Abstract
The eggplant (Solanum melongena L.) genome is the least investigated among the economically most important solanaceous crops. Extensive use of molecular markers will improve eggplant germplasm enhancement and breeding. Microsatellites, or simple sequence repeats, have proved to be very useful for eggplant germplasm management and breeding, but there is limited availability of these polymorphic, codominant, and highly repeatable markers in eggplant. We developed a genomic DNA library enriched with AG/CT, which allowed the identification of 55 new genomic microsatellites. Variation parameters of microsatellite loci analyzed showed high average values. The potential of these markers for fingerprinting was assessed in a collection of 24 accessions, of which 22 correspond to S. melongena from different types (landraces, heirlooms, modern F1 hybrids, and obsolete cultivars) and origins, and two to each of the cultivated relatives S. aethiopicum and S. macrocarpon. The multivariate (cluster and PCoA) analyses clearly differentiated four main clusters: (a) two outgroups formed by S. aethiopicum and S. macrocarpon accessions, (b) S. melongena accessions derived mostly from the Mediterranean basin, Central Europe, Africa, and America (‘occidental’ eggplants), and (c) S. melongena accessions derived mostly from Eastern and Southeastern Asia (‘oriental’ eggplants). However, no apparent association pattern was found for accessions of the different types. Observed heterozygosity (H o) values were low, although hybrid cultivars had higher values (H o = 0.12) than non-hybrid materials (H o = 0.02). The new set of eggplant microsatellite markers has proved highly informative and useful for studying the diversity, relationships, and genetic characteristics of an eggplant collection. These markers will be useful for germplasm management and breeding in eggplant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eggplant (Solanum melongena L., Solanaceae) is widely grown in many temperate and tropical regions of the world. The major goals of ongoing breeding programs include yield, resistance to biotic and abiotic stress, fruit quality, postharvest quality, nutritional value, and local market preferences (Daunay 2008). The efficient use of the genetic resources available is critical in order to obtain new improved cultivars that satisfy these new requirements. A recent example comes from the application of modern tools to characterize and use exotic germplasm, including wild and cultivated relatives, for the improvement of antioxidant phenolics content in eggplant (Stommel and Whitaker 2003; Prohens et al. 2007; Mennella et al. 2010).
A wide diversity of local eggplant varieties, adapted to different environmental conditions (mostly open field), uses, and preferences, many of which are stored in germplasm collections (Daunay 2008), coexist with modern cultivars (mostly F1 hybrids) specifically adapted to greenhouse cultivation (Muñoz-Falcón et al. 2008a, 2009a). Although eggplant production is increasingly based on modern hybrid cultivars, selections of some locally adapted materials have attained prominence in the last decades and are marketed as heirloom varieties (Daunay 2008; Muñoz-Falcón et al. 2008b, 2009b). Although many differences exist among local varieties from specific regions of the world, two large groups of eggplant varieties are generally considered by breeders: “occidental” or “Western” eggplants (from the Middle East, Africa, Europe, and America) and “oriental” or “Asian” eggplants (from Eastern and Southeastern Asia) (Chadha 1993; Hallard 1996; Daunay and Janick 2007; Daunay 2008; Bohme et al. 2008).
The availability of molecular tools for the fingerprinting and study of diversity and relationships of germplasm and breeding material is essential for adopting effective plant breeding strategies (Collard and Mackill 2008; Xu and Crouch 2008). Molecular markers have also been shown to be good tools for the indirect selection of qualitative and quantitative traits, pedigree analysis, determination of the degree of heterozygosis, establishment of genetic maps, or development of introgression lines (Staub et al. 1996; Dekkers and Hospital 2002). Unfortunately, the eggplant genome is the least investigated among the economically most important cultivated Solanaceae, and attempts to use markers developed in other related crops (e.g. tomato, pepper, potato) have shown important limitations, and in some cases have proven inefficient (Nunome et al. 2009; Frary et al. 2005). For this reason, the use of molecular markers in eggplant breeding has been limited compared to other relevant crops of the same family (Barone et al. 2009; Jo et al. 2010; Danan et al. 2011).
Several studies of genetic diversity in eggplant have been carried out using random amplified polymorphic DNA (RAPD) (Karihaloo et al. 1995; Nunome et al. 2001; Koundal et al. 2006; Singh et al. 2006), amplified fragment length polymorphisms (AFLP) (Mace et al. 1999; Nunome et al. 2001; Furini and Wunder 2004; Prohens et al. 2005; Koundal et al. 2006; Muñoz-Falcón et al. 2008b, 2009b), simple sequence repeat (SSR) (Nunome et al. 2003a, b; Behera et al. 2006; Stàgel et al. 2008; Nunome et al. 2009; Muñoz-Falcón et al. 2009b, 2011), and inter simple sequence repeat (ISSR) (Isshiki et al. 2008) markers. These studies show a relatively low frequency of polymorphism among eggplant cultivars, which is probably caused by the genetic bottleneck associated with its domestication in the Indo-Burma region, an area outside the natural range of its wild ancestor S. incanum L. (Lester and Hasan 1991; Weese and Bohs 2010). Therefore, the development of new molecular markers of interest for the management of genetic resources and breeding is a priority.
Microsatellites, or SSRs, are one of the best available marker choices for eggplant genetic studies and breeding due to their high level of polymorphism, high reproducibility, multiallelic nature, codominant inheritance, locus specificity, abundance, and random distribution throughout the genome (Powell et al. 1996; Varshney et al. 2005; Kalia et al. 2011). A number of microsatellite markers are publicly available in eggplant, either genomic microsatellites from SSR-enriched genomic libraries, or genic (expressed sequence tag; EST) microsatellites from in-silico analysis of EST databases (Nunome et al. 2003a, b; Stàgel et al. 2008). The most recent work reported the identification of 1,120 SSRs, of which only 620 were polymorphic (Nunome et al. 2009). Despite this, more markers are needed due to the low frequency of polymorphism found among eggplant cultivars.
One example of the interest in SSR markers over other molecular markers available in eggplant comes from the works of Muñoz-Falcón et al. (2009b, 2011), who have found that SSR markers are more informative than AFLPs for studying the relationships among closely related materials of the local Almagro and Listada de Gandía heirlooms, and have allowed the detection of SSR alleles specific and universal to the different selections of these heirlooms. Similarly, Demir et al. (2010) also found that a few selected SSRs discriminated a set of 19 Turkish eggplant genotypes better than RAPD markers.
The aim of this study was to develop a new set of SSR markers from an eggplant enriched genomic library and to evaluate its utility for the study of diversity and relationships in a set of eggplant materials representing different types and origins. Two related cultivated species from the secondary genepool of eggplant, the scarlet eggplant (S. aethiopicum) and the gboma eggplant (S. macrocarpon) (Schippers 2000; Daunay 2008), were also included as outgroups and to test the transferability of the new SSR markers to S. melongena relatives.
Materials and methods
Plant material and DNA extraction
Twenty-four accessions, of which 22 correspond to S. melongena and two to each of the related species S. aethiopicum and S. macrocarpom, were used for SSRs characterization (Table 1). The S. melongena accessions were chosen so that they represented different origins and morphological and molecular characteristics as assessed in previous works (Muñoz-Falcón et al. 2008b, 2009b), as well as different types, including landraces (local varieties traditionally grown in a restricted area), heirlooms (local varieties that have acquired a reputation and have wide diffusion), F1 cultivars, and two obsolete (non-hybrid) cultivars (Table 1). The plant material used in this study is either part of the germplasm collection of the Instituto de Conservacion y Mejora de la Agrodiversidad Valenciana or was obtained from seed companies.
Genomic DNA was extracted from fresh leaves of the eggplant accessions according to the CTAB method procedure (Doyle and Doyle 1987). The quality of DNA was checked on 1% agarose gels and the DNA concentrations estimated using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilminton, DE, USA).
Development of the enriched SSR library
Development of the enriched genomic library was carried out using the commercial F1 hybrid Mulata. Linker was prepared by mixing equal volumes of the oligonucleotides AdapE-forward (5′-CTCGTAGACTGCGTACC-3′) and AdapE-Reverse (5′-AATTGGTACGCAGTCTAC-3′) to a final concentration of 5 μM. The mix was denatured at 94°C for 5 min, and subsequently incubated at room temperature for 20 min.
Ten micrograms of DNA were completely digested with 60 U of EcoRI. A ligation reaction was performed in a total volume of 200 μl using 27 μl of digested DNA, 4 U of ligase T4 and 20 μl of the supplier buffer.
In order to select the fragments containing (CT) n microsatellite sequences, the PCR product was hybridized to a biotinylated (GA)9 oligonucleotide. Streptavidin-coated paramagnetic particles (Streptavidin MagneSphere Paramagnetic Particles, Promega, Sydney, Australia) were added to recover fragments potentially containing microsatellite sequences. The mix was washed several times and finally eluted with double-distilled water. An aliquot of the elute was PCR-amplified in a volume of 100 μl containing 1 μM AdapE-forward primer, 0.2 mM dNTPs, 1 mM MgCl2, 1× PCR buffer, 1 U of Taq polymerase (Roche), in a thermal cycler following the profile: 1 cycle for 5 min at 94°C, 28 cycles of 20 s at 94°C, 20 s at 60°C, and 1 min at 72°C. Finally, the PCR amplified products were column-purified (High Pure PCR Product Purification Kit, Roche Diagnostics GmbH, Mannheim, Germany) and used for the DNA library construction.
The (CT) n enriched sequences were cloned on pTZ57R/T vector (InsTAclone cloning kit, Fermentas, New York, USA) transformed through thermic shock into E. coli competent cells (strain DH5α) and plated onto selective Luria–Bertoni (LB) agar plates (50 μg/ml ampicillin). Recombinant colonies were picked up from the plates, transferred individually onto 96-well plates containing 100 μl of LB, and incubated for 12 h at 37°C.
Colony PCRs were performed using M13 primer, electrophoresed in 1.5% agarose gels, transferred to a nylon membrane (Nylon Membranes Positively Charged, Roche, Mannheim, Germany) and hybridized with a digoxigenin (Dig)-labelled (AG)15 oligonucleotide probe. Positive clones were sequenced using an ABI PRISM 377 DNA Sequencer (Applied Biosystems, Foster City, CA, USA) and the BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems). Sequences obtained were vector-trimmed and unique clones were selected. In order to detect the repeat motifs, sequences were analyzed with Websat software (Martins et al. 2009; http://wsmartins.net/websat). Primers complementary to microsatellite flanking regions were designed using the program Primer3 (Rozen and Skaletsky 2000; http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). Microsatellites were named with a three-letter code followed by a number. The first letter is a C (for COMAV; Instituto de Conservación y Mejora de la Agrodiversidad Valenciana), the second and the third are SM (for S. melongena). Since our microsatellites were developed from a genomic library, we consider not to BLAST them against the available EST-SSR datasets because a few or no hits were expected.
Microsatellite characterization
Microsatellites were amplified following the M13-tail method described by Schuelke (2000) to facilitate the incorporation of a dye label during PCR. Amplifications were performed in a total volume of 12 μl with 10 ng DNA, 1 mM MgCl2, 0.05 μM of forward primer, 0.25 μM of reverse primer, 0.2 μM fluorescent-labelled M-13 primer, 0.2 mM dNTPs, and 1 U of Taq polymerase in 1× PCR buffer. Conditions of the PCR amplification were as follows: 1 cycle for 2 min at 94°C, 35 cycles of 15 s at 94°C, 30 s at the appropriated annealing temperature (Table 2), 45 s at 72°C, followed by 10 min extension at 72°C. Microsatellite alleles were resolved on an ABI Prism 3100 DNA sequencer (Applied Biosystems) using GeneScan 3.7 software and precisely sized using GeneScan 500 LIZ molecular size standards with GenoTyper 3.7 software (Applied Biosystems).
Data analysis
Marker analysis was performed using the matrix of allele size and the program PowerMarker (Liu and Muse 2005). The following parameters were calculated: number of alleles per locus (A), polymorphic information content (PIC) values calculated as \( {\text{PIC}} = 1 - \sum\nolimits_{i = 1}^{n} {p_{i}^{2} } - \sum\nolimits_{i = 1}^{n - 1} {\sum\nolimits_{j = i + 1}^{n} {2p_{i}^{2} } } p_{j}^{2} \) (where n is the total number of alleles detected, p i the frequency of the ith allele, and p j the frequency of the jth allele) (Botstein et al. 1980), observed heterozygosity (H o), expected heterozygosity (H e), calculated as \( H_{\text{e}} = 1 - \sum\nolimits_{i = 1}^{n} {p_{i}^{2} } \) (where p i is the frequency of the ith allele) (Nei 1973), and fixation index (F is), calculated as \( F_{\text{is}} = 1 - (H_{\text{o}} /H_{\text{e}} ) \)(Wright 1965).
In order to evaluate the potential of the SSR markers obtained for diversity studies, a similarity matrix was constructed scoring the amplified fragments as present (1) or absent (0) in each microsatellite loci. Dice’s similarity values (Dice 1945) were calculated for 1,000 bootstrapped data matrices using Phyltools 1.32 software (Buntjer 1997). Subsequently, a consensus phenetic tree based on the UPGMA (Unweighted Pair Group Method with Arithmetic Mean) algorithm (Sneath and Sokal 1973) was built with the Phylip 3.62 package (Felsenstein 1989). Dice’s similarity values (Dice 1945) were used to graphically represent genetic relationships among accessions by principal coordinate analysis (PCoA) (Gower 1966) using the NTSYS 2.0 software package (Rohlf 1993).
Results
Development of microsatellite markers
A total of 896 colonies showed insert by colony PCR, and 182 of them strongly hybridized with the (GA)15 Dig-labelled probe. One hundred and sixty-nine clones gave readable sequences, of which 112 were unique sequences. We found a total of 73 microsatellites, of which 67 contained at least one (AG) n /(CT) n repeat, while different motifs were found in the remaining six SSRs. Of these, most (57) were simple microsatellites, with the number of repeats ranging from 5 to 45, five had interrupted repeats, and five had compound repeats. On the other hand, six sequences showed different microsatellite motifs (three for AT, and one for each of TTG, TAT and AAAT). Thirty-two out of 39 sequences without microsatellites had a rich AG content. Primers were designed for 73 microsatellite flanking sequences. Amplification was successful for 55 of them (75.3%), which are described in Table 2. The remaining 18 microsatellites gave complex patterns or no amplification.
SSR polymorphism
All the microsatellites developed (55) amplified discrete bands in the set of eggplant accessions studied as well as in the accessions of the related species S. aethiopicum and S. macrocarpon. When we took into account only S. melongena accessions, 41 of the microsatellites (74.5%) were polymorphic, but this number increased to 47 (85.5%) when the two accessions of the related species S. aethiopicum and S. macrocarpon were included. Most SSRs with a low number of CT/AG repeats (n ≤ 11) were monomorphic in the materials analyzed. Three of the primers (CSM15, CSM33, and CSM71) had banding patterns corresponding to the presence of two loci. Since the difference in size was great enough to distinguish them unambiguously in all cases, they were not discarded from the study and were tagged with an A or B letter to discriminate them (e.g., CSM15A, and CSM15B).
Table 3 shows the parameters of variability studied for the polymorphic SSRs. When considering only S. melongena accessions, the number of SSR alleles detected was 203, ranging from two to 15 per locus, with an average of 4.7 alleles per locus. The allelic frequencies (p) varied from 0.02 to 0.95, with a mean value of 0.21. One hundred and forty alleles (70.0%) were considered rare (i.e., p ≤ 0.10) and 2 (1.0%) were almost fixed (p ≥ 0.90) (data not shown). The mean expected heterozygosity (H e ) was 0.52, and ranged from 0.86 in CSM36 to 0.09 in CSM13 and CSM33a. Mean observed heterozygosity (H o) was 0.06, and ranged from 0.00 for 16 markers, for which no heterozygous individuals were found, to 0.24 for CSM36 marker. The H o value for F1 hybrids was 0.12, while for the non-hybrid materials it was 0.02. Wright’s fixation index (F is) had an average value of 0.88, ranging from 0.41 to 1.00. F is for F1 hybrids was 0.64, while for the rest of non-hybrid materials it was 0.96.
The most informative marker (CSM36; PIC = 0.85) was able to distinguish the highest number of S. melongena accessions, whereas the least informative markers (CSM13 and CSM33A; PIC = 0.08) were only able to distinguish one accession (ASI-S-1) from the rest. Taking into account all loci across the S. melongena accessions, the average PIC was 0.47.
When we considered all the accessions (i.e., S. melongena plus the relatives S. aethiopicum and S. macrocarpon), the number of alleles detected increased to 273, with a mean of 5.46 alleles per locus. Markers CSM63, CSM23, CSM71A, CSM75, CSM53, CSM60 and CSM35, which were monomorphic in S. melongena, had one or two different alleles in these S. melongena relatives. When these two accessions were added to the calculation of variation parameters, mean expected (H e = 0.51) and observed (H o = 0.06) heterozygosity showed very slight variation. A small increase in Wright’s fixation index (F is) was also detected (0.90), while the average PIC remained unchanged (0.47).
Multivariate analysis
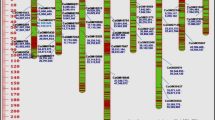
SSR-based genetic distance values between accessions ranged from 0.26 between AFR-S-1 and PI491260 to 0.61 between Listada de Gandia and Thai Long Green. The phenogram obtained by UPGMA cluster analysis clearly distinguished three main clusters (Fig. 1). The outgroup clusters, supported by a 100% bootstrap value, includes and differentiates the two S. aethiopicum and S. macrocarpon outgroup accessions. The third major cluster grouped 16 S. melongena accessions of different types derived from the Mediterranean basin, Central Europe, Africa, and America as well as the Indian Manjri Gota heirloom. Given that the origin of 15 accessions out of these 16 accessions can be traced back to Europe, Africa and America, we labelled this group “occidental”. The fourth cluster was composed of five S. melongena accessions derived from Eastern and Southeastern Asia, and also includes the Fairy Tale Hybrid originating from America (Tomato Growers Seeds, USA). We labelled this cluster “oriental”. Within the “occidental” cluster, robust nodes (with a bootstrap value ≥ 50%) were found connecting Florida High Bush and SUD-S-5 (85%), Listada de Gandia and Rami (70.4%), PI-491260, Almagro, and AFR-S-1 (62.6%), and B-S-5 and INRA11 Dourga (58.6%).
Hierarchical clustering analysis (UPGMA algorithm with bootstrap supporting values, 1,000 replicates) of the 24 eggplant accessions (22 of S. melongena, one of S. aethiopicum, and one of S. macrocarpon) based on Dice genetic distances calculated with 47 polymorphic SSRs. Only bootstrap values over 50% are shown. Accession codes are reported in Table 1
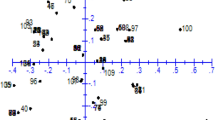
Multivariate PCoA analysis was performed in order to complement the information obtained with the cluster analysis. The first and the second coordinates accounted for 14.0 and 9.4% of the total variance respectively. The PCoA graph (Electronic Supplementary Material Fig. 1) shows a clear separation between the “occidental” group accessions, positioned at the right-hand side of the graph, and “oriental” accessions, which are plotted in the left-hand part. The commercial accession Fairy Tale Hybrid, included in the “oriental” group, plots closer to the African accession RNL-19, which forms part of the “occidental” group, rather than to other “oriental” accessions. Within the “occidental” group a differentiation is observed according to the second coordinate, so that eight accessions plot together in the upper part of the graph (high positive values of the second coordinate), another six plot in the lower part (negative values), and two others plot in between (with values close to 0 for this second coordinate) (Electronic Supplementary Material Fig. 1). No association between these three subgroups within the “occidental” group with origin or cultivar characteristics was apparent, although the two commercial F1 hybrids (Mulata and Nadia) plotted in the first subgroup.
Discussion
Genomic libraries enriched for specific dinucleotide repeats are useful for identifying highly polymorphic SSR markers (Chakraborty et al. 1997; Schug et al. 1998; Kalia et al. 2011) Using a genomic library enriched for AG/CT repeats, we were able to design 55 new genomic microsatellites that gave a successful amplification. This is an important addition to the publicly available genomic SSR markers in eggplant. Forty-one of the new SSRs proved to be polymorphic in a set of accessions of S. melongena (74.5%), and 47 (85.5%) when the related species S. aethiopicum and S. macrocarpon were considered. Studies carried out in 25 plant microsatellite libraries reported that, on average, 82.3% of loci producing PCR products are polymorphic (Squirrel et al. 2003), which is a similar value to the one obtained with our set of SSR markers and materials. This is a significant fact, given that an important genetic bottleneck is thought to have taken place during eggplant domestication and, in consequence, cultivated eggplant has a narrow genetic base (Lester and Hasan 1991; Furini and Wunder 2004; Weese and Bohs 2010). It is also remarkable that the polymorphism found by us is higher than that obtained in eggplant with genomic SSRs by Nunome et al. (2003a; 69.5%), Nunome et al. (2003b; 13.7%), and Nunome et al. (2009; 56.7%), and with EST-SSRs by Stàgel et al. (2008; 28.2%), and Nunome et al. (2009; 30.3%). The higher polymorphism of the genomic SSRs, already observed in a study on striped eggplants (Muñoz-Falcón et al. 2011), is to be expected, as these markers are mostly associated with non-coding regions, while EST-SSRs derive from expressed regions of the genome (Kalia et al. 2011).
The high average values for the number of alleles detected per locus (4.7), the expected heterozygosity (0.52) and the PIC values (0.47) obtained for S. melongena in this study indicate that the SSR markers developed can be of great utility for germplasm management and breeding programmes in eggplant. In general, the values estimated for the variation parameters obtained by us were also higher than those observed in former studies in eggplant. In this respect, Nunome et al. (2003a) obtained a mean of 3.1 alleles per locus and a H e of 0.38 when evaluating 11 S. melongena lines by means of 16 polymorphic dinucleotide genomic microsatellites. The same authors (Nunome et al. 2003b) evaluated the same 11 accessions using trinucleotide genomic microsatellites and observed that the number of alleles per locus (2.1) and H e (0.31) were even lower. This agrees with previous studies which suggested higher mutation rates in dinucleotide than in trinucleotides repeats (Chakraborty et al. 1997; Schug et al. 1998) and may also explain why our SSRs (mostly dinucleotide genomic SSRs) get relatively high values of variation parameters. Stàgel et al. (2008), when considering 11 polymorphic EST-SSRs in 38 S. melongena accessions, found a rate of 3.1 alleles per locus and an average PIC value of 0.38. Usually, genomic microsatellites tend to be more polymorphic than EST-SSRs (Kalia et al. 2011), but in this case, the results are similar to those obtained by Nunome et al. (2003a) using genomic microsatellites. This may be explained by the fact that Stàgel et al. (2008) used a higher number of accessions from a broader range of origins than Nunome et al. (2003a, b). Nunome et al. (2009) also evaluated genomic and EST-SSRs in eight eggplant accessions, obtaining a mean of 2.2 and 1.4 alleles per locus respectively. The PIC values in the Nunome et al. (2009) study were also low, being 0.27 for the genomic SSRs and 0.13 for the EST-SSRs. Demir et al. (2010), using five eggplant SSRs developed by Nunome et al. (2009) and selected for their high PIC, found an average of 4.8 alleles per locus using 20 Turkish accessions, which is similar to the average value found by us. However, if we just consider our five SSR markers with the highest PICs, the average number of alleles per locus in our study would have been 10.0, which is also a greater value than that obtained by Demir et al. (2010). The fact that we have used a wide diversity of materials, with different types and different origins, from four continents, may also have contributed to the high values of the variation parameters. However, if we exclude the clearly distinct “oriental” accessions from the analyses, the average values of number of alleles per locus and PICs of the 15 “occidental” accessions are still high (3.2 and 0.32 respectively), suggesting that the methodology used is useful for developing highly polymorphic SSR markers.
The results of this study also show a low level of observed heterozygosity (H o) in S. melongena. The mean F is was close to 1 (0.88) indicating an evident deficiency of heterozygotes. Similar results were reported by Nunome et al. (2003a, b) and Muñoz-Falcón et al. (2009a, b) in different S. melongena materials. This suggests a high level of inbreeding, probably due to the mostly autogamous nature of eggplant (Quagliotti 1979; Pessarakli and Dris 2004). A low level of observed heterozygosity is detected even in commercial hybrids (H o = 0.12). This provides evidence that the present breeding programme methods use a narrow elite genepool for the development of new hybrid cultivars, resulting in an overall reduction of the heterozygosity of the hybrids (Muñoz-Falcón et al. 2009a). Given that heterosis for yield traits has been detected in eggplant when crossing genetically distant parents (Sidhu et al. 2004; Rodríguez-Burruezo et al. 2008), the results obtained suggest that introduction of new germplasm in eggplant breeding programmes could be useful for increasing the heterozygosity and heterosis of hybrids.
The evaluation of the SSR markers developed as potential tools for fingerprinting has been demonstrated, as all the accessions used have had a unique SSR fingerprint. In fact, SSRs have proved very useful for studying variation among closely related materials of eggplant (Muñoz-Falcón et al. 2009b, 2011). Their usefulness for establishing relationships among the materials has been studied by means of UPGMA clustering and PCoA analysis. The cluster analysis clearly differentiates four groups. The outgroups include the scarlet (S. aethiopicum) and gboma (S. macrocarpon) eggplants clusters, which are mainly cultivated in Africa. The third and fourth clusters include, respectively, what we have called “occidental” and “oriental” accessions. In general, eggplants from Europe, Africa, Middle East and America are morphologically different from Asian eggplants (Chadha 1993; Hallard 1996; Daunay and Janick 2007). This is evidence that a genetic differentiation between “occidental” and “oriental” eggplants has occurred, which may have important implications for conservation of genetic resources and breeding. In this respect, it remains to be studied whether hybrids between the two types of eggplant present heterosis for yield and potential commercial interest. However, although “occidental” and “oriental” accessions were clearly separated, the relationship among the accessions belonging to each group is in general unclear, and the subclusters formed appear to show no association based on the origin or type of material.
The fact that Manjri Gota, an Indian heirloom, groups with the “occidental” accessions, derived from local Indian germplasm, is not a surprise. In former studies (Muñoz-Falcón et al. 2008b), the Manjri Gota accession we have used was found to be morphologically and molecularly similar to Mediterranean accessions. S. melongena was domesticated in the Indo-Burma center of origin (Lester and Hasan 1991; Weese and Bohs 2010), from where it was introduced into the Middle East, Africa, and Europe (Prohens et al. 2005; Daunay 2008).This may lead us to speculate that, among others, materials genetically similar to Manjri Gota were brought from India into western regions of the Old World and through the action of microevolutive forces gave rise to the materials of eggplant typical of the Middle East, Africa, and Europe. In any case, further research should be done to investigate the reason for the clustering of Manjri Gota with “occidental” eggplants. Also, the clustering of Fairy Tale Hybrid, which is an F1 hybrid with small and elongated fruits and is morphologically similar to other “oriental” eggplants (Muñoz-Falcón et al. 2009a) suggests that it might have had materials derived from Asian eggplants in its parentage. Further molecular work may help to clarify this issue.
Prior studies have suggested that Asian varieties show wider morphological and genetic diversity than Western types (Lester and Hasan 1991; Weese and Bohs 2010). Here we have found that the diversity measured as H e (Nei 1973) of the “occidental” and “oriental” groups established by us was similar (0.48 and 0.43 for “occidental” and “oriental” groups, respectively). This is probably due to the fact that the diversity of Asian eggplants was much more underrepresented in the “oriental” group than the diversity of Western types was in the “occidental” group. In this respect, most of the “oriental” accessions evaluated are commercial hybrids and no heirlooms typical of this region are represented (Lester and Hasan 1991; Daunay 2008). A wider diversity would probably have been found if more Asian landraces and heirlooms had been available and included in this study.
The complete level of transferability of microsatellites to the related scarlet (S. aethiopicum) and gboma (S. macrocarpon) eggplants is of great relevance for the breeding of these neglected crops, in which few genetic improvement efforts have been undertaken up to now (Lester and Thitai 1989; Schippers 2000; Seck 2000). The availability of these SSR markers will help in the conservation of genetic resources, as well as in studying the diversity, establishing relationships, and breeding of both African eggplant crops. It will also facilitate the construction of interspecific genetic linkage maps, and will help to accelerate the introgression of useful genes of eggplant relatives into the more economically important S. melongena.
In conclusion, the 55 newly developed eggplant microsatellite markers developed using the enriched genomic library strategy have proved highly informative and useful for studying the diversity and relationships of a set of eggplant materials, and represent a significant improvement in the available eggplant genomic resources. This new set of molecular tools as well as the information derived from its application to a collection of eggplant materials will be useful for germplasm management and breeding research in eggplant.
References
Barone A, Di Matteo A, Carputo D, Frusciante L (2009) High-throughput genomics enhances tomato breeding efficiency. Curr Genomics 10:1–9
Behera TK, Sharma P, Singh BK, Kumar G, Kumar R, Mohapatra T, Singh NK (2006) Assessment of genetic diversity and species relationships in eggplant (Solanum melongena L.) using STMS markers. Sci Hortic 107:352–357
Bohme M, Pinker I, Pietzsch R, Zude M (2008) Growing and fruiting of eggplant genotypes (Solanum melongena L.) under greenhouse conditions in Europe. Acta Hortic 769:69–76
Botstein D, White RI, Skolnich M, Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am J Hum Genet 32:324–331
Buntjer JB (1997) Phylogenetic computer tools (PhylTools), version 1.32 for Windows. Laboratory of Plant Breeding, Wageningen University, Wageningen
Chadha ML (1993) Improvement of brinjal. In: Chadha KL, Kalloo G (eds) Advances in horticulture vol. 5—vegetable crops: part 1. Malholtra Publishing House, New Delhi, pp 105–135
Chakraborty R, Kimmel M, Strivers DN, Davison LJ, Deka R (1997) Relative mutation rates at di- tri- and tetranucleotide microsatellite loci. Proc Natl Acad Sci USA 94:1041–1046
Collard BCY, Mackill DJ (2008) Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos Trans R Soc B Biol Sci 363(1491):557–572
Danan S, Veyrieras JB, Lefebvre V (2011) Construction of a potato consensus map and QTL meta-analysis offer new insights into the genetic architecture of late blight resistance and plant maturity traits. BMC Plant Biol 11:16
Daunay MC (2008) Eggplant. In: Prohens J, Nuez F (eds) Handbook of plant breeding: vegetables II. Springer, New York, pp 163–220
Daunay MC, Janick J (2007) History and iconography of eggplant. Chron Hortic 47(3):16–22
Dekkers JCM, Hospital F (2002) The use of molecular genetics in the improvement of agricultural populations. Nat Rev Genet 3(1):22–32
Demir K, Bakir M, Sarıkamış G, Acunalp S (2010) Genetic diversity of eggplant (Solanum melongena) germplasm from Turkey assessed by SSR and RAPD markers. Genet Mol Res 9(3):1568–1576
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26:6
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Felsenstein J (1989) PHYLIP: phylogeny inference package (version 3.2). Cladistics 5:164–166
Frary A, Xu Y, Liu J, Mitchell S, Tedeschi E, Tanksley S (2005) Development of a set of PCR-based anchor markers encompassing the tomato genome and evaluation of their usefulness for genetics and breeding experiments. Theor Appl Genet 111:291–312
Furini A, Wunder J (2004) Analysis of eggplant (Solanum melongena)-related germplasm: morphological and AFLP data contribute to phylogenetic interpretations and germplasm utilization. Theor Appl Genet 108:197–208
Gower JC (1966) Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53:325–328
Hallard J (1996) L’aubergine au Japon. PHM Rev Hortic 374:55–56
Isshiki S, Iwata N, Khan MR (2008) ISSR variations in eggplant (Solanum melongena L.) and related Solanum species. Sci Hortic 117:186–190
Jo YD, Kim YM, Park MN, Yoo JH, Park M, Kim BD, Kang BC (2010) Development and evaluation of broadly applicable markers for Restorer-of-fertility in pepper. Mol Breed 25:187–201
Kalia RK, Rai MK, Kalia S, Singh R, Dhawan AK (2011) Microsatellite markers: an overview of the recent progress in plants. Euphytica 177:309–334
Karihaloo JL, Brauner S, Gottlieb LD (1995) Random amplified polymorphic DNA variation in the eggplant, Solanum melongena L. (Solanaceae). Theor Appl Genet 90:767–770
Koundal M, Sharma DR, Mohapatra T, Koundal KR (2006) Comparative evaluation of RAPD and AFLP based genetic diversity in brinjal (Solanum melongena L.). J Plant Biochem Biotechnol 15:15–19
Lester RN, Hasan SMZ (1991) Origin and domestication of the brinjal eggplant, Solanum melongena, from S. incanum in Africa and Asia. In: Hawkes JG, Lester RN, Nee M, Estrada N (eds) Solanaceae III: taxonomy, chemistry, evolution. The Linnean Society of London, London, pp 369–387
Lester RN, Thitai GNW (1989) Inheritance in Solanum aethiopicum, the scarlet eggplant. Euphytica 40:67–74
Liu K, Muse S (2005) Powermarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129
Mace ES, Lester RN, Gebhardt CG (1999) AFLP analysis of genetic relationships among the cultivated eggplant Solanum melongena L., and wild relatives (Solanaceae). Theor Appl Genet 99:626–633
Martins WS, Lucas DCS, Neves KFS, Bertioli DJ (2009) WebSat—a web software for microsatellite marker development. Bioinformation 3(6):282–283
Mennella G, Rotino GL, Fibiani M, D’Alessandro A, Francese G, Toppino L, Cavallanti F, Acciarri N, Lo Scalzo R (2010) Characterization of health-related compounds in eggplant (Solanum melongena L.) lines derived from introgression lines of allied species. J Food Agric Chem 58:7597–7603
Muñoz-Falcón JE, Prohens J, Rodríguez-Burruezo A, Nuez F (2008a) Potential of local varieties and their hybrids for the improvement of eggplant production in the open field and greenhouse cultivation. J Food Agric Environ 6(1):83–88
Muñoz-Falcón J, Prohens J, Vilanova S, Nuez F (2008b) Characterization, diversity, and relationships of the Spanish striped (Listada) eggplants: a model for the enhancement and protection of local heirlooms. Euphytica 164:405–419
Muñoz-Falcón JE, Prohens J, Vilanova S, Nuez F (2009a) Diversity in commercial varieties and landraces of black eggplants and implications for broadening the breeders gene pool. Ann Appl Biol 154:453–465
Muñoz-Falcón JE, Prohens J, Vilanova S, Ribas F, Castro A, Nuez F (2009b) Distinguishing a protected geographical indication vegetable (Almagro eggplant) from closely related materials with selected morphological traits and molecular markers. J Sci Food Agric 89:320–328
Muñoz-Falcón JE, Vilanova S, Plazas M, Prohens J (2011) Diversity, relationships and genetic fingerprinting of the Listada de Gandía eggplant landrace using genomic SSRs and EST-SSRs. Sci Hortic 129(2):238–246
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Nunome T, Ishiguro K, Yoshida T, Hirai M (2001) Mapping of fruit shape and color development traits in eggplant (Solanum melongena L.) based on RAPD and AFLP markers. Breed Sci 51:19–26
Nunome T, Suwabe K, Ohyama A, Fukuoka H (2003a) Identification and characterization of microsatellites in eggplant. Plant Breed 122:256–262
Nunome T, Suwabe K, Ohyama A, Fukuoka H (2003b) Characterization of trinucleotide microsatellites in eggplant. Breed Sci 53:77–83
Nunome T, Negoro S, Kono I, Kanamori H, Miyatake K, Yamaguchi H, Ohyama A, Fukuoka H (2009) Development of SSR markers derived from SSR-enriched genomic library of eggplant (Solanum melongena L.). Theor Appl Genet 119:1143–1153
Pessarakli MM, Dris R (2004) Pollination and breeding of eggplants. J New Seeds 2(1):218–219
Powell W, Machray GC, Provan J (1996) Polymorphism revealed by simple sequence repeats. Trends Plant Sci 7:215–222
Prohens J, Blanca JM, Nuez F (2005) Morphological and molecular variation in a collection of eggplant from a secondary center of diversity: implications for conservation and breeding. J Am Soc Hortic Sci 130:54–63
Prohens J, Rodríguez-Burruezo A, Raigón MD, Nuez F (2007) Total phenolics concentration and browning susceptibility in a collection of different varietal types and hybrids of eggplant: implications for breeding for higher nutritional quality and reduced browning. J Am Soc Hortic Sci 132:638–646
Quagliotti L (1979) Floral biology of Capsicum and Solanum melongena. In: Hawkes JG, Lester RN, Skelding AD (eds) The biology and taxonomy of the Solanaceae. Academic Press, New York, pp 399–419
Rodríguez-Burruezo A, Prohens J, Nuez F (2008) Performance of hybrids between local varieties of eggplant (Solanum melongena) and its relation to the mean of parents and to morphological and genetics distances among parents. Eur J Hortic Sci 73:76–83
Rohlf FJ (1993) NTSYS-pc numerical taxonomy and multivariate analysis system. version 1.8. Exeter Software, Setauket, New York
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Schippers RR (2000) African indigenous vegetables. An overview of the cultivated species. CAB International, Wallingford
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18:233–234
Schug MD, Hutter CM, Wetterstrand KA, Gaudette MS, Mackay TFC, Aquadro CF (1998) The mutation rates of di-, tri- and tetranucleotide repeats in Drosophila melanogaster. Mol Biol Evol 5:1751–1760
Seck A (2000) Breeding procedures and results of indigenous vegetables: examples of African eggplant Solanum aethiopicum and okra Abelmoschus spp. Acta Hortic 522:195–208
Sidhu AS, Bal SS, Behera TK, Rani M (2004) An outlook in hybrid eggplant breeding. J New Seeds 6(2/3):15–29
Singh AK, Singh M, Singh AK, Singh R, Kumar S, Kalloo G (2006) Genetic diversity within the genus Solanum (Solanaceae) as revealed by RAPD markers. Curr Sci 90(5):711–716
Sneath PHA, Sokal RR (1973) Numerical taxonomy. W.H. Freeman, San Francisco
Squirrel J, Hollingsworth PM, Woodhead M, Russell J, Lowe AJ, Gibby M, Powell W (2003) How much effort is required to isolate nuclear microsatellites from plants. Mol Ecol 12:1339–1348
Stàgel A, Portis E, Toppino L, Rotino GL, Lanteri S (2008) Gene-based microsatellite development for mapping and phylogeny studies in eggplant. BMC Genomics 9:357
Staub JE, Serquen FC, Gupta M (1996) Genetic markers, map construction and their application in plant breeding. HortScience 31:729–741
Stommel JR, Whitaker BD (2003) Phenolic acid content and composition of eggplant fruit in a germplasm core subset. J Am Soc Hortic Sci 128:704–710
Varshney RK, Graner A, Sorrels ME (2005) Genetic microsatellite markers in plants: features and applications. Trends Biotechnol 23:48–55
Weese TL, Bohs L (2010) Eggplant origins: out of Africa, into the Orient. Taxon 59:49–56
Wright S (1965) The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19:395–420
Xu YB, Crouch JH (2008) Marker-assisted selection in plant breeding: from publications to practice. Crop Sci 48(2):391–407
Acknowledgments
This work was funded by the Ministerio de Ciencia y Tecnología (AGL2009-07257 and RF-2008-00008-00-00), Generalitat Valenciana (ACOMP/2011/032) and Universitat Politècnica de València (PAID-05-10-2318).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vilanova, S., Manzur, J.P. & Prohens, J. Development and characterization of genomic simple sequence repeat markers in eggplant and their application to the study of diversity and relationships in a collection of different cultivar types and origins. Mol Breeding 30, 647–660 (2012). https://doi.org/10.1007/s11032-011-9650-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-011-9650-2