tolerant genotypes (data not shown). One day after applying excess Mn2+, the susceptible parents Franklin and Naso Nijo already showed numerous tiny brown spots, and leaf chlorosis occurred 2 days later. At the end of the 1-mM Mn2+ treatment, both sensitive parents showed severe leaf chlorosis (score of 7). In contrast, both Yerong and TX9425 showed much better tolerance than Franklin and Naso Nijo. At the end of the Mn2+ treatment, only brown spots appeared on the leaves of tolerant parents Yerong and TX9425 with no obvious chlorosis. TX9425 was less tolerant than Yerong (Fig. 1).
Toxicity symptoms on the oldest fully expanded leaf of the parent genotypes at the end of the 1-mM Mn2+ treatment (11-day-old plants). Genotypes tolerant to Mn2+ toxicity: Yerong (a, chlorosis score 2.9), TX9425 (b, chlorosis score 3.2). Genotypes sensitive to Mn2+ toxicity: Franklin (c, chlorosis score 6.6), Naso Nijo (d, chlorosis score 6.7)
A wide variation (2.0–7.5) in plant survival index (mainly leaf chlorosis) after the Mn2+ treatment was found in the DH lines from both populations (Fig. 2A, C). The Yerong × Franklin population also showed a wide variation in chlorophyll content, with the SPAD reading ranging from 14 to 31 (Fig. 2B). The plant survival scores in both populations (Fig. 2A, C) and the chlorophyll content of the Yerong × Franklin population (Fig. 2B) showed normal distribution. Transgressive segregation in the plant survival scores was found in both populations, with some lines surpassing their parents. A significant regression (R2 = 0.62) between leaf chlorophyll content and plant survival index was found in the Yerong × Franklin population (Fig. 2D).
QTL for chlorophyll content and plant survival under Mn2+ stress
Four significant QTL for plant survival under Mn2+ stress (QSur.yf.1H, QSur.yf.3H, QSur.yf.4H, and QSur.yf.6H) were identified in the Yerong/Franklin DH population at the seedling stage (Table 1, Fig. 3). The Yerong alleles contributed to the tolerance at all the loci, except for the QSur.yf.1H locus where the Franklin allele improved the plant survival. These four QTL explained up to 40% of the phenotypic variation, with individual QTL explaining between 6.1 and 21% of the phenotypic variation. The major QTL for plant survival (QSur.yf.3H) was located on chromosome 3H, with the closest marker being Bmag0013 (Table 1).
Two significant QTL (QLC.yf.3H and QLC.yf.6H) controlling the severity of leaf chlorosis under Mn2+ stress were identified on chromosomes 3H and 6H, respectively (Table 1, Fig. 3). The Yerong alleles were responsible for mild leaf chlorosis (higher chlorophyll content) at both QTL. The major QTL (QLC.yf.3H) explained 22% of the phenotypic variation (Table 1). This QTL was co-located with QSur.yf.3H for plant survival under Mn2+ stress (Fig. 3). The minor QTL (QLC.yf.6H) explained 7.5% of the phenotypic variation. This QTL was located at a similar position to that for plant survival QTL (QSur.yf.6H). To test the relationship between chlorophyll content and plant survival scores, QTL analysis was conducted for the chlorophyll content using the plant survival scores as covariate, resulting in no significant QTL for the chlorophyll content.
Validation of the major QTL for plant survival in the TX9425/Naso Nijo population
Given that chlorophyll content is closely correlated with plant survival score (Fig. 2D), we used plant survival as a suitable proxy to validate the QTL in a different population. In the TX9425/Naso Nijo DH population, only one significant QTL for plant survival under Mn2+ stress was identified. The TX9425 alleles increased tolerance to manganese toxicity at QSur.tx.3H locus, explaining 15% of the phenotypic variation (Table 1). This QTL was co-located with QSur.yf.3H (cf. Fig. 3).
Discussion
Waterlogging stress is one of the major abiotic yield-limiting factors in crops, and tolerance to this stress is likely to be a complex trait controlled by several mechanisms and complicated by various confounding factors. Because of the low efficiency of direct selection for waterlogging-tolerant plants in the field (Zhou 2010), various indirect criteria have been investigated. Among them, the severity of leaf chlorosis has been used to estimate the capacity for plant survival under waterlogging stress (Li et al. 2008; Zhou 2011). In our previous report, the severity of leaf chlorosis showed a significant inverse correlation with Mn2+ tolerance, and Mn2+ tolerance was significantly correlated with waterlogging tolerance in field conditions (Huang et al. 2015). In this experiment, a significant regression (R2 = 0.62) between the severity of leaf chlorosis and plant survival under Mn2+ stress was also found in the Yerong × Franklin DH population (Fig. 2).
An understanding of the genetic background and mechanisms enhancing Mn2+ tolerance is crucial in the research and breeding programmes. Several transporters have been reported to be responsible for Mn accumulation and sequestration (Pittman 2005; Pedas et al. 2008; Cailliatte et al. 2010; Sasaki et al. 2012; Chen et al. 2013). However, no QTL has been reported for the tolerance to Mn2+ toxicity in barley. Most of the previous studies have targeted Mn2+-use efficiency (Pallotta et al. 2000; McDonald et al. 2001; Hebbern et al. 2005), with a QTL identified on the short arm of chromosome 4H (Pallotta et al. 2000). In the study reported here, barley tolerance to Mn2+ toxicity was investigated. Four significant QTL for plant survival (QSur.yf.1H, QSur.yf.3H, QSur.yf.4H, and QSur.yf.6H) and two for the severity of leaf chlorosis (QLC.yf.3H and QLC.yf.6H) under Mn2+ stress in the seedling stage were identified in the Yerong/Franklin population. Among them, QSur.yf.3H contributed more than 20% of the phenotypic variation for tolerance to Mn toxicity in the Yerong/Franklin population, whereas other QTL each explained less than 10%. To our surprise, only one minor QTL controlling plant survival under Mn2+ stress (QSur.yf.4H) was located at a similar position as the major QTL for waterlogging tolerance in the Yerong/Franklin population based on the plant survival scores after 9 weeks of waterlogging (Zhou 2011). The major QTL on chromosome 3H (QSur.yf.3H) and the minor QTL on 6H (QSur.yf.6H) are likely to be responsible for waterlogging tolerance at the early treatment stages because QTL for waterlogging tolerance on 3H and 6H were identified after 3 weeks and 2–5 weeks of waterlogging treatment, respectively (Zhou 2011).
TX9425 is a landrace from China showing better tolerance to waterlogging stress than Naso Nijo as a susceptible genotype (Zhou et al. 2007; Xu et al. 2012). However, the waterlogging tolerance of TX9425 is not as good as that of Yerong (Huang et al. 2015). In addition, TX9425 showed lower tolerance to Mn2+ toxicity (Fig. 1) and had higher shoot Mn2+ concentration under Mn2+ stress than Yerong (Huang et al. 2015). TX9425 appeared to have a high capacity to sequester Mn2+, maintaining higher Mn content in the shoot than susceptible genotype Naso Nijo (Huang et al. 2015). A DH population from the cross of TX9425/Naso Nijo was tested for Mn2+ tolerance in the present study, and only one major QTL for plant survival was identified on chromosome 3H. This QTL was at the similar position to the QTL for plant survival and leaf chlorosis (QSur.yf.3H and QLC.yf.3H) identified in the Yerong/Franklin population. Therefore, it is likely that the better tolerance of Yerong compared with TX9425 was due to other minor QTL absent in TX9425, i.e. QSur.yf.1H, QSur.yf.4H, and QSur.yf.6H.
Mn2+ toxicity can trigger oxidative stress in plant cells (Demirevska-Kepova et al. 2004) because high Mn concentration in the cytosol inhibits the non-cyclic photophosphorylation process (Lidon and Teixeira 2000), thus promoting an increase in the production of ROS (specifically, hydroxyl radicals as the most aggressive of all ROS species). This Mn2+ toxicity-induced ROS accumulation may have consequences not only for structural integrity and organisation of the chloroplast lamellae (and, hence, photosynthetic machinery operation) but also for plant ionic relations. Of specific interest is a potential Mn impact on the cytosolic K+ homeostasis. ROS (especially hydroxyl radicals) are known to be potent activators of various K+-permeable channels (e.g. Demidchik et al. 2003, 2007, 2010; Zepeda-Jazo et al. 2011), prompting ROS stress-induced K+ depletion in the cytosol. Importantly, K+ is an activator of more than 70 key metabolic enzymes (Dreyer and Uozumi 2011), including those for chlorophyll biosynthesis and photosynthetic CO2 assimilation. According to the new barley reference genome sequence and annotations (Mascher et al. 2017), a candidate gene (HORVU3Hr1G068040.1) that codes for KAT3 was found in the region of the major QTL identified in the present study on chromosome 3H. KAT3 is a member of the Shaker family of voltage-gated potassium channels (Sharma et al. 2013) that are expressed in rice guard cells (Hwang et al. 2013).
The other candidate gene HORVU3Hr1G068200.1 that is also located in this QTL region appears to be more relevant to Mn2+ toxicity tolerance. This gene is identified as AK369780 in the GenBank database, a member of heavy metal-associated domain superfamily (HMA). Most of the known HMA transporters were involved in accumulation, transportation, or detoxification of zinc, copper, and cadmium (Takahashi et al. 2012; Deng et al. 2013; Satoh-Nagasawa et al. 2013; Huang et al. 2016). However, HORVU3Hr1G068200.1 has not been assigned any putative function yet. The gene ontology (GO) data suggest that certain portions of the gene are related to heavy metal ion transport (GO:0030001) and metal ion binding (GO:0046872). This gene remains to be investigated further for its possible involvement in the regulation of Mn accumulation and detoxification.
In conclusion, the major QTL for Mn2+ toxicity tolerance was identified on chromosome 3H in the Yerong/Franklin DH population and validated in TX9425/Naso Nijo DH population. Three minor QTL were also found in the Yerong/Franklin population. All the QTL likely contribute to waterlogging tolerance in the early growth stage.
References
Ahmed F, Rafii MY, Ismail MR, Juraimi AS, Rahim HA, Asfaliza R, Latif MA (2012) Waterlogging tolerance of crops: breeding, mechanism of tolerance, molecular approaches, and future prospects. Biomed Res Int 2013:963525
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59(1):313–339. https://doi.org/10.1146/annurev.arplant.59.032607.092752
Cailliatte R, Schikora A, Briat J-F, Mari S, Curie C (2010) High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 22(3):904–917. https://doi.org/10.1105/tpc.109.073023
Chen Z, Fujii Y, Yamaji N, Masuda S, Takemoto Y, Kamiya T, Yusuyin Y, Iwasaki K, Kato S-i, Maeshima M, Ma JF, Ueno D (2013) Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. J Exp Bot 64(14):4375–4387. https://doi.org/10.1093/jxb/ert243
Demidchik V, Cuin T, Svistunenko D, Smith S, Miller A, Shabala S, Sokolik A, Yurin V (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci 123(9):1468–1479. https://doi.org/10.1242/jcs.064352
Demidchik V, Maathuis F (2007) Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol 175(3):387–404. https://doi.org/10.1111/j.1469-8137.2007.02128.x
Demidchik V, Shabala S, Coutts K, Tester M, Davies J (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+- permeable channels in plant root cells. J Cell Sci 116(1):81–88. https://doi.org/10.1242/jcs.00201
Demirevska-Kepova K, Simova-Stoilova L, Stoyanova Z, Hölzer R, Feller U (2004) Biochemical changes in barley plants after excessive supply of copper and manganese. Environ Exp Bot 52(3):253–266. https://doi.org/10.1016/j.envexpbot.2004.02.004
Deng F, Yamaji N, Xia J, Ma J (2013) A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol 163(3):1353–1362. https://doi.org/10.1104/pp.113.226225
Dreyer I, Uozumi N (2011) Potassium channels in plant cells. FEBS J 278(22):4293–4303. https://doi.org/10.1111/j.1742-4658.2011.08371.x
Hebbern CA, Pedas P, Schjoerring JK, Knudsen L, Husted S (2005) Genotypic differences in manganese efficiency: field experiments with winter barley (Hordeum vulgare L.) Plant Soil 272(1):233–244. https://doi.org/10.1007/s11104-004-5048-9
Huang X, Deng F, Yamaji N, Pinson SRM, Fuji-Kashino M, Danku J, Douglas A, Guerinot ML, Salt DE, Ma J (2016) A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat Commun 7:12138. https://doi.org/10.1038/ncomms12138
Huang X, Shabala S, Shabala L, Rengel Z, Wu X, Zhang G, Zhou M (2015) Linking waterlogging tolerance with Mn2+ toxicity: a case study for barley. Plant Biol 17(1):26–33. https://doi.org/10.1111/plb.12188
Hwang H, Yoon J, Kim HY, Min MK, Kim J, Choi E, Lan W, Bae Y, Luan S, Cho H, Kim B (2013) Unique features of two potassium channels, OsKAT2 and OsKAT3, expressed in rice guard cells. PLoS One 8(8):e72541. https://doi.org/10.1371/journal.pone.0072541
Kearsey MJ, Farquhar AG (1998) QTL analysis in plants: where are we now? Heredity 80(2):137–142. https://doi.org/10.1046/j.1365-2540.1998.00500.x
Khabaz-Saberi H, Barker SJ, Rengel Z (2012) Tolerance to ion toxicities enhances wheat (Triticum aestivum L.) grain yield in waterlogged acidic soils. Plant Soil 354(1-2):371–381. https://doi.org/10.1007/s11104-011-1073-7
Khabaz-Saberi H, Rengel Z (2010) Aluminum, manganese, and iron tolerance improves performance of wheat genotypes in waterlogged acidic soils. J Plant Nutr Soil Sci 173(3):461–468. https://doi.org/10.1002/jpln.200900316
Khabaz-Saberi H, Setter T, Waters I (2006) Waterlogging induces high to toxic concentrations of iron, aluminum, and manganese in wheat varieties on acidic soil. J Plant Nutr 29(5):899–911. https://doi.org/10.1080/01904160600649161
Kirk G, Solivas J, Alberto M (2003) Effects of flooding and redox conditions on solute diffusion in soil. Eur J Soil Sci 54(3):617–624. https://doi.org/10.1046/j.1365-2389.2003.00532.x
Li H, Vaillancourt R, Mendham N, Zhou M (2008) Comparative mapping of quantitative trait loci associated with waterlogging tolerance in barley (Hordeum vulgare L.) BMC Genomics 9(1):401. https://doi.org/10.1186/1471-2164-9-401
Lidon FC, Teixeira MG (2000) Oxy radicals production and control in the chloroplast of Mn-treated rice. Plant Sci 152(1):7–15. https://doi.org/10.1016/S0168-9452(99)00179-X
Mascher M, Gundlach H, Himmelbach A, Beier S, Twardziok S, Wicker T, Radchuk V, Dockter C, Hedley P, Russell J, Bayer M, Ramsay L, Liu H, Haberer G, Zhang X, Zhang Q, Barrero R, Li L, Taudien S, Groth M, Felder M, Hastie A, Šimková H, Staňková H, Vrána J, Chan S, María Muñoz-Amatriaín, Ounit R, Wanamaker S, Bolser D, Colmsee C, Schmutzer T, Aliyeva-Schnorr L, Grasso S, Tanskanen J, Chailyan A, Sampath D, Heavens D, Clissold L, Cao S, Chapman B, Dai F, Han Y, Li H, Li X, Lin C, McCooke J, Tan C, Wang P, Wang S, Yin S, Zhou G, Poland J, Bellgard M, Borisjuk L, Houben A, Doležel J, Ayling S, Lonardi S, Kersey P, Langridge P, Muehlbauer G, Clark M, Caccamo M, Schulman A, Mayer K, Platzer M, Close T, Scholz U, Hansson M, Zhang G, Braumann I, Spannagl M, Li C, Waugh R, Stein N (2017) A chromosome conformation capture ordered sequence of the barley genome. Nature 544(7651):427–433
McDonald GK, Graham RD, Lloyd J, Lewis J, Lonergan P, Khabas-Saberi H (2001) Breeding for improved zinc and manganese efficiency in wheat and barley. In: Proceedings of the 10th Australian Agronomy Conference, Hobart, Tasmania, Australia
Pallotta MA, Graham RD, Langridge P, Sparrow DHB, Barker SJ (2000) RFLP mapping of manganese efficiency in barley. Theor Appl Genet 101(7):1100–1108. https://doi.org/10.1007/s001220051585
Pang J, Cuin T, Shabala L, Zhou M, Mendham N, Shabala S (2007) Effect of secondary metabolites associated with anaerobic soil conditions on ion fluxes and electrophysiology in barley roots. Plant Physiol 145(1):266–276. https://doi.org/10.1104/pp.107.102624
Pedas P, Ytting CK, Fuglsang AT, Jahn TP, Schjoerring JK, Husted S (2008) Manganese efficiency in barley: identification and characterization of the metal ion transporter HvIRT1. Plant Physiol 148(1):455–466. https://doi.org/10.1104/pp.108.118851
Pezeshki SR, DeLaune RD, Anderson PH (1999) Effect of flooding on elemental uptake and biomass allocation in seedlings of three bottomland tree species. J Plant Nutr 22(9):1481–1494. https://doi.org/10.1080/01904169909365729
Pittman JK (2005) Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol 167(3):733–742. https://doi.org/10.1111/j.1469-8137.2005.01453.x
Qiu F, Zheng Y, Zhang Z, Xu S (2007) Mapping of QTL associated with waterlogging tolerance during the seedling stage in maize. Ann Bot-London 99(6):1067–1081. https://doi.org/10.1093/aob/mcm055
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24(5):2155–2167. https://doi.org/10.1105/tpc.112.096925
Satoh-Nagasawa N, Mori M, Skurai K, Takahashi H, Watanabe A, Akagi H (2013) Functional relationship heavy metal P-type ATPases (OsHMA2 and OsHMA3) of rice (Oryza sativa) using RNAi. Plant Biotechnol 30(5):511–515. https://doi.org/10.5511/plantbiotechnology.13.0616a
Shabala S (2011) Physiological and cellular aspects of phytotoxicity tolerance in plants: the role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol 190(2):289–298. https://doi.org/10.1111/j.1469-8137.2010.03575.x
Shabala S, Shabala S, Cuin T, Pang J, Percey W, Chen Z, Conn S, Eing C, Wegner L (2010) Xylem ionic relations and salinity tolerance in barley. Plant J 61:839–853
Sharma T, Dreyer I, Riedelsberger J (2013) The role of K+ channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front Plant Sci 4:224
Smethurst CF, Garnett T, Shabala S (2005) Nutritional and chlorophyll fluorescence responses of lucerne (Medicago sativa) to waterlogging and subsequent recovery. Plant Soil 270(1):31–45. https://doi.org/10.1007/s11104-004-1082-x
Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanishi H (2012) The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ 35(11):1948–1957. https://doi.org/10.1111/j.1365-3040.2012.02527.x
Teakle NL, Amtmann A, Real D, Colmer TD (2010) Lotus tenuis tolerates combined salinity and waterlogging: maintaining O2 transport to roots and expression of an NHX1-like gene contribute to regulation of Na+ transport. Physiol Plant 139(4):358–374. https://doi.org/10.1111/j.1399-3054.2010.01373.x
Van Ooijen JW (2006) JoinMap 4, software for the calculation of genetic linkage maps in experimental populations. Kyazma BV Wageningen, Netherlands
Van Ooijen JW (2009) MapQTL 6, software for the mapping of quantitative trait loci in experimental populations of diploid species. Kyazma BV Wageningen, Netherlands
Voorrips RE (2002) MAPCHART: software for the graphical presentation of linkage maps and QTLs. J Heredity 93(1):77–78. https://doi.org/10.1093/jhered/93.1.77
Wang J, Yang J, Jia Q, Zhu J, Shang Y, Hua W, Zhou M (2014) A new QTL for plant height in barley (Hordeum vulgare L.) showing no negative effects on grain yield. PLoS ONE 9(2):e90144. https://doi.org/10.1371/journal.pone.0090144
Xu R, Wang J, Li C, Johnson P, Lu C, Zhou M (2012) A single locus is responsible for salinity tolerance in a Chinese landrace barley (Hordeum vulgare L.) PLoS One 7(8):e43079. https://doi.org/10.1371/journal.pone.0043079
Zepeda-Jazo I, Velarde-Buendía AM, Enríquez-Figueroa R, Bose J, Shabala S, Muñiz-Murguía J, Pottosin I (2011) Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiol 157(4):2167–2180. https://doi.org/10.1104/pp.111.179671
Zhang X, Tang B, Yu F, Li L, Wang M, Xue Y, Zhang Z, Yan J, Yue B, Zheng Y, Qiu F (2013) Identification of major QTL for waterlogging tolerance using genome-wide association and linkage mapping of maize seedlings. Plant Mol Biol Res 31(3):594–606
Zhou M (2010) Improvement of plant waterlogging tolerance. In: Mancuso S, Shabala S (eds) Waterlogging signalling and tolerance in plants. Springer, Heidelberg, pp 267–285. https://doi.org/10.1007/978-3-642-10305-6_13
Zhou M (2011) Accurate phenotyping reveals better QTL for waterlogging tolerance in barley. Plant Breed 130(2):203–208. https://doi.org/10.1111/j.1439-0523.2010.01792.x
Zhou M, Li H, Mendham N (2007) Combining ability of waterlogging tolerance in barley. Crop Sci 47:278–284. https://doi.org/10.2135/cropsci2006.02.0065
Funding
This work was supported by the Australian Research Council Linkage grant to SS, MZ, and LS (project LP120200516) and the Grains Research and Development Corporation (GRDC) of Australia.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Table S1
(PDF 265 kb)
Rights and permissions
About this article
Cite this article
Huang, X., Fan, Y., Shabala, L. et al. A major QTL controlling the tolerance to manganese toxicity in barley (Hordeum vulgare L.). Mol Breeding 38, 16 (2018). https://doi.org/10.1007/s11032-017-0767-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-017-0767-9