Abstract
Key message
We report the first study on the unique allele from wild barley that can improve waterlogging tolerance in cultivated barley with a substantially higher contribution to aerenchyma formation.
Abstract
Waterlogging is one of the major abiotic stresses that dramatically reduce barley crop yield. Direct selection on waterlogging tolerance in the field is less effective due to its viability to environment. The most effective way of selection is to choose traits that make significant contributions to the overall tolerance and are easy to score. Aerenchyma formation under waterlogging stress is one of the most effective mechanisms to provide adequate oxygen supply and overcome stress-induced hypoxia imposed on plants. In this study, a new allele for aerenchyma formation was identified from a wild barley accession TAM407227 on chromosome 4H. Compared to that identified in cultivated barley, this allele not only produced a greater proportion of aerenchyma but made a greater contribution to the overall waterlogging tolerance. The QTL explained 76.8% of phenotypic variance in aerenchyma formation with a LOD value of 51.4. Markers co-segregating with the trait were identified and can be effectively used in marker assisted selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Waterlogging dramatically reduces the yield of crops and the problem is exacerbated by the need to increase crop production to feed an increasing human population. The yield of barley is dramatically reduced under waterlogging stress (de San Celedonio et al. 2014) and the cost caused by waterlogging is more than 60 billion Euro annually in all the crops (www.dartmouth.edu/~floods/Archives/2005sum.htm). In the USA, 16% of soils are affected by waterlogging and waterlogging is also a major problem in India, Pakistan and China (Boyer 1982).
Waterlogging mainly results from heavy rainfall and poor soil drainage (Voesenek et al. 2014; Zhang et al. 2015a). Gas diffusion under waterlogging stress is ten thousand-fold slower than that in air (Armstrong 1979), resulting in a lack of oxygen in waterlogged plants (Voesenek et al. 2016). Aerenchyma formation is one of the mechanisms to overcome waterlogging stress. Aerenchyma is the gas space in cortical tissues that improves oxygen transportation from shoots to waterlogged roots (Colmer 2003). In many wetland species, aerenchyma is well developed even in drained conditions and can be further enhanced under waterlogging stress (Evans 2004). In barley, waterlogging tolerant genotypes are able to form inducible lysigenous aerenchyma under waterlogged conditions (Zhang et al. 2015b). Aerenchyma formation is also the most effective mechanism for waterlogging tolerance in barley.
The development of waterlogging tolerant varieties is an effective and economical approach to improve crop production under waterlogging conditions. However, the progress of developing waterlogging tolerant barley varieties is slow due to the complexity of waterlogging conditions resulting from different water depth, soil type, duration of waterlogging, nutrient ions and temperature (Setter and Waters 2003; Setter et al. 2009; Zhang et al. 2015b). Waterlogging tolerance is also a complex trait, controlled by many genes including some with small effects (Zhou 2010). Molecular markers have provided plant breeders with a method to improve selection accuracy and accelerate breeding programs (Ribaut and Hoisington 1998).
Many QTL for waterlogging tolerance in barley have been detected (Li et al. 2008; Zhang et al. 2016b; Zhou et al. 2012); however, accurate phenotyping remains the main challenge for improving waterlogging tolerance in breeding. Different traits were used in different studies, such as leaf scoring system, aerenchyma formation and other agronomic traits (Zhang et al. 2016a). The leaf scoring system and aerenchyma formation under waterlogging conditions have been shown to be the most reliable method for screening waterlogging tolerance in barley (Zhang et al. 2016b; Zhou 2011).
Wild relatives of cultivated crop species are often used as the donor parents in breeding because of their tolerance to biotic and abiotic stresses. Wild relatives of maize are able to form constitutive aerenchyma under aerated conditions (Mano and Omori 2013). This favourable trait for waterlogging tolerance has been successfully used to improve waterlogging tolerance of maize (Mano and Omori 2013, 2015). Higher root porosity and lower radial oxygen loss from a wild relative Hordeum marinum were successfully transferred into cultivated wheat to improve waterlogging tolerance (Malik et al. 2011). The wild barley TAM407227 showed significantly higher potential for enhancing waterlogging tolerance in barley (Zhang et al. 2015b). Compared with waterlogging tolerant cultivated barley, TAM407227 performed much better with regard to the tolerance to waterlogging with a greater proportion of aerenchyma formation under waterlogging conditions.
In this study, a new linkage map between cultivated barley Franklin and wild barley TAM407227 was constructed. A number of different QTL for different traits under waterlogging and control conditions were detected from this population. Importantly, a new major allele showed much greater effect on aerenchyma formation and waterlogging tolerance and is an ideal candidate gene for use in barley breeding programs.
Materials and methods
Evaluation of waterlogging tolerant traits
A total of 163 double haploid (DH) lines from the cross between Franklin and wild barley accession TAM407227 (H. spontaneum) were used in this study. TAM407227 was introduced from Australian Grains Genebank and showed better waterlogging tolerance and aerenchyma formation than the cultivated tolerant barley Yerong (Fig. 1) (Zhang et al. 2015b). Franklin is a malting barley but susceptible to waterlogging stress. DH lines and parent varieties were grown in a field screening facility (Figure S1). Twenty seeds of each line/variety were sown in a 60-cm row with 15 cm between rows. The controls were sown in well-drained beds. Three replicates were applied for both waterlogging treatment and controls. The trial was sown in late April and waterlogging treatment began at the three-leaf stage. Waterlogging tolerance was scored based on plant survival and leaf senescence (0 = plants died from waterlogging, 10 = not affected by waterlogging) (Zhou 2011). At maturity, different traits were measured for each DH line and parent variety under both control and waterlogging conditions. The traits include plant height under control (CPH) and waterlogging (WPH), the number of tillers under control (CT) and waterlogging (WT), and grain yield under control (CY) and waterlogging (WY) conditions. Relative changes (waterlogging/control) in differences are also used as waterlogging tolerance indicators.
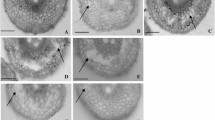
Light micrographs of cross section of adventitious roots demonstrating aerenchyma formation (arrows) after 7 days of waterlogging treatment in different DH lines: TAMF42 (a), TAMF56 (b), and TAMF135 (c). Wild barley TAM407227 (d) had larger proportion of aerenchyma than Franklin (e) and Yerong (f). Bar 100 µm. The scores for aerenchyma formation are a = 0, b = 1 c = 4, d = 4 e = 0, f = 3
Aerenchyma formation
Aerenchyma formation of DH lines and parents were detected based on the method by Zhang et al. (2016b). Plants were grown in 50-L bins, filled with pine bark/loam-based potting mixture with premixed slow release fertiliser. At the three-leaf stage, adventitious roots were sampled in each DH line after 7 days of waterlogging. Approximately 2 cm long root segments were taken from the mature zone and about 6 cm from the root apex. Cross sections were cut by free-hand using razor blades and examined under a bright field light microscope (Olympus BX41). The proportion of aerenchyma was visually scored based on digital images from Olympus DP20: 0 = no aerenchyma, 4 = well-formed aerenchyma (Fig. 1) (Zhang et al. 2016b). The experiment was repeated three times in the glasshouse in January–March, 2016.
Genetic map construction
Diversity Arrays Technology (DArT) and SNP markers were developed and conducted by Triticarte Pty. Ltd. A total of around 15,000 DArT markers and 14,500 SNP markers were shown to be polymorphic between Franklin and TAM407227. JoinMap 4.0 was used in this study to construct the linkage map (Van Ooijen and Kyazma 2009). Before map construction, markers with more than 10% missing data and duplicate markers (markers located at same/similar positions) were deleted. The relatively lower density map was used to conduct the preliminary QTL analysis. Further high-density mapping was conducted in the region on 4H where the major QTL was located.
QTL analysis
The software package MapQTL 6.0 was used to identify different QTL (Van Ooijen and Kyazma 2009). After interval mapping (IM), the closest marker at each QTL was selected as a cofactor in the multiple QTL model (MQM). A logarithm of the odds (LOD) threshold value of 3.0 was used to detect the presence of a QTL. To determine the effects of waterlogging tolerance on other traits (WPH, WCPH, WT, WCT, WY and WCY), different QTL were re-analysed by using various traits as covariates. The percentage of variance explained by each QTL (R 2) was obtained with restricted MQM mapping. The linkage maps showing the QTL positions were made with MAPCHART (Voorrips 2002). The sequence of flanking SNP markers were used to check the position of QTL on the barley physical map (http://barleygenomeapplications.com/default_2.aspx) and the barley Morex reference genome.
Results
Waterlogging tolerance related traits of DH lines
Franklin and TAM407227 showed significant difference (P < 0.01) in plant height, tiller number and grain yield under both waterlogging and control conditions (Table 1) with TAM407227 showing significantly higher aerenchyma formation as well as waterlogging tolerance based on plant survival (P < 0.01) (Fig. 1). Compared with Franklin, TAM407227 had significantly higher plant height, more tillers, but lower yield under control conditions (P < 0.01). However, in terms of relative change of traits, TAM407227 was less affected by waterlogging stress. In DH populations, waterlogging stress reduced plant height, tiller number and yield significantly (P < 0.01). DH lines showed a wide segregation in waterlogging tolerance (Table S1). Figure 2 shows frequency distributions of waterlogging tolerance based on plant survival and aerenchyma formation in all DH lines. As shown in Table 2, waterlogging tolerance showed very high positive correlations with both grain yield (r = 0.70, P < 0.01) and aerenchyma formation (r = 0.63, P < 0.01) under waterlogging stress. Waterlogging tolerance was also significantly correlated with both plant height and tiller number under waterlogging stress as well as with relative plant height, relative tiller numbers and relative grain yield (Table 2).
Figure 3 shows the correlation between waterlogging tolerance and aerenchyma formation under waterlogging stress. Aerenchyma formation was clearly grouped into two clusters, one with the scores of more than 2 and the other with the scores of less than 2. In general, nearly all the lines with high scores of aerenchyma formation showed good waterlogging tolerance. However, a few lines with low scores of aerenchyma also showed good waterlogging tolerance, indicating the possible existence of some other tolerance mechanisms.
QTL for waterlogging tolerance
A total of 19 QTL were identified for different traits under control and waterlogging conditions (Table 3; Fig. 4). Three QTL for waterlogging tolerance were based on plant survival. One major QTL was on chromosome 4H at 98.8 cM, with a LOD value of 19.2, explaining 34.6% of the phenotypic variance. Other two minor QTL for waterlogging tolerance were found on chromosomes 6H and 7H, determining 6.3 and 5.3% of the phenotypic variance, respectively. Only one major QTL for aerenchyma formation under waterlogging conditions was identified on chromosome 4H, explaining 76.8% of the phenotypic variation with a LOD value of 51.4. This QTL was located at the same position of a QTL for waterlogging tolerance. A high-density map on chromosome 4H was further constructed (Fig. 5) and the QTL was mapped to the region between 97.5 and 99.10 cM on the published consensus map (http://barleygenomeapplications.com) with around 20 markers co-segregating with the traits, which can be used in breeding programs and help to identify candidate genes.
Six other QTL for WPH, WCPH, WY, WCY, WT and WCT were also identified on chromosome 4H at the same position as those for waterlogging tolerance and aerenchyma formation. Another cluster of QTL was found on chromosome 3H at 110 cM based on the barley physical map, controlling CPH, WPH, WCPH, CY and WY (Fig. 4).
The contribution of aerenchyma formation to overall waterlogging tolerance
Figure 4 shows that the QTL for aerenchyma formation under waterlogging stress is located on a similar position to the major QTL for waterlogging tolerance, WPH, WCPH, WT, WCT, WY and WCY. To confirm their relationships between aerenchyma formation and other waterlogging tolerance-related traits, the scores for aerenchyma formation was used as a covariate while analysing QTL for other traits. Aerenchyma formation under waterlogging made a significant contribution to waterlogging tolerance, as the major QTL on 4H for traits that are used as the indices for waterlogging tolerance became insignificant (Table 4; Fig. 6). In contrast, the QTL for these traits on other chromosomes were not affected by using aerenchyma formation as a covariate. Waterlogging scores based on plant healthiness (combined leaf chlorosis and plant survival) also showed to be a good indicator of grain yield under waterlogging condition. When using the tolerance scores as covariates, QTL for grain yield, plant height and tiller numbers under waterlogging on 4H all became insignificant (Table 4). Similarly, waterlogging scores contributed less to QTL on other chromosomes for these traits.
Discussion
A new allele for aerenchyma formation from wild barley can be more effective in breeding for waterlogging tolerance in barley
A total of three QTL for waterlogging tolerance were identified from this population with all three tolerance alleles being from wild barley. The major allele controlling waterlogging tolerance from wild barley was located on chromosome 4H at 98.8 cM on the barley physical map. This QTL was located on the same position as the QTL identified from several other populations (Zhou 2011; Zhou et al. 2012). This QTL identified from the current population explained much better phenotypic variation (34.6%) than those from other populations, including Yerong/Franklin 23.9% (Zhou 2011) and YYXT/Franklin 7.0% (Zhou et al. 2012). The minor QTL on 6H was not identified in previous reports. The minor QTL on 7H is at a similar position to that identified for leave chlorosis in the Yerong/Franklin and TX9425/Franklin populations (Li et al. 2008). However, this QTL from the Yerong/Franklin population became nonsignificant after further long-term waterlogging treatment (Zhou 2011). A QTL for waterlogging tolerance was reported at 125 cM on 7H from a Chinese landrace (Xu et al. 2012), which is far away from the QTL identified in this study (71 cM on chromosome 7H).
A high-density map of chromosome 4H provided enough markers for further marker assisted selection to improve waterlogging tolerance in barley. The location of QTL on chromosome 4H is the main region controlling waterlogging tolerance in barley and 58 candidate genes have been identified (http://barleygenomeapplications.com/default_2.aspx). Among all the identified 58 candidate genes, the NAC domain transcription factor and glutathione-S-transferase genes were also candidate genes identified for lysigenous aerenchyma formation in maize (Rajhi et al. 2011; Zhang et al. 2016a). The sequence of nearest marker (3255355S4) for aerenchyma formation on chromosome 4H was used to search for barley genome sequences using the Morex reference genome. The marker 3255355S4 was blasted to Morex_contig_1572026. The Morex_contig_1572026 sequence was then searched on NCBI blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Results suggested the gene MIR171_1 (Genbank ID: JX195502.1) is a possible candidate gene for aerenchyma formation and waterlogging tolerance in barley. MIR171 was involved in the regulation of metabolic adaptations to the waterlogging conditions in maize (Zhang et al. 2008). Further experiments will be required to confirm the genes for aerenchyma formation and waterlogging tolerance in barley.
The region on chromosome 4H controlling aerenchyma formation identified from the population of Franklin/TAM407227 is at the same position as that from both Yerong/Franklin and YYXT/Franklin populations (Zhou 2011; Zhou et al. 2012). The allele originating from wild barley TAM407227 not only exhibited a higher percentage of phenotypic variation [76.8% in TAM407227 vs 44% in Yerong (Zhang et al. 2016b) and 39% in YYXT (Broughton et al. 2015)], but also made a much greater contribution to waterlogging tolerance than the allele from cultivated barley varieties. Of the total percentage of phenotypic variation determined by three significant QTL (46.2%, Table 3), the allele on 4H contributed 34.6% (75% of total contribution). In contrast, the allele from Yerong contributed 23.9% to the overall waterlogging tolerance and 42% of all the contributions by four QTL (Zhou 2011). The allele from YYXT contributed only 5.2% to the overall waterlogging tolerance, which is only 11% of all the contributions by four QTL (Zhou et al. 2012). Together this further confirms that aerenchyma formation is one of the most effective mechanisms for waterlogging tolerance (Armstrong 1979). However, some of the waterlogging tolerant DH lines did not form large amounts of aerenchyma, indicating the possible existence of other mechanisms involved in waterlogging tolerance, such as development of adventitious roots (Mano et al. 2005), formation of the barrier to radial oxygen loss (Colmer and Voesenek 2009), or increased tolerance to elemental or metabolite toxicity (Shabala et al. 2014). These mechanisms play more important roles in waterlogging tolerance in cultivated barley, while in wild barley TAM407227 the allele controlling aerenchyma formation was shown to be most effective in improving waterlogging tolerance and thus can be effectively used in future breeding programs. Similar results have been reported in other crops. Wild relatives of maize are able to form aerenchyma without waterlogging stress (Mano et al. 2006). Wild relatives of wheat showed higher root porosity and lower radial oxygen loss under waterlogging conditions (Malik et al. 2009). These favourable traits of waterlogging tolerance in wild relatives of maize and wheat have been successfully transferred to cultivated maize and wheat (Malik et al. 2011; Mano and Omori 2013).
Selecting for waterlogging tolerance
Higher yield under waterlogging stress is always an important target in plant breeding in high rainfall areas. However, the heritability of yield under waterlogging conditions is relatively low (Collaku and Harrison 2005) and, therefore, difficult to be directly used in breeding programs. The genes contributing to high yield under abiotic stresses might be the same as those controlling higher yield under well-drained conditions with nothing to do with stress tolerance (Jones 2007). Thus, relative changes in different paremeters (stressed/control) are always used as indicators for stress tolerance. QTL for yield and other useful agronomic traits (plant height and tiller number) under control and waterlogging conditions were also identified. Two QTL for WPH and WCPH were identified at the same positions on chromosomes 3H and 4H. A QTL for CPH was identified at the same position of a QTL for WPH and WCPH on chromosome 3H. When aerenchyma formation and waterlogging tolerance were used as covariates, QTL for WCPH became insignificant. However, the QTL for WPH on chromosome 3H was not affected by aerenchyma formation and waterlogging tolerance. This suggested the importance of using relative changes in different paremeters (stressed/control) as indicators for waterlogging tolerance.
In this work the correlation coefficient between waterlogging tolerance and WY was the highest among all the traits (r = 0.7, P < 0.01) and the QTL for WY and WCY on chromosome 4H is the same position of QTL for waterlogging tolerance based on plant survival. The QTL for WY and WCY on chromosome 4H could not be detected when aerenchyma formation and waterlogging tolerance were used as covariates. This further confirmed the effectiveness of using aerenchyma formation and waterlogging tolerance as the selection criteria to improve the yield under waterlogging conditions. The QTL for waterlogging tolerance on chromosome 7H is in the same position as the QTL for yield under hypoxia in barley (Bertholdsson et al. 2015; Zhang et al. 2016a).
Plant architecture traits, such as plant height and tillers, are reported to be possible target traits to improve yield (Khush 2001). A QTL on chromosome 3H at around 110 cM was identified for CPH, WPH, WCPH, CY and WY. This region also controls drought tolerant QTL, such as plant height and peduncle length under drought stress (Korff et al. 2008; Zhang et al. 2016a). QTL analysis for CY using CPH as a covariate suggested that plant height did not have a significant effect on yield. This position on chromosome 3H provided a useful resource for breeders to improve yield of barley.
In conclusion, a new allele for aerenchyma formation under waterlogging stress was identified from a wild barley accession. This allele showed a much better ability in forming aerenchyma and was the major contributor to waterlogging tolerance. A high-density linkage map helped identify several co-segregating markers that can be directly used in breeding programs.
Author contribution statement
XZ conducted the experiment and wrote the paper; YF, helped genotyping; SS, AK, LS contributed to writing; PJ, HH helped phenotyping; MZ designed the experiments and wrote the paper.
References
Armstrong W (1979) Aeration in higher plants, vol 7. Advances in Botanical Research, London
Bertholdsson NO, Holefors A, Macaulay M, Crespo-Herrera LA (2015) QTL for chlorophyll fluorescence of barley plants grown at low oxygen concentration in hydroponics to simulate waterlogging. Euphytica 201:357–365. doi:10.1007/s10681-014-1215-0
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Broughton S, Zhou G, Teakle N, Matsuda R, Zhou M, O’Leary R, Colmer T, Li C (2015) Waterlogging tolerance is associated with root porosity in barley (Hordeum vulgare L.). Mol Breed 35:1–15. doi:10.1007/s11032-015-0243-3
Collaku A, Harrison SA (2005) Heritability of waterlogging tolerance in wheat. Crop Sci 45:722–727
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
de San Celedonio RP, Abeledo LG, Miralles D (2014) Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil 378:265–277. doi:10.1007/s11104-014-2028-6
Evans DE (2004) Aerenchyma formation. New Phytol 161:35–49
Jones HG (2007) Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance. J Exp Bot 58:119–130. doi:10.1093/jxb/erl118
Khush GS (2001) Green revolution: the way forward. Nat Rev Genet 2:815–822
Korff M, Grando S, Del Greco A, This D, Baum M, Ceccarelli S (2008) Quantitative trait loci associated with adaptation to Mediterranean dryland conditions in barley. Theor Appl Genet 117:653–669. doi:10.1007/s00122-008-0787-2
Li H, Vaillancourt R, Mendham N, Zhou M (2008) Comparative mapping of quantitative trait loci associated with waterlogging tolerance in barley (Hordeum vulgare L.). BMC Genom 9:401. doi:10.1186/1471-2164-9-401
Malik AI, English JP, Colmer TD (2009) Tolerance of Hordeum marinum accessions to O2 deficiency, salinity and these stresses combined. Ann Bot 103:237–248. doi:10.1093/aob/mcn142
Malik AI, Islam AKMR, Colmer TD (2011) Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): evaluation of four H. marinum–wheat amphiploids. New Phytol 190:499–508. doi:10.1111/j.1469-8137.2010.03519.x
Mano Y, Omori F (2013) Flooding tolerance in interspecific introgression lines containing chromosome segments from teosinte (Zea nicaraguensis) in maize (Zea mays subsp. mays). Ann Bot 112:1125–1139
Mano Y, Omori F (2015) Flooding tolerance in maize (Zea mays subsp. mays) F1 hybrids containing a QTL introgressed from teosinte (Zea nicaraguensis). Euphytica 205:255–267. doi:10.1007/s10681-015-1449-5
Mano Y, Muraki M, Fujimori M, Takamizo T, Kindiger B (2005) Identification of QTL controlling adventitious root formation during flooding conditions in teosinte (Zea mays ssp. huehuetenangensis) seedlings. Euphytica 142:33–42. doi:10.1007/s10681-005-0449-2
Mano Y, Omori F, Takamizo T, Kindiger B, Bird RM, Loaisiga CH (2006) Variation for root aerenchyma formation in flooded and non-flooded maize and teosinte seedlings. Plant Soil 281:269–279. doi:10.1007/s11104-005-4268-y
Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, Nakazono M (2011) Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol 190:351–368. doi:10.1111/j.1469-8137.2010.03535.x
Ribaut JM, Hoisington D (1998) Marker-assisted selection: new tools and strategies. Trends Plant Sci 3:236–239. doi:10.1016/S1360-1385(98)01240-0
Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253:1–34
Setter TL, Waters I, Sharma SK, Singh KN, Kulshreshtha N, Yaduvanshi NP, Ram PC, Singh BN, Rane J, McDonald G, Khabaz-Saberi H, Biddulph TB, Wilson R, Barclay I, McLean R, Cakir M (2009) Review of wheat improvement for waterlogging tolerance in Australia and India: the importance of anaerobiosis and element toxicities associated with different soils. Ann Bot 103:221–235. doi:10.1093/aob/mcn137
Shabala S, Shabala L, Barcelo J, Poschenrieder C (2014) Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ 37:2216–2233. doi:10.1111/pce.12339
Van Ooijen JW, Kyazma BV (2009) MapQTL 6. Software for the mapping of quantitative trait loci in experimental populations of diploid species. Kyazma BV, Wageningen
Voesenek LACJ, van Veen H, Sasidharan R (2014) Learning from nature: the use of non-model species to identify novel acclimations to flooding stress. AoB Plants. doi:10.1093/aobpla/plu016
Voesenek LACJ, Sasidharan R, Visser EJW, Bailey-Serres J (2016) Flooding stress signaling through perturbations in oxygen, ethylene, nitric oxide and light. New Phytol 209:39–43. doi:10.1111/nph.13775
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78. doi:10.1093/jhered/93.1.77
Xu R, Wang J, Li C, Johnson P, Lu C, Zhou M (2012) A single locus is responsible for salinity tolerance in a Chinese landrace barley (Hordeum vulgare L.). PLoS One 7:e43079. doi:10.1371/journal.pone.0043079
Zhang Z, Wei L, Zou X, Tao Y, Liu Z, Zheng Y (2008) Submergence-responsive MicroRNAs are potentially involved in the regulation of morphological and metabolic adaptations in maize root cells. Ann Bot 102:509–519. doi:10.1093/aob/mcn129
Zhang X, Huang X, Zhou M, Shabala L, Koutoulis A, Shabala S (2015a) Plant breeding for flood tolerance: advances and limitations. In: Dhankher OP, Jaiwal PK, Singh RP (eds) Genetic manipulation in plants for mitigation of climate change. Springer, Berlin, pp 43–72
Zhang X, Shabala S, Koutoulis A, Shabala L, Johnson P, Hayes D, Nichols D, Zhou M (2015b) Waterlogging tolerance in barley is associated with faster aerenchyma formation in adventitious roots. Plant Soil 394:355–372. doi:10.1007/s11104-015-2536-z
Zhang X, Shabala S, Koutoulis A, Shabala L, Zhou M (2016a) Meta-analysis of major QTL for abiotic stress tolerance in barley and implications for barley breeding. Planta. doi:10.1007/s00425-016-2605-4
Zhang X, Zhou G, Shabala S, Koutoulis A, Shabala L, Johnson P, Li C, Zhou M (2016b) Identification of aerenchyma formation-related QTL in barley that can be effective in breeding for waterlogging tolerance. Theor Appl Genet 129:1167–1177. doi:10.1007/s00122-016-2693-3
Zhou M (2010) Improvement of plant waterlogging tolerance. In: Mancuso S, Shabala S (eds) Waterlogging signalling and tolerance in plants. Springer, Heidelberg, pp 267–285. doi:10.1007/978-3-642-10305-6_13
Zhou M (2011) Accurate phenotyping reveals better QTL for waterlogging tolerance in barley. Plant Breed 130:203–208. doi:10.1111/j.1439-0523.2010.01792.x
Zhou M, Johnson P, Zhou G, Li C, Lance R (2012) Quantitative trait loci for waterlogging tolerance in a barley cross of Franklin× YuYaoXiangTian Erleng and the relationship between waterlogging and salinity tolerance. Crop Sci 52:2082–2088. doi:10.2135/cropsci2012.01.0008
Acknowledgements
This work was supported by the Australian Research Council Linkage grant (LP120200516) and Grains Research & Development Corporation (GRDC) of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Communicated by Gary Muehlbauer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2017_2910_MOESM1_ESM.pptx
Supplementary Figure 1: The system of screening waterlogging tolerance in the field. A: Field preparation; B: 4 weeks after waterlogging; C: Tam407227 (left) and Franklin (right) (PPTX 975 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Fan, Y., Shabala, S. et al. A new major-effect QTL for waterlogging tolerance in wild barley (H. spontaneum). Theor Appl Genet 130, 1559–1568 (2017). https://doi.org/10.1007/s00122-017-2910-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-017-2910-8