Abstract
Saikai 35 was bred from a cross between TD0101 as the female, which was created by chromosome-doubling of a good-tasting and bacterial wilt resistant diploid variety, Inca-no-mezame, and Sakurafubuki as the male, the latter of which has H1 and Ry chc genes showing resistance to potato cyst nematode (PCN) and Potato virus Y (PVY), respectively. All favorable traits were combined into Saikai 35, although marketable yield in the spring cropping was 20.4–21.0% lower than those of major double-cropping varieties. Saikai 35 is particularly useful for having Solanum phureja-derived cytoplasm (S/ε), which resulted in high male and female fertility. In addition, sets of very tightly linked DNA markers sandwiching H1 and Ry chc are available. Therefore, Saikai 35 is being released as a breeding line, which can confer efficiently PCN and PVY resistance genes.
Resumen
Saikai 35 es el resultado de la cruza entre TD0101 como hembra, que se creó por duplicidad de cromosomas de una variedad diploide de buen sabor y resistente a la marchitez bacteriana, Inca-no-mezame, y Sakurafubuki como macho, que tiene genes de H1 y Rychc que muestran resistencia al nematodo de quiste de la papa (PCN) y al virus Y de la papa (PVY), respectivamente. Se combinaron todos los caracteres favorables en Saikai 35, aunque el rendimiento comercial en el cultivo de primavera fue 20.4–21.0% más bajo que las principales variedades de doble cultivo. Saikai es particularmente útil por tener citoplasma derivado de Solanum phureja (S/ε), que da como resultado alta fertilidad masculina y femenina. Además, están disponibles juegos de marcadores de DNA fuertemente ligados en sándwich con H1 y Rychc. De aquí que Saikai 35 se está liberando como una línea de mejoramiento, que puede conferir eficientemente genes de resistencia a PCN y PVY.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various diseases and pests damage potato production. Genetic resistances are favorable compared with chemical controls. Although resistance genes are not always available to all diseases and pests, various resistance genes are known and genetically mapped in the potato genome (Gebhardt and Valkonen 2001). Some of them are highly effective in protecting against causal organisms, and have been incorporated with ease into cultivars.
In Japan, pathotype Ro1 of potato cyst nematodes [Globodera rostochiensis (Woll.) Behrens] has become increasingly a serious problem, particularly in seed potato production areas where once cyst nematodes are found, seed potatoes are not allowed to be transported outside the infected areas (Mori et al. 2007). A single dominant gene H1, introduced from the accession CPC1673 of Solanum tuberosum L. ssp. andigena Hawkes, confers perfect resistance to the pathotype Ro1 (Huijsman 1955). The presence of H1 is now a prerequisite to the release of new cultivars. H1 was mapped to potato chromosome 5 (Pineda et al. 1993; Gebhardt et al. 1993). Recently, a set of tightly linked markers, N146 and N195, were developed which sandwich H1 with the recombination frequencies of 0.109% and 0.207%, respectively (Takeuchi et al. 2009).
Twelve viruses are known to infect and damage potatoes in Japan (Maoka et al. 2010). Among them, Potato virus Y (PVY) is the most important, and thus, resistance to PVY is highly desirable. Three main strains have been distinguished: common strain (PVYO), tobacco veinal necrosis strain (PVYN) and stipple streak strain (PVYC) (German 2001), among which PVYN is problematic because it often causes a virtually symptomless infection on potato but causes a severe necrosis on nearby tobacco foliage. Extreme resistance genes to PVY have been known from three different sources: Ry sto from S. stoloniferum Schlechtd. et Bché. (Cockerham 1943), Ry adg from S. tuberosum ssp. andigena (Munoz et al. 1975) and Ry chc from S. chacoense Bitt. (Asama et al. 1982). These three genes inherit monogenically with a dominant fashion and show strain nonspecific resistance (Ross 1958; Munoz et al. 1975; Hosaka et al. 2001). Ry chc was identified in one of the leading Japanese cultivars, Konafubuki (Hosaka et al. 2001), and mapped to the most distal end of chromosome 9 (Sato et al. 2006). The Ry chc -linked RAPD marker 38–530 has been used for marker-assisted selection, although the recombination frequency was relatively high, 16.3% in the tetraploid population (Hosaka et al. 2001) and 0.9% in the diploid population (Sato et al. 2006). Recently, Takeuchi et al. (2009) developed a set of tightly linked markers, Ry186 and Ry364, which sandwich Ry chc with recombination frequencies of 0.203% and 0.085%, respectively.
For use as a parent in breeding, male and female fertility is an important concern, because breeders often encountered sterility problems in genotypes with desirable resistance traits. The common potato (S. tuberosum, 2n = 4x = 48) has at least seven different cytoplasmic sterility factors ([ASF s], [Fm s], [In s], [SM s], [Sp s], [TA s] and [VSA s]) that condition sterilities in the presence of dominant chromosomal genes (ASF, Fm, In, SM, Sp, TA and VSA) (Grun et al. 1977). The cytoplasmic genome of potato is characterized by having T-type chloroplast DNA (Hosaka 1986) and β-type mitochondrial DNA (Lössl et al. 1999). Although the cytoplasmic sterility factors likely reside on mitochondrial DNA (Hosaka et al. 1988; Lössl et al. 2000), β-type mitochondrial DNA so far shows complete association with T-type chloroplast DNA (hereinafter, T/β cytoplasm) (Lössl et al. 2000). T/β cytoplasm is predominant in the common potato (Hosaka and Hanneman 1988; Waugh et al. 1990; Powell et al. 1993; Bryan et al. 1999; Provan et al. 1999; Lössl et al. 2000), so that sterility problems are unavoidable when T/β cytoplasm is present. Besides such intrinsic sterility, some specific male sterility in association with the cytoplasmic genome has been known. Cultivars carrying Ry sto , released mainly in Germany (Ross 1986), show male sterility caused by association with the characteristic mitochondrial genome derived from S. stoloniferum (Lössl et al. 2000). S. demissum Lindl. is a hexaploid Mexican wild species and the most frequently used wild species in potato breeding as a source of resistance to the most serious disease, late blight (Phytophthora infestans) (Rudorf 1950; Ross 1986; Plaisted and Hoopes 1989). It can be crossed directly with the common potato only when used as a female and forms pentaploid hybrids (Black 1943; Cooper and Howard 1952; Irikura 1968). The resultant pentaploid F1 hybrids are non-functional as males, and usually produce seeds only if backcrossed with the pollen of S. tuberosum. The male sterility of the backcrossed progeny persists even after ten or more successive generations of backcrossing (Dionne 1961). The cytoplasmic genomes of S. stoloniferum and S. demissum introduced into cultivars were characterized to be W/γ and W/α, respectively (Lössl et al. 2000).
Inca-no-mezame is the first diploid variety released in Japan (Mori et al. 2009). It was selected for tuber formation under long day from hybrid plants composed with the Andean potato-derived (75%) nuclear genome and S. phureja cytoplasm (S/ε cytoplasm). It produces smaller tubers than normal tetraploid cultivars, resulting in lower yield. Nevertheless, due to its excellent taste, it was officially released in 2001. The potato breeding program of Nagasaki Agricultural and Forestry Technical Development Center aims to breed potato varieties adopted for double-cropping in warm regions. As the period between cropping seasons (spring cropping from early February to mid-May and fall cropping from early September to late November) is fairly short, short tuber dormancy is required. We planned to combine desirable characters of Inca-no-mezame (good taste, bacterial wilt resistance, short tuber dormancy and S/ε cytoplasm) with H1 and Ry chc genes. The resultant Saikai 35 has all these traits and also high male and female fertility, although tuber yield was slightly lower than major varieties grown in double-cropping regions. In this paper, we report that Saikai 35 is a highly useful breeding line for conferring potato cyst nematode and PVY resistance genes. Saikai 35 will be available for distribution from the US Potato Genebank.
Materials and Methods
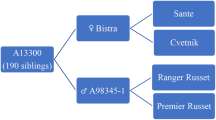
Saikai 35 and the other cultivars and advanced breeding lines used in this study are listed in Table 1. The pedigree of Saikai 35 is shown in Fig. 1. Inca-no-mezame was chromosome-doubled using a tuber-disc method (Komura and Ohbayashi 2002), resulting in TD0101 that acquired improved male and female fertility (Mukojima et al. 2003). A pollen parent, Sakurafubuki, is a cultivar for starch production and possesses H1, originally derived from a German variety Tunika, and Ry chc , derived from S. chacoense via Konafubuki (Murakami et al. 1995).
For breeding double-cropping varieties, two clonal generations were grown every year: a spring season crop using clear plastic film-covered rows (mulching) and a fall season crop. After seedling selection, superior genotypes were grown and selected in fields as follows: a first clonal selection (one hill), a line selection (eight hills in one row), a preliminary yield trial (three rows with ten hills each), and then, several seasons of yield trials (four rows with ten hills each, three replications). Seed tubers were planted early February for spring season cropping, and early September for fall season cropping, with a planting density of 65 cm between rows and 30 cm between plants for a first clonal selection, or 25 cm for all later generation plantings. Fertilizer at 126, 112, 112 and 28 kg ha−1 of N, P2O5, K2O and MgO, respectively, was applied.
Initial screening for the presence of H1 and Ry chc genes was carried out using molecular markers PCN (Tanaka and Komura 2000) and 38–530 (Hosaka et al. 2001), respectively.
Growth and yield performances were examined from 2005 to 2010 at one location (Unzen, Nagasaki) and compared with Dejima and Nishiyutaka, the two most prevalent double-cropping cultivars. Marketable yields (kg/a) were compared; marketable sizes were >30 g in the spring season cropping and >40 g in the fall season cropping. Specific gravity was calculated as weight in air (weight in air—weight in water)−1, using 15 tubers of approximately 100 g size.
Taste of steamed potatoes was evaluated 1 month after harvest from the spring crop and 2 weeks after harvest from the fall crop by the same three members as sensory panelists. Taste was scored from 1 (bad) to 5 (very good) with Dejima as a standard (score 4), and the three season means were compared. Carotenoid contents were measured from three tubers with a size of approximately 100 g by analytical high performance liquid chromatography as described in Kobayashi et al. (2008). Data was obtained from tubers of the spring crop of 2010.
Disease and pest resistances were evaluated by biological assays in the fields or by artificial inoculation. Five plants in a row were grown with two replications in a heavily infested field with potato cyst nematodes in mid-May. Each of three randomly chosen plants in the row was evaluated by rating from 0 (no cyst) to 4 (many cysts), and the mean was calculated as Infection index from 0 (no cyst) to 100 (many cysts on all tested plants). For a PVY resistance assay, ten plants per genotype were grown in pots and inoculated with PVYO or PVYN to three leaves per plant in a greenhouse. One month later, upper leaves were examined by ELISA test and transmission percentages from inoculated leaves to upper leaves were obtained. For a bacterial wilt resistance assay, eight plants in a row were grown with two replications in fall seasons, and percentages of diseased plants were obtained. For a late blight resistance assay, five plants in a row were grown with three replications in spring seasons under natural infection without fungicide application. Plants were separately scored from 0 (<2% leaflets infected) to 6 (100% leaflets infected) and the mean was obtained as the Infection index. For a common scab resistance assay, five plants in a row were grown with two replications in a heavily infested field in spring and fall seasons. Harvested tubers were visually inspected and Infection indices from 0 (no lesions) to 100 (serious defects as Nishiyutaka) were obtained.
Diagnostic marker bands for H1 and Ry chc were detected using a pair of N146 and N195, and a pair of Ry186 and Ry364 markers, respectively (Table 2) (Takeuchi et al. 2009). To both sets of markers, the granule-bound starch synthase I gene (GBSS) marker was included as a positive control to check whether the PCR was performed correctly or not. PCR reaction was set in the volume of 10 μl consisting of 2 μl of template DNA (approximately 5 ng/μl), 5 μl of Ampdirect® Plus (Shimadzu Co., Japan), 0.25 units of Taq DNA polymerase (BIOTAQ™ HS DNA Polymerase, Bioline Ltd., UK) and 0.3 μM primers (Table 2). Thermal cycling was performed using Veriti® 96-well thermal cycler (Applied Biosystems) (one cycle of 10 min at 95°C, followed by 35 cycles of 30 s at 94°C, 30 s at 55°C and 1.5 min at 72°C, and then, terminated with one cycle of 5 min at 72°C). A newly developed multiplex PCR method for simultaneous detection of diagnostic DNA markers of H1, Ry chc and resistance genes to Potato virus X (Rx1) and late blight (R1 and R2) was also tested by the protocol of Mori et al. (2011). PCR products were separated by electrophoresis on a 1.4% agarose gel in 1× TAE buffer, stained in 2.5 μl of Midori Green DNA Stain (Nippon Genetics Europe GmbH, Germany) per 100 ml of 1× TAE buffer for 30 min with gentle shaking, followed by de-staining in used 1× TAE buffer for 30 min with gentle shaking. Photographic images were captured over UV lamp.
Pollen stainability was examined for limited genotypes in 2010. A sample of 247–360 pollen grains was stained with aceto-carmine and calculated by \( 100 \times ({\text{the}}\;{\text{number}}\;{\text{of}}\;{\text{stained}}\;{\text{pollen}}\;{\text{grains}}/{\text{the}}\;{\text{number}}\;{\text{of}}\;{\text{total}}\;{\text{pollen}}\;{\text{grains}}). \)
Results and Discussion
Breeding Process
In May of 2002, TD0101 was crossed as a female with pollen of Sakurafubuki. Twenty pollinations set 13 berries containing 1,007 seeds. Four hundred seeds (T02128 family) were sown in September of 2002, and 159 tuber-setting genotypes were selected. In February of 2003, one tuber per genotype was planted in a field, from which 14 genotypes were selected on the basis of general appearance of the tuber shape and size distribution. By a line selection in the fall of 2003, agronomic traits were evaluated, and H1 and Ry chc genotypes were estimated using molecular markers PCN and 38–530 (Fig. 2), respectively. The marker analysis indicated five genotypes had both PCN and 38–530 markers. By the spring cropping of 2004, one clone with better taste and agronomic traits (clone T02128-14) was selected. Yield trials were continued for several years and agronomic performances and disease resistances were examined. Clonal identity was upgraded to Chokei 126 in 2005, and to Saikai 35 in February of 2006.
Morphological Description and Growth Habit
Photographs of Saikai 35 are shown in Fig. 3. Saikai 35 was slightly taller and had a shorter tuber dormancy period than Dejima and Nishiyutaka (Table 3). Vine maturity of Saikai 35 was medium to late, which was slightly earlier than those of Dejima and Nishiyutaka. Saikai 35 had purple flowers, and its tubers were characterized by yellow flesh and skin with red-colored shallow eyes.
Tuber Yield
Saikai 35 produced lower marketable yields from spring cropping (6 year mean = 331 kg/a) than Dejima and Nishiyutaka (416 and 419 kg/a, respectively), and fall cropping as well (185 kg/a vs. 271 and 183 kg/a, respectively) (Table 4). The number of marketable tubers per hill of Saikai 35 was larger, but the mean tuber weight was lower, which resulted in lower yields. However, the specific gravity of Saikai 35 (1.085 in spring cropping and 1.082 in fall cropping) was significantly higher than those of Dejima (1.068) and Nishiyutaka (1.066) in the spring cropping, and Nishiyutaka (1.063) in the fall cropping (Table 4).
Tuber Quality
Saikai 35 tasted as good (a score of 4.8) as Inca-no-mezame (5.0), and better than Dejima (4.0) and Nishiyutaka (2.6). Saikai 35 contained carotenoids (58.8 ± 4.46 μg lutein and 17.4 ± 4.99 μg of zeaxanthin per 100 g fresh weight), which likely contributed to the yellow flesh and good taste, because Dejima contained 48.1 ± 2.26 μg lutein and no zeaxanthin per 100 g fresh weight (no data was available from Nishiyutaka).
Disease and Pest Resistances
Saikai 35 was extremely resistant to potato cyst nematode (pathotype Ro1), PVY strains PVYO and PVYN, and also resistant to bacterial wilt (Table 5).
DNA Marker Analysis
Saikai 35 showed marker bands PCN and 38–530, and was confirmed by biological assays as a H1 and Ry chc holder as described above. Genetic segregations of these marker bands indicated that Saikai 35 was likely simplex for these resistance genes (Table 6). More accurate diagnostic markers also supported that Saikai 35 has both H1 and Ry chc genes (Fig. 4).
Diagnostic marker bands for identification of Ry chc (A) and H1 (B) genes. Multiplex PCR method (Mori et al. 2011) can identify simultaneously five resistance genes (in C, a late blight resistance gene R2 was not possessed by any genotypes). The granule-bound starch synthase I gene (GBSS) marker is included as a positive control to check whether the PCR was performed correctly or not. The left-most lane in each gel contained HindIII-digested λDNA. 1 Nishiyutaka, 2 Dejima, 3 Haru-akari, 4 Pike, 5 10H15, 6 10H16, 7 10H17, 8 Saikai 37, 9 Saikai 38, 10 Saikai 39, 11 Chokei 131, 12 Aikei 172, 13 Saikai 35
Crossability with Saikai 35 and its Derived Progenies
Saikai 35 showed very high pollen stainability (80.7%). It was crossed as a male onto seven cultivars or breeding lines, which produced berries with 35.4–68.2% berry setting rates and 84.5–255.9 seeds per berry (Table 7). When Saikai 35 was used as a female, berry setting rates were 46.8–58.6% and 126.9–135.3 seeds per berry were obtained. These results indicated Saikai 35 had high male and female fertility.
Male fertility of the progenies from Saikai 35 was examined (Table 8). Aikei 172 was derived from Saikai 35 as a male and has typical S. tuberosum cytoplasm (T/β) (Table 1). It showed high pollen stainability (77.2%). The crosses of Aikei 172 as a male onto four genotypes produced berries with 9.0–30.1% berry setting rates and 24.6–37.2 seeds per berry.
Saikai 39 and T04204-13, derived from Saikai 35 as a male, have S. demissum-derived cytoplasm (W/α). Both produced abundant pollen grains, and the pollen stainability of Saikai 39 was 79.6%, implying high male fertility. Nevertheless, the pollen of Saikai 39 failed to set berries completely onto four cultivars and one breeding line, while T04204-13 showed relatively low berry setting rates (4.3–13.7%). Such no or very low function of the pollen from these two genotypes might be attributed to the interaction of a cytoplasmic factor or factors of S. demissum with nuclear factors contributed by Saikai 35 (Dionne 1961).
Saikai 37 and Chokei 131 were derived from Saikai 35 as a female, thus both having the same S/ε cytoplasm as Saikai 35. The pollen stainability of Saikai 37 was 87.9%. These two genotypes yielded high male fertility with 28.1–55.9% berry setting rates and 101.5–219.3 seeds per berry (Table 8).
These suggest that the S. phureja-derived S/ε cytoplasm did not cause male sterility by interaction with nuclear genomic factor(s). Consequently, Saikai 35 showed good male and female fertility, and as long as Saikai 35 was used as a female, resulting progenies maintained good male fertility.
Usefulness of Saikai 35
We successfully combined H1 and Ry chc genes with the favorable traits of Inca-no-mezame, resulting in Saikai 35, a genotype with good taste, short tuber dormancy and resistances to potato cyst nematodes, PVY and bacterial wilt. However, the tuber yield in the spring cropping was significantly lower than those of major cultivars.
Ry chc shows extreme resistance to any PVY strains so far tested. From progenies of Saikai 35, advanced breeding lines with PVY resistance have already been selected (Saikai 37, Saikai 38, Saikai 39, and Chokei 131) (Table 1). A set of very tightly linked markers sandwiching Ry chc (Takeuchi et al. 2009) can facilitate marker-assisted selection (Fig. 4). Clones such as10H15, 10H17 and Aikei 172 have been selected as possible multiple resistance genotypes based on diagnostic DNA markers (Fig. 4). Therefore, Saikai 35 is particularly useful as a PVY resistance gene donor. An additional advantage of Saikai 35 is that has S/ε cytoplasm, which can avoid male sterility problems frequently caused by the cytoplasm of common potato (T/β), or strictly caused by S. demissum cytoplasm (W/α) and S. stoloniferum cytoplasm (W/γ). In this context, we recommend to use Saikai 35 as a female to assure good male fertility in the progeny.
References
Asama, K., H. Ito, N. Murakami, and T. Itoh. 1982. New potato variety “Konafubuki” (in Japanese). The Bulletin of Hokkaido Prefectural Agricultural Experiment Stations 48: 75–84.
Black, W. 1943. Inheritance of resistance to two strains of blight (Phytophthora infestans de Bary) in potatoes. Transactions of the Royal Society of Edinburgh 61: 137–147.
Bryan, G.J., J. McNicoll, G. Ramsay, R.C. Meyer, and W.S. De Jong. 1999. Polymorphic simple sequence repeat markers in chloroplast genomes of Solanaceous plants. Theoretical and Applied Genetics 99: 859–867.
Cockerham, G. 1943. Potato breeding for virus resistance. Annals of Applied Biology 30: 105–108.
Cooper, J.P., and H.W. Howard. 1952. The chromosome numbers of seedlings from the cross Solanum demissum × tuberosum backcrossed by S. tuberosum. Journal of Genetics 50: 511–521.
Dionne, L.A. 1961. Cytoplasmic sterility in derivatives of Solanum demissum. American Potato Journal 38: 117–120.
Gebhardt, C., and J.P.T. Valkonen. 2001. Organization of genes controlling disease resistance in the potato genome. Annual Review of Phytopathology 39: 79–102.
Gebhardt, C., D. Mugniery, E. Ritter, F. Salamini, and E. Bonnel. 1993. Identification of RFLP markers closely linked to the H1 gene conferring resistance to Globodera rostochiensis in potato. Theoretical and Applied Genetics 85: 541–544.
German, T.L. 2001. Potato virus Y. In Compendium of potato disease, 2nd ed, ed. W.R. Stevenson, R. Loria, G.D. Franc, and D.P. Weingartner, 69–71. St. Paul: APS Press.
Grun, P., C. Ochoa, and D. Capage. 1977. Evolution of cytoplasm factors in tetraploid cultivated potatoes (Solanacease). American Journal of Botany 64: 412–420.
Hosaka, K. 1986. Who is the mother of the potato? - restriction endonuclease analysis of chloroplast DNA of cultivated potatoes. Theoretical and Applied Genetics 72: 606–618.
Hosaka, K., and R.E. Hanneman Jr. 1988. The origin of the cultivated tetraploid potato based on chloroplast DNA. Theoretical and Applied Genetics 76: 172–176.
Hosaka, K., G.A. de Zoeten, and R.E. Hanneman Jr. 1988. Cultivated potato chloroplast DNA differs from the wild type by one deletion – evidence and implications. Theoretical and Applied Genetics 75: 741–745.
Hosaka, K., Y. Hosaka, M. Mori, T. Maida, and H. Matsunaga. 2001. Detection of a simplex RAPD marker linked to resistance to potato virus Y in a tetraploid potato. American Journal of Potato Research 78: 191–196.
Huijsman, C.A. 1955. Breeding for resistance to the potato root eelworm. 2. Data on the inheritance of resistance in Andigenum-Tuberosum crosses obtained in 1954. Euphytica 4: 133–140.
Irikura, Y. 1968. Studies on interspecific crosses of tuber-bearing Solanums. I. Overcoming cross-incompatibility between Solanum tuberosum and other Solanum species by mean of induced polyploids and haploids (in Japanese). Hokkaido Agricultural Experiment Station Shuho 92: 21–37.
Kobayashi, A., A. Ohara-Takada, S. Tsuda, C. Matsuura-Endo, N. Takada, Y. Umemura, T. Nakao, T. Yoshida, K. Hayashi, and M. Mori. 2008. Breeding of potato variety “Inca-no-hitomi” with a very high carotenoid content. Breeding Science 58: 77–82.
Komura, K., and K. Ohbayashi. 2002. Production of a hybrid progeny between doubled Andes-aka (6x) and the potato variety (in Japanese). Kyushu-Nogyo-Kenkyu 64: 46.
Lössl, A., N. Adler, R. Horn, U. Frei, and G. Wenzel. 1999. Chondriome type characterization of potato: mt α, β, γ, δ, ε and novel plastid-mitochondrial configurations. Theoretical and Applied Genetics 99: 1–10.
Lössl, A., M. Götz, A. Braun, and G. Wenzel. 2000. Molecular markers for cytoplasm in potato: Male sterility and contribution of different plastid-mitochondrial configurations to starch production. Euphytica 116: 221–230.
Maoka, T., S. Sugiyama, Y. Maruta, and T. Hataya. 2010. Application of cDNA microarray for simultaneous detection of 12 potato viruses. Plant Disease 94: 1248–1254.
Mori, K., Y. Sakamoto, N. Mukojima, S. Tamiya, T. Nakao, T. Ishii, and K. Hosaka. 2011. Development of a multiplex PCR method for simultaneous detection of diagnostic DNA markers of five disease and pest resistance genes in potato. Euphytica 180: 347–355.
Mori, M., S. Tsuda, N. Mukojima, A. Kobayashi, C. Matsuura-Endo, A. Ohara-Takada, and I.S.M. Zaidul. 2007. Breeding of potato cyst nematode resistant varieties in Japan. In Potato production and innovative technologies, ed. A.J. Haverkort and B.V. Anisimov, 328–339. The Netherlands: Wageningen Academic Publishers.
Mori, M., A. Ohara-Takada, Y. Umemura, T. Maida, T. Kimura, N. Takada, A. Kobayashi, S. Tsuda, T. Nakao, T. Yoshida, C. Matsuura-Endo, and K. Hayashi. 2009. Breeding of diploid potato variety “Inca no mezame” with orange in the tuber flesh color (in Japanese). Breeding Research 11: 53–58.
Mukojima, N., T. Nakao, K. Mori, and K. Komura. 2003. Change of characteristics of 2x potato by chromosome doubling treatment (in Japanese). Kyushu-Nogyo-Kenkyu 65: 37.
Munoz, F.J., R.L. Plaisted, and H.D. Thurston. 1975. Resistance to potato virus Y in Solanum tuberosum ssp. andigena. American Potato Journal 52: 107–115.
Murakami, N., H. Matsunaga, K. Senda, Y. Okuyama, M. Iritani, K. Asama, Y. Mitsui, and K. Shimizu. 1995. A new potato variety “Konamuso (=Sakurafubuki)” (in Japanese). The Bulletin of Hokkaido Prefectural Agricultural Experiment Stations 68: 1–16.
Pineda, O., M.W. Bonierbale, and R.L. Plaisted. 1993. Identification of RFLP markers linked to the H1 gene conferring resistance to the potato cyst nematode Globodera rostochiensis. Genome 36: 152–156.
Plaisted, R.L., and R.W. Hoopes. 1989. The past record and future prospects for the use of exotic potato germplasm. American Potato Journal 66: 603–627.
Powell, W., E. Baird, N. Duncan, and R. Waugh. 1993. Chloroplast DNA variability in old and recently introduced potato cultivars. Annals of Applied Biology 123: 403–410.
Provan, J., W. Powell, H. Dewar, G. Bryan, G.C. Machray, and R. Waugh. 1999. An extreme cytoplasmic bottleneck in the modern European cultivated potato (Solanum tuberosum) is not reflected in decreased levels of nuclear diversity. Proceedings of the Royal Society, London, B 266: 633–639.
Ross, H. 1958. Inheritance of extreme resistance to virus Y in Solanum stoloniferum and its hybrids with Solanum tuberosum. In Proceedings of Third Conference on Potato Virus Diseases, 204–211.
Ross, H. 1986. Potato breeding-problems and perspectives. Berlin: Verlag Paul Parey.
Rudorf, W. 1950. Methods and results of breeding resistant strains of potatoes. American Potato Journal 27: 332–339.
Sato, M., K. Nishikawa, K. Komura, and K. Hosaka. 2006. Potato virus Y resistance gene, Ry chc , mapped to the distal end of potato chromosome 9. Euphytica 149: 367–372.
Sukhotu, T., O. Kamijima, and K. Hosaka. 2004. Nuclear and chloroplast DNA differentiation in Andean potatoes. Genome 47: 46–56.
Takeuchi, T., J. Sasaki, T. Suzuki, H. Horita, S. Hiura, S. Iketani, R. Fujita, and K. Senda. 2009. DNA markers for efficient selection of disease and pests resistance genes in potato (in Japanese). Hokkaido Nogyo-Shiken-Kaigi-Shiryo 2008: 1–26.
Tanaka, T., and K. Komura. 2000. Development of a genetic diagnosis technique for detection of resistant feature to Globodera rostochiensis in potato (in Japanese). The Bulletin of Nagasaki Prefectural Agricultural and Forestry Experiment Stations 26: 1–18.
Waugh, R., D.R. Glendinning, N. Duncan, and W. Powell. 1990. Chloroplast DNA variation in European potato cultivars. Potato Research 33: 505–513.
Acknowledgments
We thank coworkers in the Central Agricultural Experiment Station and the Kitami Agricultural Experiment Station, Hokkaido Research Organization, for performance of or assistance in biological assays of PVY and PCN resistances, respectively, and K. Mizoue, S. Sakai, Y. Kanasaki, N, Shikaya, S. Ohmachi, T. Matsushima and Y. Mukaida, Nagasaki Agricultural and Forestry Technical Development Center, for technical assistance. This study was supported in part by the Authorization Test Project, Agriculture, Forestry and Fisheries Res. Council, and by Calbee Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mori, K., Mukojima, N., Nakao, T. et al. Germplasm Release: Saikai 35, a Male and Female Fertile Breeding Line Carrying Solanum Phureja-Derived Cytoplasm and Potato Cyst Nematode Resistance (H1) and Potato Virus Y Resistance (Ry chc ) Genes. Am. J. Pot Res 89, 63–72 (2012). https://doi.org/10.1007/s12230-011-9221-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-011-9221-4