Abstract
Spring wheat (Triticum aestivum L.) breeding goals in western Canada include good agronomic characteristics and good end-use quality, and also moderate to elevated resistance to diseases of economic importance. In this study, we aimed to identify quantitative trait loci (QTL) associated with resistance to common bunt (Tilletia tritici and Tilletia laevis), tan spot (Pyrenophora tritici-repentis), leaf rust (Puccinia triticina), and stripe rust (Puccinia striiformis f. sp. tritici). A total of 167 recombinant inbred lines (RILs) derived from a cross between two spring wheat cultivars, ‘Attila’ and ‘CDC Go’, were evaluated for reactions to the four diseases in nurseries from three to eight environments, and genotyped with the Wheat 90K SNP array and three gene-specific markers (Ppd-D1, Vrn-A1, and Rht-B1). The RILs exhibited transgressive segregation for all four diseases, and we observed several lines either superior or inferior to the parents. Broad-sense heritability varied from 0.25 for leaf rust to 0.48 for common bunt. Using a subset of 1203 informative markers (1200 SNPs and 3 gene-specific markers) and average disease scores across all environments, we identified two QTLs (QCbt.dms-1B.2 and QCbt.dms-3A) for common bunt, and three QTLs each for tan spot (QTs.dms-2B, QTs.dms-2D, and QTs.dms-6B), leaf rust (QLr.dms-2D.1, QLr.dms-2D.2, and QLr.dms-3A), and stripe rust (QYr.dms-3A, QYr.dms-4A, and QYr.dms-5B). Each QTL individually explained between 5.9 and 18.7% of the phenotypic variation, and altogether explained from 21.5 to 26.5% of phenotypic and from 52.2 to 86.0% of the genetic variation. The resistance alleles for all QTLs except one for stripe rust (QYr.dms-5B) were from CDC Go. Some of the QTLs are novel, while others mapped close to QTLs and/or genes reported in other studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 85% of spring wheat in Canada is produced in the western prairie provinces of Manitoba, Saskatchewan, and Alberta (McCallum and DePauw 2008). The primary target traits of spring wheat breeders in western Canada is developing early maturing and short stature cultivars with high-yield potential and grain protein content, combined with other end-use qualities, and resistance to diseases. Currently, cultivars to be released in the region must be at least intermediately resistant to stem rust (Puccinia graminis f. sp. tritici), leaf rust (Puccinia triticina), yellow (stripe) rust (Puccinia striiformis f. sp. tritici), common bunt (caused by two very closely related fungi, Tilletia tritici and Tilletia laevis), and Fusarium head blight (caused mainly by Fusarium graminearum) (http://www.pgdc.ca). Breeding for resistance to diseases involves (i) identification of sources of resistance, (ii) introgressing the new sources of resistance from the resistant parents into the genetic background of other parents, and (iii) selecting progenies showing acceptable combinations of resistance to diseases and other agronomic and quality traits. Breeding for disease resistance is often challenging for at least two reasons. First, breeders often need to pyramid diverse sources of resistance to multiple diseases in to the same genetic background. Second, the inheritance of each disease is often both qualitative and quantitative (Faris et al. 1996, 1997; Faris and Friesen 2005; Singh et al. 2007; Chu et al. 2008, 2010; Singh et al. 2016b), which complicates the selection process. Qualitative resistance is controlled by a single gene with a major effect, but resistance regulated by single genes often loses their effectiveness over time due to changes in pathogen populations. Quantitative resistance is controlled by multiple minor effect genes or quantitative trait loci (QTLs) with small additive effects, which are more durable (Singh et al. 2008), but require the introgression of multiple genes or QTLs that confer resistance for a given disease.
Currently, over 73 leaf rust (Lr) and 65 stripe rust (Yr) resistance genes have been reported in the literature on almost every chromosome (McIntosh et al. 2012; Dakouri et al. 2013; Park et al. 2014). Several gene combinations have been reported to contribute some level of resistance to rusts in many of the cultivars grown in western Canada (McCallum et al. 2007, 2012; Randhawa et al. 2012). However, only few gene combinations (pyramids) have provided good levels of durable resistance in global spring wheat cultivars released since the mid-1980s. Such combinations include the slow-rusting Lr34/Yr18 gene on chromosome 7DS (Suenaga et al. 2003; Spielmeyer et al. 2005; Lagudah et al. 2009), Lr46/Yr29 on 1BL (Singh et al. 1998; William et al. 2003), Sr2/Yr30 on 3BS (Singh et al. 2005), Yr17/Lr37/Sr38 cluster on 2AS (Helguera et al. 2003; Milus et al. 2015), and Lr67/Yr46 on 4DL (Hiebert et al. 2010; Herrera-Foessel et al. 2011).
At least 15 monogenic and race-specific genes (named from Bt1 to Bt15) conferring resistance to common bunt (Goates 1996) (also known as stinking smut and covered smut (Gaudet and Puchalski 1989)), have also been reported in wheat. Both incidence and severity of common bunt have been controlled largely by introgressing Bt10 and Bt8 (Menzies et al. 2006; McCallum and DePauw 2008; Hiebert et al. 2011), but the vulnerability of such major genes through intense selection pressure on the pathogen is a concern (Wang et al. 2009). QTLs associated with resistance to common bunt have also been reported on some chromosomes, including 1B and 7A (Galaev et al. 2006; Fofana et al. 2008; Wang et al. 2009). Tan spot, caused by Pyrenophora tritici-repentis, is the most destructive leaf spotting disease of wheat in Canada and other major wheat-growing countries (Faris et al. 1997; Friesen and Faris 2004). The virulence of P. tritici-repentis (Ptr) depends on the production of Ptr ToxA, Ptr ToxB, and/or Ptr ToxC host-specific toxins by the different races of the fungus (Ciuffetti et al. 1998, 2010; Lamari et al. 2003). Fungal isolates of races 2, 3, and 5 produce Ptr ToxA, Ptr ToxC, and Ptr ToxB, respectively (Strelkov and Lamari 2003; Lamari and Strelkov 2010). Isolates of races 1, 6, and 7 each produce two toxins, with race 1 producing Ptr ToxA and Ptr ToxC, race 6 producing Ptr ToxB and Ptr ToxC, and race 7 producing Ptr ToxA and Ptr ToxB. Race 8 isolates produce all the three toxins (Faris et al. 2013). Fungal isolates producing both Ptr ToxA and Ptr ToxC are highly abundant in the Canadian prairies, while those producing Ptr ToxB are extremely rare in this region (Lamari et al. 1998, 2003; Aboukhaddour et al. 2013).
We have been evaluating the performance of wheat cultivars and mapping populations at the University of Alberta, Edmonton, Canada for a wide range of agronomic traits and diseases. One of the mapping populations was a recombinant inbred line (RIL) population derived from a cross between two spring wheat cultivars, ‘Attila’ and ‘CDC Go’. ‘Attila’ is an awned, medium-yielding, semi-dwarf, and early-maturing cultivar (in South Asiatic regions) developed by the International Maize and Wheat Improvement Center from CM85836-50Y-0M-0Y-3M-0Y (Tadesse et al. 2010). It has been released in several countries with different local names and grown on millions of hectares throughout the world. ‘Attila’ carries at least two additive genes for slow-rusting resistance to leaf rust and three for stripe rust, such as Yr27 (Rosewarne et al. 2008) and Lr46/Yr29 (Rosewarne et al. 2006; Datta et al. 2009), showed moderate to high levels of field resistance to both leaf and stripe rusts, and has been frequently used as a slow-rusting donor parent in international spring wheat breeding programs (Rosewarne et al. 2008). ‘CDC Go’ is a Canadian Western Red Spring wheat cultivar characterized by strong straw, medium height, relatively late maturity (in western Canada), high yield, and high test weight and thousand kernel weight (Asif et al. 2015); it is also resistant to common bunt, and moderately resistant to resistant to both leaf and stripe rust (Randhawa et al. 2012; Perez-Lara et al. 2017). ‘CDC Go’, however, does not have the Lr34/Yr18 resistance allele (Randhawa et al. 2012). We previously used the ‘Attila’ × ‘CDC Go’ RIL population to map QTLs associated with agronomic traits both under conventional and organic management systems (Asif et al. 2015; Zou et al. 2017a, b). This RIL population has also shown good segregation for different wheat diseases, but information on the genetics of disease resistance in this population has not been previously reported. Here, we present QTLs associated with common bunt, tan spot, leaf rust, and stripe rust resistance in the ‘Attila’ × ‘CDC Go’ RIL population using the Wheat 90K SNP array.
Materials and methods
Phenotyping
The present study was conducted on a biparental mapping population of 167 RILs developed from a cross between two spring wheat cultivars—‘Attila’ (CM85836-50Y-0M-0Y-3M-0Y) and ‘CDC Go’. The RIL population was developed as described in one of our previous papers (Asif et al. 2015). The RIL population and the two parents, along with two susceptible (‘AC Barrie’ and ‘AC Crystal’) and two resistant (‘Lillian’ and ‘Carberry’) checks were evaluated eight times for their reaction to stripe rust in disease screening nurseries at Creston, British Columbia (49° 6′ 28.32″ N, 116° 34′ 4.67″ W) in 2011, 2013, and 2014; at Lethbridge Research Centre, Alberta (49.6989° N, 112.7636° W) from 2012 to 2015, and at Ellerslie Research Station, Edmonton, Alberta (53° 25′ 35.30″ N, 113° 32′ 59.03″ W) in 2015.
The RIL population, the two parents, and checks also were evaluated for 3 years (2012–2014) for reaction to leaf rust, common bunt, and tan spot at the Crop Research Facility of the University of Alberta, South Campus (53° 19′ N, 113° 35′ W), Alberta, Canada. The following cultivars were used as susceptible/moderately susceptible and moderately resistant/resistant checks: (i) leaf rust nurseries: ‘AC Barrie’ and ‘Park’ as moderately susceptible to susceptible checks, and ‘Peace’ and ‘Carberry’ as moderately resistant to resistant checks; (ii) tan spot nurseries: ‘AC Barrie’, ‘Unity’, and ‘Glenlea’ as moderately susceptible checks, and ‘Neepawa’ as moderately resistant check; and (iii) for common bunt nurseries: ‘Glenlea’ and ‘Neepawa’ as moderately susceptible checks; ‘AC Barrie’ and ‘Unity’ as moderately resistant and resistant checks, respectively. In Creston and Lethbridge, plots consisted of a single 1-m long row per genotype, arranged in a randomized complete block (RCB) design with two to three replicates per trial, depending on seed and space availability. In Edmonton, we grew hill-plots of ten seeds per genotype spaced 25 cm apart, with a similar RCB design. Details on disease evaluation methodology have been described in our recent paper (Perez-Lara et al. 2017). Visual disease assessments (disease scores) for leaf rust and stripe rust were done on a scale of 1 (no visible sign or symptom = resistant) to 9 (leaf area totally covered with spores = highly susceptible) on a plot basis when the spreader rows reached maximum infection. Tan spot reaction was recorded on a scale of 1 to 9 at milk-stage as described above. For common bunt, all heads of each genotype in a hill plot were examined for infection at the dough stage and recorded in percentages as the ratio of the number of infected heads to the total number of head per hill plot.
Genotyping and linkage maps
DNA extraction, genotyping, and linkage analyses were done as described in our previous study (Perez-Lara et al. 2016), and the linkage maps are summarized by Zou et al. (2017a). Briefly, a total of 5665 of the 81,587 SNPs and 3 functional markers initially used for genotyping the RIL population were incorporated into 27 linkage groups that covered all chromosomes except chromosomes 3D and 4D (Supplemental Table 1); none of the SNPs were polymorphic on chromosomes 3D and 4D. Markers that cosegregated in the linkage maps were excluded from the final dataset, which reduced the final number of markers retained for QTL analyses to 1200 SNPs and 3 functional markers (Ppd-D1, Vrn-A1a, and Rht-B1). The total map length for the 19 chromosomes, excluding 3D and 4D, was 3442 cM, with each chromosome varying between 14 cM on 1D and 325 cM on 5B (Zou et al. 2017a).
Statistical analyses
In each environment (year), outlier disease scores were checked on row basis using box plot and frequency distribution histograms in MiniTab v14, and some extreme values were deleted prior to any statistical analyses. Least square means and F statistics were computed for combined disease scores across all environments using PROC MIXED, whereas plot-based broad-sense heritability was computed using PROC ILM in SAS version 9.3 (SAS Institute Inc. Cary, USA). Genotypes (entries) were considered fixed, while environments, replications, replication within environment, and entry × environment interactions were considered random effects. Both test for normality and the frequency distribution were computed using MiniTab v14. Inclusive composite interval mapping (ICIM) was performed on disease scores averaged across all environments with QTL IciMapping v4.1 (Li et al. 2007; Meng et al. 2015) using a mean replacement for missing data, 1 cM walking distance, a minimum logarithm of odds (LOD) score of 2.5, and an additive model to determine the effect of individual QTL. QTL names were designated following the International Rules of Genetic Nomenclature (http://wheat.pw.usda.gov/ggpages/wgc/98/Intro.htm), which consisted of three letters for trait acronym, lab designation (dms = Dean Michael Spaner), and chromosome. Genetic maps and QTL graphs were drawn using MapChart v2.1 (Voorrips 2002).
Results
Disease evaluation
Tan spot, leaf rust, and stripe rust mean disease scores among the checks varied from 1.1 to 3.8 (in the 1 to 9 scale) for the resistant checks and from 5.3 to 7.4 for the susceptible checks. For common bunt, mean scores varied from 1.8 to 3.8% for the resistant checks and from 7.4 to 34.3% for susceptible checks (data not shown). Table 1 provides a summary of descriptive and F statistics. For tan spot, stripe rust, and leaf rust, RILs with mean disease scores ≤3.0 were considered resistant, 3.1–5.0 moderately resistant, 5.1–7.0 moderately susceptible, and 7.1–9.0 susceptible. Based on such categorization, both parents (‘Attila’ and ‘CDC Go’) exhibited moderate resistance to leaf rust (4.2 for both parents) and stripe rust (3.3–3.5), but moderately susceptible to tan spot (5.4–5.8). However, common bunt infection in ‘CDC Go’ was 9%, which was half of the 18% observed for ‘Attila’.
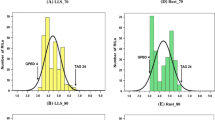
The 167 RILs showed highly variable reactions to the four diseases, varying from 4.0 to 8.5 for tan spot, from 2.5 to 5.8 for leaf rust, from 1.8 to 7.2 for stripe rust, and from 1.8 to 37.2% for common bunt (Table 1). RILs differed (p < 0.0001) for their reactions to all four diseases. Broad-sense heritability varied from 0.25 for leaf rust to 0.48 for common bunt (Table 2). The distribution of disease scores averaged across all environments was normal (P > 0.050) for both tan spot and leaf rust, but it deviated from normality (P ≤ 0.018) both for common bunt and stripe rust. Overall, the RIL population exhibited transgressive segregation for all four diseases and several RILs that were superior or inferior to the parents were observed (Fig. 1). A total of 11 and 33 RILs were found to be resistant with a disease score rating of ≤3.0 for leaf rust and stripe rust, respectively, but only 6 RILs were resistant to both rusts.
QTL mapping
Using inclusive composite interval mapping and disease scores averaged across all environments, we identified a total of 11 QTLs (Table 3; Fig. 2) associated with resistances to tan spot (3), common bunt (2), leaf rust (3), and stripe rust (3). The three QTLs for resistance to tan spot mapped at 264 cM on 2B (QTs.dms-2B), at 3 cM on 2D (QTs.dms-2D), and at 27 cM on 6B (QTs.dms-6B), which individually explained between 7 and 10% (Table 3) and altogether accounted for 24.0% of the phenotypic and 52.2% of the genetic variance for tan spot (Table 2). RILs with ‘CDC Go’ alleles at the two flanking markers of all three QTLs for tan spot showed between 0.6 and 0.9 (on 1 to 9 scale) less tan spot scores than those with ‘Attila’ alleles.
Chromosomal position of QTLs associated with common bunt (black), tan spot (pink), leaf rust (red), and stripe rust (blue) across combined environments. Map position in centiMorgans (cM) is shown on the left side of the chromosomes, with each horizontal line representing a marker. QTLs are shown on the right side of each linkage group, with bars indicating their 95% genetic confidence interval. Details of each QTL is given in Table 3, while the markers and linkage map is given in Supplemental Table 1
ICIM uncovered two QTLs associated with common bunt resistance (Table 3) that mapped at 52 cM on the second linkage group of chromosome 1B (QCbt.dms-1B.2) and at 202 cM on chromosome 3A (QCbt.dms-3A) that altogether accounted for 26.5% of the phenotypic and 55.2% of the genetic variance across all environments (Table 2). QCbt.dms-1B.2 is a moderate effect QTL that mapped between BS00086854_51 and wsnp_Ex_c5679_9976893 on chromosome 1B, had a LOD score of 7.2 and individually explained 18.7% the phenotypic variance. QCbt.dms-3A was a minor effect QTL that mapped between RAC875_c17453_896 and RAC875_c57584_240 on 3A and explained 7.9% of the phenotypic variance across all combined environments. On average, RILs with the ‘CDC Go’ alleles at the two flanking markers for QCbt.dms-1B.2 and QCbt.dms-3A had 7.1% and 2.9%, respectively, less common bunt disease score than those with ‘Attila’ alleles.
Three QTLs associated with resistance to leaf rust were mapped at 39 cM on 2D (QLr.dms-2D), at 2 cM on 2D second linkage group (QLr.dms-2D.2), and at 17 cM on 3A (QLr.dms-3A) that altogether explained 21.5% of phenotypic and 86.0% of genetic variance across all environments (Table 2). Each QTL for resistance to leaf rust individually explained between 5.9 and 8.6% of phenotypic variance across combined environments (Table 3). RILs with ‘CDC Go’ alleles at the flanking markers of all three QTLs for leaf rust scored from 0.3 to 0.5 less leaf rust disease score than those with ‘Attila’ alleles. The three QTLs associated with resistance to stripe rust were mapped at 300 cM on 3A (QYr.dms-3A), at 123 cM on 4A (QYr.dms-4A), and at 191 cM on 5B (QLr.dms-5B). Each QTL for stripe rust resistance individually explained between 6.7 and 8.5% (Table 3) and altogether accounted for 23.1% of the phenotypic and 82.5% of the genetic variance across all combined environments (Table 2). The resistant alleles for QYr.dms-3A and QYr.dms-4A originated from ‘CDC Go’, while that of QYr.dms-5B from ‘Attila’. RILs with the resistance alleles at the two flanking markers of every QTL for stripe rust had between 0.5 and 0.7 less stripe rust disease scores than those with susceptible alleles.
Discussion
Resistance to common bunt and tan spot
Here, we report three and two QTLs associated with resistance to tan spot and common bunt accounting for 52.2 and 55.2% of the total genetic variance, respectively (Table 2). Given our medium heritability estimates for tan spot (0.46) and common bunt (0.48), nearly 45–48% of the genetic variation of these two wheat diseases remained unexplained, which may be partly due to limitations of the current linkage map (wider gaps on marker distribution in some chromosomes; lack of SNP polymorphism on chromosomes 3D and 4D, and very low genome coverage on the D genome as whole) (Zou et al. 2017a) and/or presence of other minor effect QTLs that remained undetected in the current phenotypic data. Furthermore, tan spot infection in wheat fields occurs in association with Stagonospora nodorum and Zymoseptoria tritici (Singh et al. 2006), which may have partly contributed to elevated tan spot disease scoring in the current study.
One of the QTLs associated with common bunt was a minor effect QTL that mapped on chromosome 3A (QCbt.dms-3A) and accounted nearly for 8% of the phenotypic variation (Table 3). We are not aware of any mapping studies that reported either single major effect gene(s) or QTL on chromosome 3A that confers resistance to common bunt in wheat. It is possible that QCbt.dms-3A may be a novel minor effect QTL that has not been reported elsewhere. In addition, we also identified a moderate effect QTL on chromosome 1B (QCbt.dms-1B.2), which accounted for nearly 19% of the phenotypic variation for common bunt disease reaction. Several previous studies have reported genes and QTLs associated with resistance to common bunt on chromosome 1B (Fofana et al. 2008; Wang et al. 2009; Dumalasová et al. 2012; Singh et al. 2016a). One of the previous studies used a doubled haploid (DH) spring wheat population derived from a cross between ‘RL4452’ × ‘AC Domain’ and reported three QTLs associated with resistance to common bunt (Fofana et al. 2008). In that study, the authors observed a continuous frequency distribution and transgressive segregation for common bunt and reported two QTLs on chromosome 1B (QCbt.crc-1B.1 and QCbt.crc-1B.2) that together explained 29%, and another QTL on chromosome 7A that accounted for 3% of the phenotypic variation. QCbt.crc-1B.1 mapped on the short arm of chromosome 1B between XGwm374.1 and XWmc818, had a LOD score of 9.0 and accounted for 21% of the phenotypic variance, while QCbt.crc-1B.2 mapped between GluB1 and XGwm274 on the long arm of chromosome 1B, had a LOD score of 3.6 and explained 8% of the phenotypic variance. Our results, together with others suggest the presence of a moderate to major effect QTL associated with common bunt resistance on chromosome 1B irrespective of the genetic background of the mapping populations, the type of markers and marker density. However, directly comparing the two studies is difficult due to the use of diverse marker types and lack of physical position for all flanking markers.
At least 15 monogenic and race specific genes (named from Bt1 to Bt15) conferring resistance to common bunt have also been reported in wheat (Goates 1996), of which Bt4, Bt5 and Bt6 mapped on chromosome 1B (Sears et al. 1960; Scmidt et al. 1969). In a winter wheat DH population derived from a cross between ‘Blizzard’ × ‘8405-JC3C’, a single gene associated with common bunt resistance has been reported on the short arm of chromosome 1B (Wang et al. 2009). Using a DH population derived from a cross between ‘Trintella’ and ‘Piko’, another group mapped a single major effect gene conferring resistance to common bunt around the centromere region on chromosome 1B, flanked by Xgwm273 (Dumalasová et al. 2012). That gene had a LOD score of 38 and explained up to 30% of the phenotypic variance for common bunt disease severity. Common bunt disease severity in the ‘Trintella’ × ‘Piko’ DH population showed bimodal distribution, which clearly support the presence of a major effect gene or QTL segregating in that population. In the present study, common bunt disease scores in the ‘Attila’ × ‘CDC Go’ RIL population showed somewhat a bimodal distribution (Fig. 1) that may partly explain the moderate effect QCbt.dms-1B.2 QTL identified on chromosome 1B (Table 3). Again, direct comparisons of the position of genes and QTLs reported in the current study with previous studies were not possible due to differences in the types of markers used and lack of physical positions of the flanking markers reported in the different studies. For tan spot, we identified three minor effect QTLs on chromosomes 2B (QTs.dms-2B), 2D (QTs.dms-2D), and 6B (QTs.dms-6B), which individually explained between 6.9 and 10.0% of the phenotypic variance for tan spot disease reaction. Previous studies have reported genes and QTLs associated with tan spot resistance on several chromosomes, including 2B (Friesen and Faris 2004; Gurung et al. 2011), 2D (Gurung et al. 2011), and 6B (Singh et al. 2016b). Ptr ToxB is a proteinaceous host-selective toxin that induces chlorosis in wheat lines harboring the dominant Tsc2 gene (Strelkov et al. 1999; Lamari et al. 2003), which mapped on the short arm of chromosome 2B (Friesen and Faris 2004; Abeysekara et al. 2010). Recently (Perez-Lara et al. 2017), our group evaluated 81 spring wheat cultivars registered (released) in western Canada for reaction to tan spot isolates in field nurseries and for sensitivity to the three Ptr toxins in a greenhouse, and genotyped them with a subset of 19,919 of the Wheat 90K single-nucleotide polymorphic (SNP) array and 11 gene-specific markers. Using genome-wide association analysis, we uncovered clusters of 30 SNPs on chromosome 2B (from 17 to 39 cM interval) that were significantly (p value ranging from 4.5 × 10−5 to 2.9 × 10−10) associated with Ptr ToxB; each SNP individually explained from 21.3 to 44.9% of the phenotypic variation of Ptr ToxB, with an overall average of 28.9%. In the current study, the two flanking markers (Kukri_c148_1346 and GENE-1343_556) for QTs.dms-2B fell within the confidence interval that we reported for Ptr ToxB in the association mapping panel, which suggests that the two regions are the same. However, the genetic position of QTs.dms-2B (262–270 cM) is different from Ptr ToxB QTL position that we reported using association analysis (Perez-Lara et al. 2017), because the linkage map used in the current study was developed using the 167 RILs derived from ‘Attila’ × ‘CDC Go’, while the linkage map used in our GWAS study was a consensus map developed based on multiple mapping populations available to our programs.
At least three minor effect QTLs associated with Ptr ToxB have also been reported on the short arm of chromosomes 2A, the long arms of both 2B and 4A (Friesen and Faris 2004), which suggests that the sensitivity of wheat genotypes to Ptr ToxB depends not only on the Tsc2 gene on chromosome 2B, but also on additional minor effect QTLs that are located on different chromosomes. Using a DH population derived from a cross between ‘CPI133872’ and ‘Janz’, Zwart and colleagues (Zwart et al. 2010) reported a major effect QTL on chromosome 3D, the recessive tsn1 gene on 5BL that confers insensitivity to Ptr ToxA, and five environment specific QTLs on chromosomes 2B, 2D, 3A, 4B, and 5A (Zwart et al. 2010). Based on an association mapping study conducted on 567 spring wheat landraces from the United States Department of Agriculture National Small Grains Collection, several genomic regions, including chromosomes 2B and 2D, that individually explained between 1.3 and 5.9% of the phenotypic variance for race 1 and/or race 5 isolates have been reported (Gurung et al. 2011). An associating mapping study conducted on a set of bread wheat germplasm from CIMMYT has also reported 9 genomic regions associated with tan spot resistance, including chromosome 6B (Singh et al. 2016b). Therefore, the QTLs that we identified for tan spot on chromosomes 2B, 2D, and 6B may be on the same genomic regions with those genes and QTLs reported in previous studies. However, we were not able to make direct comparisons among the different studies for diverse reasons: (i) most studies were conducted using different types of markers, mapping populations, and linkage mapping software’s, which makes direct comparison based on genetic position unreliable; (ii) physical positions for most flanking markers reported in multiple studies, including most SNPs used in the present study, not yet available; (iii) there is even some level of discrepancy (disagreement) on loci order and genetic positions of several SNPs from the Wheat 90K SNP array depending on the mapping population; and (iv) for SNPs where physical positions are available, there are also some level of disagreement with physical positions given in publicly available database, such as Triticeae toolbox (https://triticeaetoolbox.org/wheat/) and Ensembl Plants (http://plants.ensembl.org/Triticum_aestivum/Info/Index).
Resistance to leaf and stripe rust
‘Attila’ was reported to possess moderate to high level of field resistance to both leaf and stripe rusts (Rosewarne et al. 2006; Datta et al. 2009) and has been frequently used as a slow-rusting donor parent in international spring wheat breeding programs (Rosewarne et al. 2008; Datta et al. 2009). ‘CDC Go’ has also been reported to be moderately resistant to leaf rust, and resistant or moderately resistant to stem rust (http://www.agric.gov.ab.ca) and stripe rust (McCallum et al. 2012; Randhawa et al. 2012). In our disease evaluation nurseries, both ‘Attila’ and ‘CDC Go’ showed moderate level of resistance to both leaf and stripe rusts, which agrees with previous studies. Using ICIM, we identified 3 QTLs associated with resistance to leaf rust on the short arms of both chromosomes 2D (QLr.dms-2D.1 and QLr.dms-2D.2) and 3A (QLr.dms-3A), which individually explained between 5.9 and 8.6% of the phenotypic variance (Table 3). Previous mapping studies have reported several single genes associated with leaf rust resistance on chromosome 2D, which includes Lr2a, Lr2b and Lr2c (Dyck and Samborski 1974), Lr15 (Luig and McIntosh 1968), Lr22a, Lr22b and Lr22c (Rowland and Kerber 1974; Dyck 1979), Lr39 and Lr41 (Singh et al. 2004), and Lr54 (Marais et al. 2005). However, the observed continuous leaf rust disease scores distribution with a single peak in the ‘Attila’ × ‘CDC Go’ RIL population does not support the presence of major effect single genes. Several QTLs associated with leaf rust resistance have also been reported on chromosome 2D. One of the QTLs located on chromosome 2D was QLrid.osu-2D, which explained between 21.5 and 26.4% of the phenotypic variance for leaf rust infection in a RIL population derived from ‘CI13227’ × ‘Suwon92’ (Xu et al. 2005). A major effect QTL on the short arm of chromosome 2A (QYr.ufs-2A), along with three minor effect QTLs on chromosomes 2D (QYr.ufs-2D ), 5B (QYr.ufs-5B), and 6D (QYr.ufs-6D) have been reported (Agenbag et al. 2012). QYr.ufs-2D was located on the short arm of chromosome 2D that is believed to be the position of Yr16 and explained between 4.7 and 10.3% of the phenotypic variance for stripe rust on individual experiments. An environment specific QTL associated with leaf rust has also been reported on the short arm of chromosome 2D distal to Xwmc25.2 (Buerstmayr et al. 2014). Three other minor effect QTLs for leaf rust resistance were reported on chromosomes 1B, 2A, and 2D (Rosewarne et al. 2012). A recent meta-analysis compiled 144 QTLs reported in 19 studies conducted between 1999 and 2015 using 20 mapping populations (Soriano and Royo 2015). That study reported a total of 35 meta-QTLs associated with leaf rust resistance, of which three meta-QTLs mapped on chromosome 2D (MQTL9, MQTL10, and MQTL1) and two meta-QTLs mapped on 3A (MQTL12, MQTL13, and MQTL14), with each meta-QTL consisting of clusters of two to six QTLs (Soriano and Royo 2015). MQTL14 mapped around Xmwg570 on chromosome 3A, and the individual QTLs explained between 19 and 30% of leaf rust resistance (Maccaferri et al. 2008). Overall, results from the various studies, together with ours, clearly revealed the presence of several genomic regions on chromosome 2D conferring resistance to leaf rust, which does not seem the case for chromosome 3A.
For stripe rust resistance, we identified three QTLs at 300 cM on 3A (QYr.dms-3A), at 123 cM on 4A (QYr.dms-4A), and at 191 cM on 5B (QLr.dms-5B (Table 3). Each QTL associated with stripe rust resistance individually explained between 6.7 and 8.5% of the phenotypic variance. Although we are not aware of stripe rust resistance gene assigned to chromosome 3A, a few minor effect QTLs for stripe rust have been reported on chromosome 3A. Using composite interval mapping in the Avocet × Saar population, for example, a QTL associated with resistance to stripe rust was reported on the short arm of 3A, which colocalized with a QTL for powdery mildew resistance (Lillemo et al. 2008). In another study, a minor effect QTL (QYr.ifa-3AS) that explained 5.6% of the phenotypic variance for stripe rust severity was reported on the short arm of chromosome 3A in three of five experiments in the ‘Capo’ × ‘Arina’ population (Buerstmayr et al. 2014). Another study (Rosewarne et al. 2012) has also reported minor effect QTLs for stripe rust resistance on several chromosomes, including 3A.
Stripe rust resistance genes Yr51 (Randhawa et al. 2014) and Yr60 (Herrera-Foessel et al. 2015) are two genes reported on chromosome 4A. Yr60 is located on the long arm of 4A and conferred moderate levels of resistance at both seedling and adult plant stages against two Mexican races of P. striiformis (Herrera-Foessel et al. 2015). The distribution of stripe rust disease score in the present study, however, was continuous with a single peak, which did not support the segregation of single gene with major effect. Recently, two QTLs on chromosomes 4A (QYrel.wak-4A) and 6B (QYrfi.wak-6B) that explained between 15 and 16% of the phenotypic variance for stripe rust disease severity in a RIL population derived from ‘Eltan’ and ‘Finch’ have been reported (Klarquist et al. 2016). Yr19 (Chen et al. 1995) and Yr47 that mapped distal to Lr52 (Bansal et al. 2011) are the single genes that confer resistance to stripe rust resistance on chromosome 5B. Some QTLs that confer an adult stage stripe rust resistance have also been reported on chromosome 5B, which includes Yrco.wpg-5B and QYrbr.wpg-5B (Case et al. 2014).
Conclusions
Using combined disease scores across three to eight environments, we identified a total of 11 QTLs associated with resistance to four wheat diseases, which included two for common bunt and three each for tan spot, leaf rust, and stripe rust. Each QTL showed either minor or moderate effect and individually explained between 5.9 and 18.7% of phenotypic variance, and altogether accounted from 21.5 to 26.5% of phenotypic and from 52.2 to 86.0% of genetic variance. Even though the identified QTLs are of minor to moderate effect, they provide useful information to spring wheat breeders aiming to pyramid such types of genomic regions for developing wheat cultivars with durable levels of disease resistance. Some of the QTLs identified in the present study were novel, while others were located on the same genomic regions as previously reported QTLs. Direct comparisons on QTL positions across multiple studies was difficult due to differences in marker platforms, lack of common set of markers, and/or physical positions of flanking markers reported in different studies.
References
Abeysekara NS, Friesen TL, Liu ZH, McClean PE, Faris JD (2010) Marker development and saturation mapping of the tan spot Ptr ToxB sensitivity locus Tsc2 in hexaploid wheat. Plant Genome 3:179–189
Aboukhaddour R, Turkington TK, Strelkov SE (2013) Race structure of Pyrenophora triciti-repentis (tan spot of wheat) in Alberta, Canada. Can J Plant Pathol 35:256–268
Agenbag GM, Pretorius ZA, Boyd LA, Bender CM, Prins R (2012) Identification of adult plant resistance to stripe rust in the wheat cultivar Cappelle-Desprez. Theor Appl Genet 125:109–120
Asif M, Yang RC, Navabi A, Iqbal M, Kamran A, Lara EP, Randhawa H, Pozniak C, Spaner D (2015) Mapping QTL, selection differentials, and the effect of Rht-B1 under organic and conventionally managed systems in the Attila × CDC go spring wheat mapping population. Crop Sci 55:1129–1142
Bansal UK, Forrest KL, Hayden MJ, Miah H, Singh D, Bariana HS (2011) Characterisation of a new stripe rust resistance gene Yr47 and its genetic association with the leaf rust resistance gene Lr52. Theor Appl Genet 122:1461–1466
Buerstmayr M, Matiasch L, Mascher F, Vida G, Ittu M, Robert O, Holdgate S, Flath K, Neumayer A, Buerstmayr H (2014) Mapping of quantitative adult plant field resistance to leaf rust and stripe rust in two European winter wheat populations reveals co-location of three QTL conferring resistance to both rust pathogens. Theor Appl Genet 127:2011–2028
Case AJ, Naruoka Y, Chen XM, Garland-Campbell KA, Zemetra RS, Carter AH (2014) Mapping stripe rust resistance in a BrundageXCoda winter wheat recombinant inbred line population. PLoS One 9:e91758
Chen XM, Jones SS, Line RF (1995) Chromosomal location of genes for stripe rust resistance in spring wheat cultivars Compair, Fielder, Lee, and Lemhi and interactions of aneuploid wheats with races of Puccinia striiformis. Phytopathology 85:375–338
Chu CG, Chao S, Friesen TL, Faris JD, Zhong S, SS X (2010) Identification of novel tan spot resistance QTLs using an SSR-based linkage map of tetraploid wheat. Mol Breed 25:327–338
Chu CG, Friesen TL, SS X, Faris JD (2008) Identification of novel tan spot resistance loci beyond the known host-selective toxin insensitivity genes in wheat. Theor Appl Genet 117:873–881
Ciuffetti LM, Francl LJ, Ballance GM, Bockus WW, Lamari L, Meinhardt SW, Rasmussen JB (1998) Standardization of toxin nomenclature in the Pyrenophora tritici-repentis/wheat interaction. Can J Plant Pathol 20:421–424
Ciuffetti LM, Manning VA, Pandelova I, Betts MF, Martinez JP (2010) Host-selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora tritici-repentis-wheat interaction. New Phytol 187:911–919
Dakouri A, McCallum BD, Radovanovic N, Cloutier S (2013) Molecular and phenotypic characterization of seedling and adult plant leaf rust resistance in a world wheat collection. Mol Breed 32:663–677
Datta D, Nayar SK, Prashar M, Bhardwaj SC (2009) Inheritance of temperature-sensitive leaf rust resistance and adult plant stripe rust resistance in common wheat cultivar PBW343. Euphytica 166:277–282
Dumalasová V, Simmonds J, Bartoš P, Snape J (2012) Location of genes for common bunt resistance in the European winter wheat cv. Trintella. Euphytica 186:257–264
Dyck PL (1979) Identification of the gene for adult-plant leaf rust resistance in Thatcher. Can J Plant Sci 59:499–501
Dyck PL, Samborski DJ (1974) Inheritance of virulence in Puccinia recondita of alleles at the Lr2 locus for resistance in wheat. Can J Genet Cytol 16:323–332
Faris JD, Anderson JA, Francl LJ, Jordahl JG (1996) Chromosomal location of a gene conditioning insensitivity in wheat to a necrosis-inducing culture filtrate from Pyrenophora tritici-repentis. Phytopathology 86:459–463
Faris JD, Anderson JA, Francl LJ, Jordahl JG (1997) RFLP mapping of resistance to chlorosis induction by Pyrenophora tritici-repentis in wheat. Theor Appl Genet 94:98–103
Faris JD, Friesen TL (2005) Identification of quantitative trait loci for race-nonspecific resistance to tan spot in wheat. Theor Appl Genet 111:386–392
Faris JD, Liu Z, SS X (2013) Genetics of tan spot resistance in wheat. Theor Appl Genet 126:2197–2217
Fofana B, Humphreys DG, Cloutier S, McCartney CA, Somers DJ (2008) Mapping quantitative trait loci controlling common bunt resistance in a doubled haploid population derived from the spring wheat cross RL4452 × AC domain. Mol Breed 21:317–325
Friesen TL, Faris JD (2004) Molecular mapping of resistance to Pyrenophora tritici-repentis race 5 and sensitivity to Ptr ToxB in wheat. Theor Appl Genet 109:464–471
Galaev AV, Babayantz LT, Sivolap YM (2006) Molecular marker mapping of resistance gene to common bunt transferred from Aegilops cylindrica into bread wheat. Cytol Genet 40:3–11
Gaudet DA, Puchalski BL (1989) Races of common bunt (Tilletia caries and T. foetida) of wheat in western Canada. Can J Plant Pathol 11:415–418
Goates BJ (1996) Common bunt and dwarf bunt. In: Wilcoxson RD, Saari EE (eds) Bunt and smut diseases of wheat: concepts and methods of disease management. CIMMYT, Mexico, pp 12–25
Gurung S, Mamidi S, Bonman JM, Jackson EW, del Rio LE, Acevedo M, Mergoum M, Adhikari TB (2011) Identification of novel genomic regions associated with resistance to Pyrenophora tritici-repentis races 1 and 5 in spring wheat landraces using association analysis. Theor Appl Genet 123:1029–1041
Helguera M, Khan IA, Kolmer J, Lijavetzky D, Zhong-qi L, Dubcovsky J (2003) PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Sci 43:1839–1847
Herrera-Foessel SA, Lagudah ES, Huerta-Espino J, Hayden MJ, Bariana HS, Singh D, Singh RP (2011) New slow-rusting leaf rust and stripe rust resistance genes Lr67 and Yr46 in wheat are pleiotropic or closely linked. Theor Appl Genet 122:239–249
Herrera-Foessel SA, Singh RP, Lan CX, Huerta-Espino J, Calvo-Salazar V, Bansal UK, Bariana HS, Lagudah ES (2015) Yr60, a gene conferring moderate resistance to stripe rust in wheat. Plant Dis 99:508–511
Hiebert CW, Fetch TG, Zegeye T, Thomas JB, Somers DJ, Humphreys DG, McCallum BD, Cloutier S, Singh D, Knott DR (2011) Genetics and mapping of seedling resistance to Ug99 stem rust in Canadian wheat cultivars ‘Peace’ and ‘AC Cadillac’. Theor Appl Genet 122:143–149
Hiebert CW, Thomas JB, McCallum BD, Humphreys DG, DePauw RM, Hayden MJ, Mago R, Schnippenkoetter W, Spielmeyer W (2010) An introgression on wheat chromosome 4DL in RL6077 (Thatcher*6/PI 250413) confers adult plant resistance to stripe rust and leaf rust (Lr67). Theor Appl Genet 121:1083–1091
Klarquist EF, Chen XM, Carter AH (2016) Novel QTL for stripe rust resistance on chromosomes 4A and 6B in soft white winter wheat cultivars. Agronomy 6
Lagudah ES, Krattinger SG, Herrera-Foessel S, Singh RP, Huerta-Espino J, Spielmeyer W, Brown-Guedira G, Selter LL, Keller B (2009) Gene-specific markers for the wheat gene Lr34/Yr18/Pm38 which confers resistance to multiple fungal pathogens. Theor Appl Genet 119:889–898
Lamari L, Gilbert J, Tekauz A (1998) Race differentiation in Pyrenophora tritici-repentis and survey of physiologic variation in western Canada. Can J Plant Pathol 20:396–400
Lamari L, Strelkov SE (2010) The wheat/Pyrenophora tritici-repentis interaction: progress towards an understanding of tan spot disease. Can J Plant Pathol 32:4–10
Lamari L, Strelkov SE, Yahyaoui A, Orabi J, Smith RB (2003) The identification of two new races of Pyrenophora tritici-repentis from the host center of diversity confirms a one-to-one relationship in tan spot of wheat. Phytopathology 93:391–396
Li H, Ye G, Wang J (2007) A modified algorithm for the improvement of composite interval mapping. Genetics 175:361–374
Lillemo M, Asalf B, Singh RP, Huerta-Espino J, Chen XM, He ZH, Bjørnstad Å (2008) The adult plant rust resistance loci Lr34/Yr18 and Lr46/Yr29 are important determinants of partial resistance to powdery mildew in bread wheat line Saar. Theor Appl Genet 116:1155–1166
Luig NH, McIntosh RA (1968) Location and linkage of genes on wheat chromosome 2D. Can J Genet Cytol 10:99–105
Maccaferri M, Mantovani P, Tuberosa R, Deambrogio E, Giuliani S, Demontis A, Massi A, Sanguineti MC (2008) A major QTL for durable leaf rust resistance widely exploited in durum wheat breeding programs maps on the distal region of chromosome arm 7BL. Theor Appl Genet 117:1225–1240
Marais GF, McCallum B, Snyman JE, Pretorius ZA, Marais AS (2005) Leaf rust and stripe rust resistance genes Lr54 and Yr37 transferred to wheat from Aegilops kotschyi. Plant Breed 124:538–541
McCallum BD, DePauw RM (2008) A review of wheat cultivars grown in the Canadian prairies. Can J Plant Sci 88:649–677
McCallum BD, Fetch T, Chong J (2007) Cereal rust control in Canada. Aust J Agric Res 58:639–647
McCallum BD, Humphreys DG, Somers DJ, Dakouri A, Cloutier S (2012) Allelic variation for the rust resistance gene Lr34/Yr18 in Canadian wheat cultivars. Euphytica 183:261–274
McIntosh RA, Dubcovsky WJ, Morris CA, Appels R, Xia XC (2012) Catalogue of gene symbols for wheat: 2012 supplement. KOMUGI Integrated Wheat Science Database Available online at http://www.shigen.nig.ac.jp/wheat/komugi/genes/symbolClassList.jsp
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. The Crop J 3:269–283
Menzies JG, Knox RE, Popovic Z, Procunier JD (2006) Common bunt resistance gene Bt10 located on wheat chromosome 6D. Can J Plant Sci 86:1409–1412
Milus EA, Lee KD, Brown-Guedira G (2015) Characterization of stripe rust resistance in wheat lines with resistance gene Yr17 and implications for evaluating resistance and virulence. Phytopathology 105:1123–1130
Park RF, Mohler V, Nazari K, Singh D (2014) Characterisation and mapping of gene Lr73 conferring seedling resistance to Puccinia triticina in common wheat. TAG Theor Appl Genet 127:2041–2049
Perez-Lara E, Semagn K, Chen H, Iqbal M, N’Diaye A, Kamran A, Navabi A, Pozniak C, Spaner D (2016) QTLs associated with agronomic traits in the cutler × AC Barrie spring wheat mapping population using single nucleotide polymorphic markers. PLoS One 11:e0160623
Perez-Lara E, Semagn K, Tran AN, Ciechanowska I, Chen H, Iqbal M, N’Diaye A, Pozniak C, Strelkov SE, Hucl PJ, Graf RJ, Randhawa H, Spaner D (2017) Population structure and genomewide association analysis of resistance to disease and insensitivity to Ptr toxins in Canadian spring wheat using 90K SNP array. Crop Sci 57:1522–1539
Randhawa H, Puchalski BJ, Frick M, Goyal A, Despins T, Graf RJ, Laroche A, Gaudet DA (2012) Stripe rust resistance among western Canadian spring wheat and triticale varieties. Can J Plant Sci 92:713–722
Randhawa M, Bansal U, Valárik M, Klocová B, Doležel J, Bariana H (2014) Molecular mapping of stripe rust resistance gene Yr51 in chromosome 4AL of wheat. Theor Appl Genet 127:317–324
Rosewarne GM, Singh RP, Huerta-Espino J, Herrera-Foessel SA, Forrest KL, Hayden MJ, Rebetzke GJ (2012) Analysis of leaf and stripe rust severities reveals pathotype changes and multiple minor QTLs associated with resistance in an Avocet × Pastor wheat population. Theor Appl Genet 124:1283–1294
Rosewarne GM, Singh RP, Huerta-Espino J, Rebetzke GJ (2008) Quantitative trait loci for slow-rusting resistance in wheat to leaf rust and stripe rust identified with multi-environment analysis. Theor Appl Genet 116:1027–1034
Rosewarne GM, Singh RP, Huerta-Espino J, William HM, Bouchet S, Cloutier S, McFadden H, Lagudah ES (2006) Leaf tip necrosis, molecular markers and β1-proteasome subunits associated with the slow rusting resistance genes Lr46/Yr29. Theor Appl Genet 112:500–508
Rowland GG, Kerber ER (1974) Telocentric mapping in hexaploid wheat of genes for leaf rust resistance and other characters derived from Aegilops squarrosa. Can J Genet Cytol 16:137–144
Scmidt JW, Morris R, Johnson VA (1969) Monosomic analysis for bunt resistance in derivatives of Turkey and Oro Wheats1. Crop Sci 9:286–288
Sears E, Schaller C, Briggs F (1960) Identification of the chromosome carrying the Martin gene for resistance of wheat to bunt. Can J Genet Cytol 2:262–267
Singh A, Knox RE, DePauw RM, Singh AK, Cuthbert RD, Kumar S, Campbell HL (2016a) Genetic mapping of common bunt resistance and plant height QTL in wheat. Theor Appl Genet 129:243–256
Singh PK, Crossa J, Duveiller E, Singh RP, Djurle A (2016b) Association mapping for resistance to tan spot induced by Pyrenophora tritici-repentis race 1 in CIMMYTs historical bread wheat set. Euphytica 207:515–525
Singh PK, Mergoum M, Ali S, Adhikari TB, Elias EM, Anderson JA, Glover KD, Berzonsky WA (2006) Evaluation of elite wheat germ plasm for resistance to tan spot. Plant Dis 90:1320–1325
Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Njau P, Wanyera R, Herrera-Foessel SA, Ward RW (2008) Will stem rust destroy the world's wheat crop? Adv Agron:271–309
Singh RP, Huerta-Espino J, William HM (2005) Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. Turk J Agric For 29:121–127
Singh RP, Mujeeb-Kazi A, Huerta-Espino J (1998) Lr46: a gene conferring slow-rusting resistance to leaf rust in wheat. Phytopathology 88:890–894
Singh S, Franks CD, Huang L, Brown-Guedira GL, Marshall DS, Gill BS, Fritz A (2004) Lr41, Lr39, and a leaf rust resistance gene from Aegilops Cylindrica may be allelic and are located on wheat chromosome 2DS. Theor Appl Genet 108:586–591
Singh S, Indu S, Sehgal SK, Bains NS, Guo ZG, Nelson JC, Bowden RL (2007) Molecular mapping of QTLs for Karnal bunt resistance in two recombinant inbred populations of bread wheat. Theor Appl Genet 116:147–154
Soriano JM, Royo C (2015) Dissecting the genetic architecture of leaf rust resistance in wheat by QTL meta-analysis. Phytopathology 105:1585–1593
Spielmeyer W, McIntosh RA, Kolmer J, Lagudah ES (2005) Powdery mildew resistance and Lr34/Yr18 genes for durable resistance to leaf and stripe rust cosegregate at a locus on the short arm of chromosome 7D of wheat. Theor Appl Genet 111:731–735
Strelkov SE, Lamari L (2003) Host-parasite interactions in tan spot [Pyrenophara tritici-repentis] of wheat. Can J Plant Pathol 25:339–349
Strelkov SE, Lamari L, Ballance GM (1999) Characterization of a host-specific protein toxin (Ptr ToxB) from Pyrenophora tritici-repentis. Mol Plant-Microbe Interact 12:728–732
Suenaga K, Singh RP, Huerta-Espino J, William HM (2003) Microsatellite markers for genes Lr34/Yr18 and other quantitative trait loci for leaf rust and stripe rust resistance in bread wheat. Phytopathology 93:881–890
Tadesse W, Manes Y, Singh RP, Payne T, Braun HJ (2010) Adaptation and performance of CIMMYT spring wheat genotypes targeted to high rainfall areas of the world. Crop Sci 50:2240–2248
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Wang S, Knox RE, Depauw RM, Clarke FR, Clarke JM, Thomas JB (2009) Markers to a common bunt resistance gene derived from ‘Blizzard’ wheat (Triticum aestivum L.) and mapped to chromosome arm 1BS. Theor Appl Genet 119:541–553
William M, Singh RP, Huerta-Espino J, Ortiz Islas S, Hoisington D (2003) Molecular marker mapping of leaf rust resistance gene Lr46 and its association with stripe rust resistance gene Yr29 in wheat. Phytopathology 93:153–159
Xu X, Bai G, Carver BF, Shaner GE, Hunger RM (2005) Molecular characterization of slow leaf-rusting resistance in wheat. Crop Sci 45:758–765
Zou J, Semagn K, Iqbal M, N’Diaye A, Chen H, Asif M, Navabi A, Perez-Lara E, Pozniak C, Yang RC, Randhawa H, Spaner D (2017a) Mapping QTLs controlling agronomic traits in the Attila × CDC Go spring wheat population under organic management using 90K SNP array. Crop Sci:365–377
Zou J, Semagn K, Iqbal M, N’Diaye A, Chen H, Asif M, Navabi A, Perez-Lara E, Pozniak C, Yang RC, Randhawa H, Spaner D (2017b) QTLs associated with agronomic traits in the Attila × CDC Go spring wheat population evaluated under conventional management. PLoS One 12:e0171528
Zwart RS, Thompson JP, Milgate AW, Bansal UK, Williamson PM, Raman H, Bariana HS (2010) QTL mapping of multiple foliar disease and root-lesion nematode resistances in wheat. Mol Breed 26:107–124
Acknowledgments
The authors would like to thank Klaus Strenzke, Mark Virginillo, and all other research technicians for evaluating the population across multiple wheat disease nurseries in this study. This involved a small army of individuals over the years from both the University of Alberta’s and Agriculture and Agri-Food Canada’s Lethbridge wheat breeding programs.
Funding
This research was supported by grants to the University of Alberta wheat breeding program from the Alberta Crop Industry Development Fund, Alberta Wheat Commission, Agriculture and Agri-Food Canada, Western Grains Research Foundation Endowment Fund and Core Program check-off funds to D. Spaner. This work was conducted in part within the project “Canadian Triticum Advancement Through Genomics (CTAG).” We would like to acknowledge CTAG funding provided by the Saskatchewan Ministry of Agriculture, Western Grains Research Foundation, Agriculture and Agri-Food Canada, Genome Canada, Genome Prairie, Genome Alberta, and Alberta Innovates. The study was also supported by Chinese Government Scholarship to Jun Zou and Hua Chen.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplemental Table 1
Summary of the 5667 SNPs and three gene specific markers (Ppd-D1, Rht-B1, and Vrn-A1) integrated into the linkage map of ‘Attila’ × ‘CDC Go’ RIL population and a subset of 1203 markers used for QTL mapping. (XLSX 245 kb)
Rights and permissions
About this article
Cite this article
Zou, J., Semagn, K., Chen, H. et al. Mapping of QTLs associated with resistance to common bunt, tan spot, leaf rust, and stripe rust in a spring wheat population. Mol Breeding 37, 144 (2017). https://doi.org/10.1007/s11032-017-0746-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-017-0746-1