Abstract
We analyzed the cytoplasmic diversity of CIP potato breeding germplasm. Cytoplasm types were assigned to 978 genotypes consisting of 265 foreign accessions used as input germplasm, 642 breeding lines developed by CIP, and 71 varieties released from CIP material. We found T (45 %), D (38 %), and W (11 %) to be the most frequent cytoplasm types in CIP breeding germplasm. Comparing the initial input germplasm to CIP breeding lines, the frequency of T-type cytoplasm decreased from 64 to 38 %, while those of D- and W-type cytoplasms increased from 26 to 41 % and from 6 to 14 %, respectively. We conclude that the CIP breeding program, as many others worldwide, has experienced a genetic bottleneck in terms of cytoplasmic diversity due to the unintended and continuous use of cytoplasmic-based male-sterile maternal lineages derived from Solanum demissum and S. stoloniferum in parental line and variety development. Nonetheless, the finding of male-fertile T-type breeding lines must have alleviated the problem to a certain extent, thus enabling CIP breeding progress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum L.) has a rich genetic reservoir with thousands of Andean landraces and over 100 wild tuber-bearing relatives. Potato breeders have been using this exotic germplasm since the early 1900s (Plaisted and Hoopes 1989; Ross 1986). Nevertheless, as modern potato breeding was started with only a few genotypes (Mendoza and Haynes 1974; Plaisted and Hoopes 1989), the narrow genetic base of potato is a concern for continued, long-term improvement. A founder effect is reflected in the modern potato’s cytoplasm; classical European and American varieties have exclusively T-type chloroplast DNA (Bryan et al. 1999; Douches et al. 1991; Hosaka and Hanneman 1988; Lössl et al. 2000; Powell et al. 1993; Provan et al. 1999; Waugh et al. 1990).
The T-type chloroplast DNA has been shown to be in complete association with β-type mitochondrial DNA (Lössl et al. 2000). This T/β cytoplasm is sensitive to nuclear genes that condition male sterility (Grun et al. 1977). Previous studies have shown prevalence of the T/β cytoplasm in common potato gene pools as a consequence of a forced use of these genotypes as female parents because of pollen sterility and the more attractive agronomical attributes of their derivatives in comparison with those from cytoplasm of Andean landraces (S. tuberosum Andigenum Group) (Hoopes et al. 1980; Maris 1989; Sanford and Hanneman 1982). The need to increase levels of non-T-type chloroplast DNA cytoplasm in the cultivated gene pool has been suggested previously (Provan et al. 1999). However, danger of cytoplasmic uniformity may also occur from the widespread use of cultivars with other cytoplasmic genomes associated with male sterility. For example, cultivars carrying Ry sto (a gene for resistance to Potato virus Y), released mainly in Germany (Ross 1986), exhibit complete male sterility caused by association of W-type chloroplast DNA with the characteristic mitochondrial DNA derived from S. stoloniferum Schltdl. (the W/γ cytoplasm) (Brown 1984; Lössl et al. 2000; Ortiz et al. 1993). In both T/β and W/γ cytoplasm, sterility is always characterized by visible abnormalities, such as no pollen, no or poor pollen-shedding, or various deformities of anthers (Grun 1979). In contrast, F1 and backcrossed progenies carrying S. demissum cytoplasm (W/α) produce abundant and normal-looking pollen, but this pollen is non-functional on S. tuberosum (Dionne 1961). The W/α cytoplasm is found in nearly 40 % of German varieties as a result of extensive use of this hexaploid species in German potato breeding programs as a source of resistance to the most serious disease of potato, late blight [Phytophthora infestans (Mont.) de Bary] (Lössl et al. 2000). Once male-sterile S. stoloniferum or S. demissum cytoplasm type is incorporated into parental clones, their use as female parents leads to worsen male sterility problems throughout the potato gene pool as warned by Provan et al. (1999) and Hosaka and Sanetomo (2012). Conversely, we have demonstrated that cytoplasm types derived from diploid cultivars of S. tuberosum Andigenum Group do not contribute to male or female sterility and hence would help alleviate the problems of sterility in potato breeding programs (Hosaka and Sanetomo 2012).
This highlights the importance of characterizing cytoplasm types in potato breeding programs in order to minimize negative consequences of pollen sterility which can limit the choice of male parents and lead to maternal bottlenecks. We previously developed a potato cytoplasm classification method which distinguished potato cytoplasm into six distinct types (M, P, A, W, T, and D types) (Hosaka and Sanetomo 2012). The P- and A-type cytoplasms and the T- and D-type cytoplasms are relatively distinct cytoplasm types within the M- and W-type cytoplasms, respectively, each of which has diverse cytoplasmic variations (Hosaka and Sanetomo 2009; Sukhotu et al. 2004). Andean cultivated potatoes evolved from ancestral wild species with M-type or M-derived type cytoplasm, while all the other wild species not involved in the origin of cultivated potatoes have W-type or W-derived type cytoplasm (Hosaka and Sanetomo 2012). The A-type cytoplasm is the most prevalent type in tetraploid cultivars of S. tuberosum Andigenum Group (referred to as S. tuberosum ssp. andigena by Hawkes 1990, hereinafter Andigena), while the T-type cytoplasm is the most prevalent type in the common potato (referred to as S. tuberosum ssp. tuberosum by Hawkes 1990, hereinafter Tuberosum). The P-type cytoplasm was introduced from diploid cultivars of S. tuberosum Andigenum Group (referred to as S. phureja Juz. et Buk. by Hawkes 1990, hereinafter S. phureja) (Mori et al. 2012), while the D-type cytoplasm was introduced from S. demissum into the common potato gene pool (Sanetomo and Hosaka 2011).
The International Potato Center (known by its Spanish acronym CIP) was founded in 1971 with the aim to deliver sustainable solutions to the world’s pressing problems of hunger, poverty, and the degradation of natural resources. CIP’s global priorities include sustaining root and tuber biodiversity, and breeding more nutritious, adaptable, pest- and disease-resistant varieties for developing countries. CIP’s potato breeding program and the germplasm it conserves in trust are based in Peru, one of the centers of diversity for potato (Hawkes 1990). Since its foundation, CIP has actively worked on developing breeding populations using wild and native Andean germplasm as well as foreign breeding lines from which breeding lines and true seed progenies are distributed worldwide upon request or under collaborative agreements. Many foreign breeding lines have been used as founders or incorporated during advanced cycles of selection to enhance genetic variability for quality and adaptation traits, while others are only held as stocks for distribution when required or requested by other breeding programs.
We have previously shown that the D- and W/γ-type cytoplasms are increasing in the common potato gene pool, i.e., 17.4 and 1.2 %, respectively, in Japanese collections (Hosaka and Sanetomo 2012) and 40 and 10 %, respectively, in German collections (Lössl et al. 2000). In this study, we evaluate the cytoplasmic diversity of CIP breeding germplasm and discuss its influence on potato breeding worldwide, considering that many developing countries depend upon CIP materials as immediate cultivars and as initial or supplemental breeding lines.
Materials and methods
Plant material
Nine hundred and eighty-three genotypes were selected as representative of CIP breeding germplasm. Among these, 947 were obtained as in vitro plantlets from the in-trust genebank and in vitro unit at CIP. The remaining 36 were grown in the field in Japan, and their cytoplasm types were reported by Hosaka and Sanetomo (2012). These genotypes consisted of 15 Peruvian varieties (non-CIP-bred cultivars), 207 foreign varieties, 43 foreign breeding lines, 642 CIP breeding lines, and 71 varieties released from CIP’s breeding program. Peruvian cultivars, foreign varieties, and foreign breeding lines have been used to different degrees in population breeding at CIP and are thus regarded here as “input germplasm.” CIP breeding lines were represented by samples from three populations: “B3” (highland tropics late blight resistance population) with 130 genotypes, “LTVR” (lowland tropics virus resistance population) with 206 genotypes, and “A” (ancient late blight resistance population) with 37 genotypes; two inter-population groups: “LTVR × B3” (10 genotypes), and “BW” (bacterial wilt resistance group) (56 genotypes); “TPS” (true potato seed progenitors) (25 genotypes); and early selections “ES” from before the consolidation of populations (178 genotypes). Varieties released from CIP’s population improvement program (71 genotypes) are regarded here as “output germplasm.”
Determination of cytoplasm types
Total DNA was extracted from in vitro plants (4–6 plantlets/genotype) using the “one-minute DNA extraction” method (Hosaka 2004). All procedures for multiplex polymerase chain reaction (PCR) using T, S, SAC, D, and A markers, BamHI digestion, and agarose gel electrophoresis were as described in Hosaka and Sanetomo (2012). One of the PCR primers for amplification of the A marker (5′-ACGCTTCATTAGCCCATACC-3′) was replaced by 5′-TCACCCAAGAAAAATGACTCG-3′, which amplified a 158 bp shorter fragment. T, S, SAC, and A markers are chloroplast DNA markers, whereas the D marker is a mitochondrial DNA marker (Sanetomo and Hosaka 2013).
Results
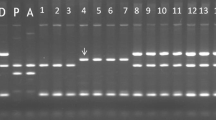
Cytoplasm types were determined for 978 out of the 983 genotypes sampled (Fig. 1). DNA of two genotypes was lost, and each of the three genotypes showed two different types of cytoplasm simultaneously suggesting a mixture of genotypes as plantlets or DNA. The characteristic band for the D marker was often observed with variable intensities. Repeated experiments or comparison with other neighboring marker band intensities could discriminate the real band from a falsely amplified band.
Considering all 978 genotypes characterized, 440 (45.0 %), 368 (37.6 %), 110 (11.2 %), 53 (5.4 %), 5 (0.5 %), and 2 (0.2 %) had cytoplasm types T, D, W, A, M, and P, respectively (Table 1). When the cytoplasms of these genotypes were compared by their improvement stages (input germplasm, breeding lines, and output germplasm), frequency of T-type cytoplasm decreased significantly from input germplasm to breeding gene pool created by CIP and its released (output) germplasm. The opposite was true for D-type cytoplasm. Less represented cytoplasm types, W and A, increase their frequencies in the breeding gene pool and released germplasm, except for W-type that was barely represented in the released germplasm. The presence of M- and P-type cytoplasms was negligible in all groups.
Inspecting CIP-developed genotypes by breeding population, we found the high percentage of T and W cytoplasm in early selections “ES” (47.2 and 15.2 %, respectively) and “LTVR” population (39.3 and 26.2 %, respectively), while the high percentage of D cytoplasm was found in “B3” population (81.5 %). As for other populations not well represented in this survey, a higher frequency of T cytoplasm relative to other cytoplasm types within the same population was found in “A” population, and “TPS” and “LTVR × B3” groups. The same was true for “BW” group with regard to D-type cytoplasm. All other cytoplasm types, namely M, P and A, were absent or barely present in CIP breeding populations as well as in the “input germplasm.” However, it is worth remarking here that though A-type cytoplasm was scarcely present, its frequency was slightly higher in CIP breeding populations, particularly in early selections “ES” (10.7 %) and “LTVR” population (4.4 %), than in the “input germplasm” (3.0 %).
We analyzed cytoplasmic types of genotypes that were used successfully as male parents. Out of the 978 genotypes, 436 were developed from 77 genotypes used as male parents for which cytoplasm type had been determined as part of this study (Table 2). Cytoplasm type was not analyzed in 234 male parents used to develop 376 genotypes. In addition, there were 82 genotypes with unknown male parents, 77 generated by means of pollen bulk, and 7 by open pollination. Out of the 77 male parents with cytoplasm type information, 48 had T-type cytoplasm and were used to develop 302 genotypes. Some of them, such as CIP breeding lines CIP 392820.1 and CIP 387170.9, were involved in crosses with up to 20 different female parents. As for the cytoplasm types of the remaining 29 male parents, 15 A and 10 D were involved in crosses with up to 26 and 5 different female parents, respectively, and in the development of 99 and 31 genotypes, respectively, while 2 W and 2 M male parents were each involved in crosses with a single female parent, respectively. The breeding line CIP 676008 (I-1039) of Indian origin and CIP breeding line CIP 378017.2, both with A-type cytoplasm, were involved in crosses with 26 and 18 different females, respectively. None of the genotypes found to have P-type cytoplasm were male parents of any of the genotypes surveyed.
Finally, cytoplasm types were compared between maternal parents and their progenies (Table 3). One hundred and forty-four maternal lineages were found among 493 genotypes, while the remaining 485 genotypes had unique female parents, unknown, or unavailable female parent information. Of the 144 maternal lineages, 24 lineages of 135 genotypes contained different cytoplasm types within each lineage. In the most extreme case, “Serrana INTA” was found as female parent in 21 genotypes according to available pedigree information, of which 15 had the same cytoplasm (W type) as “Serrana INTA,” while the remaining six had T-type (three genotypes), D-type (two genotypes), or A-type cytoplasm. Fifty-five maternal lineages of 181 genotypes consistently showed the same cytoplasm types within each lineage, while 65 maternal lineages of 177 genotypes showed the same cytoplasm types within each lineage but lacked cytoplasm type information of the maternal parent. Among genotypes with unique female parents, there were 72 that matched and 16 that did not match their female parent cytoplasm types.
Discussion
Many factors might account for inconsistencies in cytoplasm type encountered between genotypes and their maternal parents, as were found for 68 (17 %) out of 404 genotypes for which we have maternal cytoplasm type information. For instance, mistakes could have occurred during crossing program installation, i.e., mixture of plants from seed parents or labeling mistakes; cross–registration; i.e., seed parent not written as the first one for a given cross; pedigree registration on CIP pedigree database; and in vitro plant management or propagation. Whatever the cause, the overall frequency of each cytoplasm type would not be significantly affected.
We found that T (45 %), D (38 %), and W (11 %) were the most frequent cytoplasm types in CIP breeding germplasm (Table 1). Comparing the “input germplasm” to that generated by CIP breeding, we found that the frequency of T-type cytoplasm diminished almost by half (from 64 to 38 %), while those of D- and W-type cytoplasms increased almost twice (from 26 to 41 %) and more than twice (from 6 to 14 %), respectively. Compared with the Japanese gene pool (72.1 % T, 17.8 % D, 2.3 % W, 1.2 % A, 0.2 % M, and 6.4 % P; Hosaka and Sanetomo 2012), the frequency of T-type cytoplasm in CIP breeding materials was relatively low, whereas those of D- and W-type cytoplasms were surprisingly high. Two of 11 accessions with W-type cytoplasm found in Japanese collections had γ-type mitochondrial DNA, which indicated that these were S. stoloniferum-derived cytoplasm (Hosaka and Sanetomo 2012). In this study, mitochondrial DNA types were not specifically determined. However, due to the extensive use in CIP’s potato breeding of foreign materials from European origin descending from S. stoloniferum, a considerable proportion of the 110 genotypes with W-type cytoplasm is suggested to have the S. stoloniferum-derived cytoplasm, as demonstrated below.
The significant increase in D-type at the expense of T-type was very likely due to the utilization of S. demissum-derived late blight-resistant genotypes as female parents. For instance, bacterial wilt-resistant varieties of Mexican origin, such as “Atzimba” and “Cruza-148,” both with D-type cytoplasm, appeared as maternal parents of several breeding lines with resistance to bacterial wilt. These bacterial wilt-resistant lines were used as founders of the “BW” group and were generally crossed as female parents to bacterial wilt-resistant S. phureja-derived hybrids and early varieties with T-type cytoplasm of North American origin, among which are “Atlantic,” “Katahdin,” and their derivatives developed as part of the “LTVR” population (e.g., some “LT” and “XY” CIP breeding lines) (Wissar and Ortiz 1988). The “BW” group exemplifies the systematic increase in D-type cytoplasm in breeding germplasm as a result of using S. demissum as female parent. D-type frequency was significantly greater in this group (60.7 %) than in the “input germplasm” (25.7 %), and “ES” group (25.8 %) used as founders. The same was true in populations bred for late blight resistance, such as “B3” population in which D-type cytoplasm reached the highest frequency (>80 %). Other sources that contributed to late blight resistance in this population came from complex hybrids of S. acaule, S. bulbocastanum, S. phureja, and Tuberosum (ABPT hybrids, Hermsen 1994), as well as from Andigena, Andigena-derived Neotuberosum germplasm, and to a lesser degree from S. phureja. However, pedigree records show that these sources were predominantly used as male parents in crosses to Tuberosum, which may account for the absence of A- and P-type cytoplasms they would otherwise have contributed. In the development of CIP’s breeding populations, the best performing clones are introduced to the genebank at CIP after each cycle of recurrent selection and subsequently cleaned for international distribution. “B3” was not an exception with regard to best performing late blight-resistant clones. The sample of 130 breeding lines from “B3” population used in this survey included genotypes from different cycles of selection. Nonetheless, Tuberosum (T-type cytoplasm) was extensively used as maternal parent; unpredictably, we found a significantly higher frequency of D- than T-type cytoplasm in selected breeding lines from population “B3.” In fact, breeding lines with late blight resistance derived from S. demissum must have been repeatedly used as maternal parents due to the non-functional pollen of D-type cytoplasm onto Tuberosum (Dionne 1961). However, the higher frequency of selected breeding lines with D-type with respect to those of T-type cytoplasm, as shown in this sample, raises the question of the existence of a maternal effect associated with D-type cytoplasm in the inheritance of late blight resistance. Regardless of the reasons, the high frequency of D-type cytoplasm was reflected in turn in CIP “output germplasm,” in which it accounted for 49 %. The highest priority for new varieties with improved levels of resistance to late blight worldwide might account for the highest frequency of germplasm with D-type cytoplasm from S. demissum being released from CIP’s potato breeding program.
T-type cytoplasm, relatively less frequent than D type in breeding lines developed by CIP, had its highest frequency in “ES” group and the “LTVR” population, as well as in others, such as population “A” and the “TPS” group, even though they were not well represented in this survey. “ES” group and “LTVR” population showed cytoplasmic variability, as T, D, W, and A types were all present, though A-type cytoplasm was in a very low frequency (11 and 4 %, respectively). This was not entirely unexpected, as Andigena and its derivatives were used as pollen parents for introgression of extreme resistance to P. virus Y (PVY) and P. virus X (PVX) in “LTVR” population (Muñoz et al. 1975). Meanwhile, the predominance of T-type cytoplasm could be associated with the higher yields and more attractive agronomic attributes encountered in bred germplasm with this cytoplasm type as previously indicated (Maris 1989; Sanford and Hanneman 1982). However, we attribute this rather to the hybrid advantage of using Andigena adapted to short days as pollen parents in crosses to Tuberosum for exploiting heterosis at CIP as in other potato breeding programs in subtropical environments (Gopal et al. 2000). Since its inception, breeding at CIP looked toward developing populations for warm tropical climates, with resistance to globally and agroecologically important potato diseases. The combination of germplasm of different origins (“input germplasm”) within the same population in the early days of breeding may account for the cytoplasmic variability found in the group of “ES,” which subsequently would be used as founders of different populations (Wissar and Ortiz 1988).
Several genotypes with T-type cytoplasm (at least 48 out of 440 genotypes with T-type cytoplasm) showed adequate male fertility (Table 2). The existence of a fertility-restoring gene (Rt), which circumvents male sterility caused by T-type cytoplasm, was described previously (Iwanaga et al. 1991). They reported the presence of this gene in “Atlantic” in simplex dosage and suggested its absence in “Katahdin” based on the high percentage of male-sterile progeny encountered in crosses between its haploids and cultivated diploid species by Hougas and Peloquin (1962). In the present study, we found “Katahdin” (non-Rt-carrier) to have been involved in crosses with 14 different female lines and present as male parent in the pedigrees of 20 genotypes including 2 foreign varieties, 10 foreign breeding lines, 1 Peruvian variety, and 7 CIP breeding lines used in this survey. Likewise, one of its derivatives, the CIP breeding line XY.20, was also a male parent of three breeding lines with different female parents in their pedigrees. In addition, we identified 28 CIP breeding lines, 12 foreign varieties, and 8 foreign breeding lines with T-type cytoplasm involved in crosses as male parents with up to 20 different breeding lines and in the development of 302 out of the 978 genotypes surveyed here (Table 2). This may be due to the presence of the Rt gene and/or any other fertility-restoring gene system which circumvents male sterility caused by T-type cytoplasm. The presence of non-sensitive cytoplasm in the Tuberosum group must not be overlooked as another contributing factor (Ortiz et al. 1993).
These T-type cytoplasmic male-fertile breeding lines and the likely existence of many others may have had an important role in the progress of CIP’s breeding populations through their various cycles of recurrent selection. This is exemplified in the “LTVR” population. We found T-, D-, and W-type cytoplasm in high frequency in this population, while those of A, M, and P were low or null. T-type cytoplasm was present since the initiation of “LTVR” population from the input of North American commercial cultivars and breeding lines for their earliness and relative heat tolerance. On the other hand, A-type cytoplasm came from few Andigena-derived Neotuberosum cultivars that were used as female parents; nonetheless, this germplasm as well as Andigena cultivars has generally been used as male parents across potato breeding programs including CIP’s (Tarn and Tai 1983; Mendoza 1990). We postulate that the Rt gene was inherited from early varieties and breeding lines of North American origin used as founders of the “LTVR” population, and additionally, through the input of early varieties of European origin (Germany, Poland, Czechoslovakia, and Netherlands), incorporated in advanced cycles of recurrent selection, to name a few, Granola (Germany), Alpha (Netherlands), and Monalisa (Netherlands). These European and North American varieties and breeding lines when used as female parents led to the development of at least 38 male-fertile breeding lines that in turn were subsequently used in crosses with up to 20 different maternal lines generating 119 out of the 206 “LTVR” breeding lines used in this survey. Along with the input of bred germplasm of European origin, and additionally of Mexican and South American (Argentina and Chile) origin, D- and W-types were incorporated to “LTVR” population in an effort to combine resistance to Potato leaf roll virus (PLRV) present in this material, with extreme resistance to PVY and PVX, both derived from Andigena and Andigena-derived Neotuberosum germplasm (Mendoza 1990; Bonierbale et al. 2003). Materials of European and South American origin were derived from MPI lines developed at Max Planck Institute for Plant Breeding Research (MPIPZ, Germany). S. demissum was extensively used in German breeding programs as a source of late blight resistance, while S. stoloniferum was a source of PVY resistance (Ross 1986; Lössl et al. 2000). Resistance to PLRV in this long-day adapted breeding germplasm seems to have been derived from introgression of S. demissum as a fortuitous by-product of breeding for resistance to late blight (Ross 1966). The same is true for the input germplasm of Mexican origin. Actually, given the male sterility associated with D- and W-type cytoplasms, these were only used as maternal parents. To name a few, the varieties “Krasa” and “Mariella” both of European origin, and the Mexican breeding lines MEX 32 and CEX 69-1, along with some of their maternally derived genotypes, all with D-type cytoplasm, contributed to at least 26 out of 61 genotypes with D-type cytoplasm in “LTVR” population. On the other hand, “Achirana INTA” and “Serrana INTA” from Argentina and “Brda,” “Bzura,” and “Pirola” of European origin were all maternally descended from S. stoloniferum as described elsewhere (Song and Schwarzfischer 2008; Hosaka and Sanetomo 2012). These W-type varieties and their maternally derived genotypes appear in the pedigree of 31 out of 54 genotypes with W-type cytoplasm in “LTVR” population (Table 1). In addition, tetrad male sterility caused by interaction with S. stoloniferum cytoplasm has been intentionally exploited at CIP for the development of male-sterile progenitors for true potato seed (TPS) varieties (Almekinders et al. 2009).
Most Andean landraces have M-, P-, or A-type cytoplasm (Hosaka and Sanetomo 2012). These types empirically known to be associated with male fertility were rarely found in the CIP breeding germplasm. As discussed above, they have contributed significantly to the paternal diversity and thus to nuclear diversity as well. On the other hand, high percentages of functionally male-sterile W- and D-type cytoplasms encountered must certainly have limited the choice of male parents. Fortunately, T-type foreign varieties and breeding lines with male fertility and the selection of their male-fertile derivatives in CIP breeding populations have contributed significantly to reducing this risk. An Andigena origin of the Rt gene has been suggested due to the high frequency of this gene found in crosses of Tuberosum with Neotuberosum lines obtained after several cycles of selection pressure for early tuberization of Andigena under long days. Linkage or pleiotropic effects were suggested as likely reasons for this increase (Vilaro et al. 1989). In fact, the selection of increasingly precocious lines is performed at each cycle of selection in CIP’s breeding program. Hence, our findings in this survey of male-fertile breeding lines in CIP populations would add evidence to these observations. These results highlight the need to monitor and develop a marker to assist selection (MAS) of Rt gene in order to screen breeding populations and deliberately improve the frequency of male-fertile parental lines. Furthermore, we found that “Atzimba” and two of its derivative breeding lines “BR-63.65” and “CFK 69-1,” all with D-type cytoplasm, were used as male parents in crosses to five, two, and three different female breeding lines, respectively. This confirmed empirical observations that some genotypes with the D-type cytoplasm can function as pollen parents (Hosaka and Sanetomo 2012). A higher frequency of male-fertile lines will contribute as well to germplasm enhancement, facilitating the incorporation of desirable traits from 2x germplasm to 4x populations (Ortiz et al. 2009).
In conclusion, we found that CIP’s breeding germplasm as many others worldwide has experienced a genetic bottleneck in terms of cytoplasmic diversity and continuous incorporation of D- and W/γ-type cytoplasms due to the unintended and continuous use of cytoplasmic-based male-sterile maternal lineages in its breeding program. Presumably, CIP breeding activity has already been hindered to a certain extent by sterility problems. Nonetheless, male-fertile T-type breeding lines must have contributed to alleviate the problem, thus enabling progress for multiple traits in CIP breeding populations.
CIP functions as a source for distributing breeding germplasm worldwide. Our results show that most of the CIP material distributed to developing countries has T- and D-type cytoplasm. Breeders in developing countries may experience breeding constraints imposed by pollen sterility associated with these cytoplasm types. Furthermore, we point out that despite this characteristic, CIP has likely been distributing male-fertile maternal breeding lines within T-type cytoplasm material due to the presence of Rt gene. Our results highlight the importance of the analysis of cytoplasm diversity in breeding germplasm and suggest the need to develop molecular tools to select Rt-bearing breeding lines in order to improve selection of male-fertile parental donors or otherwise to reconsider breeding practices to increase cytoplasmic diversity in breeders’ gene pools.
References
Almekinders CJM, Chujoy E, Thiele G (2009) The use of true potato seed as pro-poor technology: the efforts of an international agricultural research institute to innovating potato production. Potato Res 52:275–293
Bonierbale M, Amorós W, Landeo J (2003) Improved resistance and quality in potatoes for the tropics. In: Proceedings of the 26th international horticultural congress, August 11–17. Toronto, Canada. Acta Horticulturae, vol 619, pp 15–22
Brown CR (1984) Tetrad sterility: a cytoplasmic–genic male sterility attractive to bumblebees. EAPR-abstracts of conference papers. Interlaken, July 1–6, pp 101–102
Bryan GJ, McNicoll J, Ramsay G, Meyer RC, De Jong WS (1999) Polymorphic simple sequence repeat markers in chloroplast genomes of Solanaceous plants. Theor Appl Genet 99:859–867
Dionne LA (1961) Cytoplasmic sterility in derivatives of Solanum demissum. Am Potato J 38:117–120
Douches DS, Ludlam K, Freyre R (1991) Isozyme and plastid DNA assessment of pedigrees of nineteenth century potato cultivars. Theor Appl Genet 82:195–200
Gopal J, Chahal GS, Minocha JL (2000) Progeny mean, heterosis and heterobeltiosis in Solanum tuberosum × tuberosum and S. tuberosum × andigena families under a short day sub-tropic environment. Potato Res 43:61–70
Grun P (1979) Evolution of the cultivated potato: a cytoplasmic analysis. In: Hawkes JG, Lester RN, Skelding AD (eds) The biology and taxonomy of the Solanaceae. Academic Press, London, pp 655–665
Grun P, Ochoa C, Capage D (1977) Evolution of cytoplasmic factors in tetraploid cultivated potatoes (Solanaceae). Am J Bot 64:412–420
Hawkes JG (1990) The potato—evolution, biodiversity and genetic resources. Belhaven Press, London
Hermsen JG (1994) Introgression of genes from wild species, including molecular and cellular approaches. In: Bradshaw JE, Mackay GR (eds) Potato genetics. Scottish Crop Research Institute, Invergowrie, pp 515–538
Hoopes RW, Plaisted RL, Cubillos AG (1980) Yield and fertility of reciprocal-cross tuberosum–andigena hybrids. Am Potato J 57:275–284
Hosaka K (2004) An easy, rapid, and inexpensive DNA extraction method, “One-minute DNA extraction”, for PCR in potato. Am J Potato Res 81:17–19
Hosaka K, Hanneman RE Jr (1988) The origin of the cultivated tetraploid potato based on chloroplast DNA. Theor Appl Genet 76:172–176
Hosaka K, Sanetomo R (2009) Comparative differentiation in mitochondrial and chloroplast DNA among cultivated potatoes and closely related wild species. Genes Genet Syst 84:371–378
Hosaka K, Sanetomo R (2012) Development of a rapid identification method for potato cytoplasm and its use for evaluating Japanese collections. Theor Appl Genet 125:1237–1251
Hougas RW, Peloquin SJ (1962) Exploitation of Solanum germplasm. In: Correll DS (ed) The potato and its wild relatives. Texas Research Foundation, Renner, pp 21–24
Iwanaga M, Ortiz R, Cipar MS, Peloquin SJ (1991) A restorer gene for genetic cytoplasmic male sterility in cultivated potatoes. Am Potato J 68:19–28
Lössl A, Götz M, Braun A, Wenzel G (2000) Molecular markers for cytoplasm in potato: male sterility and contribution of different plastid-mitochondrial configurations to starch production. Euphytica 116:221–230
Maris B (1989) Analysis of an incomplete diallel cross among three ssp. tuberosum varieties and seven long-day adapted ssp. andigena clones of the potato (Solanum tuberosum L.). Euphytica 41:163–182
Mendoza H (1990) Progreso en el mejoramiento de papa por resistencia, en función de la eficiencia de los procedimientos de tamizado. In: Hidalgo OA, Rincón H (eds) Avances en el mejoramiento genético de la papa en los países del cono sur. CIP, Lima, pp 47–62
Mendoza HA, Haynes FL (1974) Genetic relationship among potato cultivars grown in the United States. HortScience 9:328–330
Mori K, Mukojima N, Nakao T, Tamiya S, Sakamoto Y, Sohbaru N, Hayashi K, Watanuki H, Nara K, Yamazaki K, Ishii T, Hosaka K (2012) Germplasm release: Saikai 35, a male and female fertile breeding line carrying Solanum phureja-derived cytoplasm and potato cyst nematode resistance (H1) and Potato virus Y resistance (Ry chc ) genes. Am J Potato Res 89:63–72
Muñoz FJ, Plaisted RL, Thurston HD (1975) Resistance to potato virus Y in Solanum tuberosum ssp. andigena. Am Potato J 52:107–115
Ortiz R, Iwanaga M, Peloquin SJ (1993) Male sterility and 2n pollen in 4x progenies derived from 4x × 2x and 4x × 4x crosses in potatoes. Potato Research 36:227–236
Ortiz R, Simon P, Jansky S, Stelly D (2009) Ploidy manipulation of the gametophyte, endosperm and sporophyte in nature and for crop improvement: a tribute to Professor Stanley J. Peloquin (1921–2008). Ann Bot 104:795–807
Plaisted RL, Hoopes RW (1989) The past record and future prospects for the use of exotic potato germplasm. Am Potato J 66:603–627
Powell W, Baird E, Duncan N, Waugh R (1993) Chloroplast DNA variability in old and recently introduced potato cultivars. Ann Appl Biol 123:403–410
Provan J, Powell W, Dewar H, Bryan G, Machray GC, Waugh R (1999) An extreme cytoplasmic bottleneck in the modern European cultivated potato (Solanum tuberosum) is not reflected in decreased levels of nuclear diversity. Proc R Soc B 266:633–639
Ross H (1966) The use of wild Solanum species in German potato breeding of the past and today. Am Potato J 43:63–80
Ross H (1986) Potato breeding-problems and perspectives. Verlag Paul Parey, Berlin
Sanetomo R, Hosaka K (2011) A maternally inherited DNA marker, descended from Solanum demissum (2n = 6x = 72) to S. tuberosum (2n = 4x = 48). Breed Sci 61:426–434
Sanetomo R, Hosaka K (2013) A recombination-derived mitochondrial genome retained stoichiometrically only among Solanum verrucosum Schltdl. and Mexican polyploid of wild potato species. Genet Resour Crop Evol 60:2391–2404
Sanford JC, Hanneman RE Jr (1982) Large yield differences between reciprocal families of Solanum tuberosum. Euphytica 31:1–12
Song Y-S, Schwarzfischer A (2008) Development of STS markers for selection of extreme resistance (Ry sto ) to PVY and maternal pedigree analysis of extremely resistant cultivars. Am J Potato Res 85:159–170
Sukhotu T, Kamijima O, Hosaka K (2004) Nuclear and chloroplast DNA differentiation in Andean potatoes. Genome 47:46–56
Tarn TR, Tai GCC (1983) Tuberosum × tuberosum and tuberosum × andigena potato hybrids: comparisons of families and parents, and breeding strategies for Andigena potatoes in long-day temperate environments. Theor Appl Genet 66:87–91
Vilaro F, Plaisted RL, Hoopes RW (1989) Comparison of cytoplasmic male sterilities in progenies of tuberosum × andigena and tuberosum × neotuberosum crosses. Am Potato J 66:13–24
Waugh R, Glendinning DR, Duncan N, Powell W (1990) Chloroplast DNA variation in European potato cultivars. Potato Res 33:505–513
Wissar R, Ortiz R (1988) Mejoramiento de papa en el CIP por adaptación a climas cálidos tropicales. In: Guía de Investigación CIP 22, 51. Centro Internacional de la Papa, Lima, Perú
Acknowledgments
We thank Walter Amoros for checking pedigree of materials and CIP genebank for in vitro propagation of the materials used. This study was supported by Calbee, Inc., Tokyo, Japan, and Calbee Potato, Inc., Obihiro, Japan, and the Roots, Tubers and Bananas CRP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mihovilovich, E., Sanetomo, R., Hosaka, K. et al. Cytoplasmic diversity in potato breeding: case study from the International Potato Center. Mol Breeding 35, 137 (2015). https://doi.org/10.1007/s11032-015-0326-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0326-1