Abstract
2-Amino-3-cyano-4H-chromenes are structural core motifs that received increasing attention in the last years due to their interesting potential pharmacological properties. In this review, the synthetic methods for these compounds are classified based on the type of catalyst in the pertinent reactions. In addition, the wide range of pharmacological properties of these compounds is covered in a separate section.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
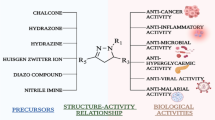
Polysubstituted 2-amino-4H-pyran-3-carbonitrile derivatives are among very important heterocyclic compounds with a wide range of interesting biological activities (Table 1) [1,2,3,4,5,6,7,8,9]. Their huge potential in drug discovery has inspired a wide array of synthetic work, and the conception of a new modified polycyclic framework based on this core structure has stimulated an extensive effort and attention. Furthermore, because of their orthogonal functional groups, these compounds can be considered as key intermediates for subsequent transformations.
Several strategies toward the synthesis of 2-amino-4H-pyran-3-carbonitriles in racemic or enantiomerically pure form have been described. The most useful and preferred method for the construction of these heterocyclic compounds is the multicomponent reaction (MCR) of aldehydes or isatin with malononitrile and β-ketoesters, diverse enolizable C-H-activated acidic compounds and phenols in the presence or absence of a catalyst (Scheme 1).
In 2015, a review article was published describing the different organocatalyzed synthetic strategies for the construction of chiral 2-amino-3-cyano-4H-chromene derivatives [10]. But despite their importance in synthetic, biological and medicinal chemistry, a comprehensive summary on the synthetic methodologies and different applications of polysubstituted 2-amino-4H-pyran-3-carbonitrile derivatives has not been reported. Hence, in this review, we aim to classify the existing synthetic methods for the construction of new 2-amino-4H-pyran-3-carbonitriles and their heterocyclic-fused analogs based on the type of the catalyst. In addition, the potential pharmacological or biological properties of these compounds will be discussed. In this regard, the content is divided into two main sections, namely description of catalytic synthesis strategies and evaluation of biological properties of scaffolds.
Synthetic entries for polysubstituted 2-amino-4H-pyran-3-carbonitriles based on the type of the catalyst
Common acid/base catalysts
Kidwai et al. [11] reported a green synthesis of substituted 2-amino-4H-chromene and benzo[e]chromene derivatives (5, 6) in good-to-high yields (87–93%). The condensation reaction of aldehydes 1, malononitrile 2 and resorcinol 3/β-naphthol 4 afforded the desired products 5/6, respectively, in aqueous K2CO3 under microwave irradiation (Scheme 2).
In a similar work, Dinh Thanh et al. [12] synthesized 2-amino-7-hydroxy-4H-chromene-3-carbonitriles 8 in moderate-to-high yields (62–92%). These three-component reactions were carried out with aromatic aldehydes 7, malononitrile 2 and resorcinol 3 using sodium carbonate as catalyst in ethanol (Scheme 3).
Kidwai et al. [13] in another paper reported the synthesis of 2-amino-6-(1H-benzo[d]imidazol-2-ylthio)-chromenes 14 in moderate-to-good yields (46–74%) by the multicomponent coupling of p-bromophenol 10 with 2-benzimidazolethiols 9, malononitrile 2 and substituted aldehydes 1 (Scheme 4). The reaction is initiated by the in situ generation of 4-(1H-benzo[d]imidazol-2-ylthio)phenol 11 from the reaction of p-bromophenol 10 with 2-benzimidazolethiols 9 in aqueous K2CO3. The Knoevenagel condensation of malononitrile 2 and the substituted aldehydes 1, the phenol ortho-C-alkylation and then subsequent nucleophilic attack by phenolic OH group to the CN affords the final 2-amino-2-chromenes 14 (Scheme 5).
Karimi-Jaberi et al. [14] reported the synthesis of a new series of 2-amino-4H-pyran-3-carbonitriles 16. In their procedure, the reaction of malononitrile 2 and α,α´-bis(arylidene) cycloalkanones 15 in ethanol with K2CO3 as a catalyst led to the desired products 16 in good-to-excellent yields (75–95%) (Scheme 6).
Karami et al. [15] reported a good procedure for the preparation of new pyrano[2,3-H]coumarin derivatives 18 from the reaction of 5,7-dihydroxy-4-substituted coumarins 17, malononitrile 2 and aromatic aldehydes 7 in the presence of K2CO3 as a basic catalyst. The pyrano[2,3-h]coumarin derivatives 18 resulted in good-to-excellent yields (78–98%) (Scheme 7).
Rbaa et al. [16] reported the synthesis of new pyran derivatives based on 8-hydroxyquinoline 21 in good-to-high yields (80–93%). Initially, p-substituted benzaldehydes 19 reacted with malononitrile 2 in the presence of calcium carbonate (CaCO3) in absolute ethanol to afford intermediates 20. Then, 2-amino-4-aryl-4H-pyrano[3,2-h]quinoline-3-carbonitriles 22 was obtained from the reaction of 8-hydroxyquinoline 21 with intermediates 20 in the same conditions (Scheme 8).
Mohan and Bahulayan synthesized the triazole-linked chromene peptidomimetics 30 from the reaction of chromene alkynes 24 with α-acyl amino acetamide azides (Ugi azides) 29 [17]. The chromene alkynes 24 were synthesized initially with grinding of β-naphthol 4, malononitrile 2 and propargylated aldehydes 23 in the presence of sodium carbonate in a solvent-free process. The α-acyl amino acetamide azides “Ugi azides” 29 were synthesized from Ugi four-component reaction of aldehydes 1, 2-chloro acetic acid 25, amines 26 and pivalonitrile 27 and then an azide substitution in the Ugi reaction product 28. Finally, 1,4-disubstituted regioisomer of the triazole peptidomimetic 30 obtained in good yields (72–82%) from the click cycloaddition reactions between the alkynes 24 and azides 29 as shown in Scheme 9.
Yao et al. [18] reported an efficient procedure for the synthesis of 7-aryl-1,1-dioxothieno[3,2-b]pyrans 32 in good-to-high yields (75–89%). The reaction of aryl aldehydes 7, tetrahydrothiophene-3-one-1,1-dioxide 31 and malononitrile 2 was carried out in the presence of ammonium acetate and led to the desired derivatives 32 under solvent-free conditions at room temperature (Scheme 10).
Damavandi [19] reported the reaction of aromatic aldehydes 7, malononitrile 2 and indolin-3-one 33 in the presence of ammonium acetate as the catalyst in ethanol. As a result, 2-amino-4,5-dihydro-4-arylpyrano[3,2-b]indole-3-carbonitriles 34 were obtained in good-to-high yields (75–91%) (Scheme 11).
Another example of the synthesis of 2-amino-3-cyano-1,4,5,6-tetrahydropyrano[3,2-c]quinolin-5-one derivatives 36 in good-to-excellent yields (80–95%) in the presence of ammonium acetate as the catalyst was reported by Lei et al. [20]. In this study, the products 36 were obtained from the one-pot condensation of 4-hydroxyquinolin-2(1H)-one 35, aldehydes 1 and malononitrile 2 in refluxing EtOH in short reaction time and excellent yields (Scheme 12).
Ghadari et al. [21] reported an interesting stepwise procedure for the preparation of the functionalized pyranoquinoxaline derivatives 40 in the presence of trimethylamine. At the first step, quinoxaline derivatives 39 were synthesized from the reaction of diaminomaleonitrile 37 with 2-hydroxy-1,4-naphthoquinone 38 in acetic acid at room temperature. Subsequently, the three-component reaction of the synthesized quinoxaline 39 with aromatic aldehydes 7 and malononitrile 2 in the presence of triethylamine in CH3CN/EtOH (2:1) produced the functionalized pyrano[f]quinoxaline derivatives 40 in good-to-high yields (71–93%) (Scheme 13).
Gein et al. [22] reported the synthesis of ethyl 6-amino-4-aryl-5-cyano-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates 43 through a four-component reaction of the sodium salt of diethyloxaloacetate 41, aldehydes 1, hydrazine hydrate 42 and malononitrile 2. The products 43 were obtained in good-to-high yields (71–92%) in acetic acid (Scheme 14).
Wang et al. [23] devised two-step multicomponent tandem synthesis of highly functionalized benzo[a]pyrano[2,3-c]phenazine derivatives 47 from microwave-assisted reaction of 2-hydroxynaphthalene-1,4-dione 38, diamines 44, aromatic aldehydes 7 and malononitrile 2 (Scheme 15). The reaction started by the condensation of 2-hydroxynaphthalene-1,4-dione 38 and diamine 44 to afford benzo[a]phenazin-5-ol 45. Condensation of aromatic aldehydes 7 with malononitrile 2 produced 2-benzylidenemalononitriles 46 which after Michael addition with benzo[a]phenazin-5-ol 45 and subsequent cyclization afforded benzo[a]pyrano[2,3-c]phenazine derivatives 47 in good-to-high yields (81–92%).
Shestopalov et al. [24] developed an efficient procedure for the synthesis of isomeric isothiazolothienopyranopyridines (53, 54) through a two-step process (Scheme 17). The synthesis started from alkylation of dithiolate 48 by ethyl 4-chloroacetoacetate and then iodomethane to generate compound 50. The reverse order of alkylation gives isomer 49. The key thienopiridine 51 furnished by cyclization of 50 in the presence of sodium ethylate in refluxing ethanol and isomeric thienopiridine 52 also was obtained from cyclization of compound 49 in refluxing trimethylamine (Scheme 16).
The resulting isomeric compounds (51, 52) were used in three-component reaction with the malononitrile 2 and aromatic aldehydes 7 to prepare a wide range of isomeric isothiazolothienopyranopyridines 53 and 54 in (75–88%) and 30% yields, respectively (Scheme 17).
Khodabakhshi et al. [25] reported a one-pot multicomponent process for the synthesis of pyrano[c]chromenes containing an aroyl group 57 in good-to-high yields (70–93%). In this process, the reaction of 4-hydroxycoumarin 55 with various aryl glyoxals 56 and malononitrile 2 in the presence of ammonium dihydrogen phosphate as a catalyst in EtOH/H2O (1:1) afforded the corresponding products 57 (Scheme 18).
Han et al. [26] synthesized 2-amino-7-methoxy-4-aryl-4H-chromene-3-carbonitrile derivatives 59 in high yields (88–93%) through condensation of β-dicyanostyrenes 46 with 3-methoxyphenol 58 in the presence of piperidine in absolute ethanol (Scheme 19). Precursor β-dicyanostyrenes 46 were prepared from the reaction of aromatic aldehydes 7 and malononitrile 2 in ethanol using KF.2H2O as a catalyst.
Molla and Hussain [27] successfully developed an efficient method for the synthesis of dihydropyrano[3,2-c]chromenes 61 in good yields (83–87%). These compounds 61 were synthesized through three-component reaction of 4-hydroxycoumarin 55, dimethylacetylenedicarboxylate (DMAD) 60 and malononitrile 2 in refluxing water in the presence of borax as a catalyst (which upon hydrolysis in water produces a hydroxyl anion (Brønsted base) and boric acid (Lewis acid) (Scheme 20).
El-Ablak et al. [28] presented the synthesis of functionalized 2-amino-7-oxo-4,5,6,7-tetrahydropyrano[2,3-c]pyrrole-3-carbonitriles 63 in good yields (72–78%). The desired products 63 were obtained by the Michael addition of 1,5-diaryl-2,3-dioxopyrrolidines 62 with α-cyanocinnamonitriles 46 in the presence of sodium ethoxide as a catalyst (Scheme 21).
Wang et al. [29] disclosed a tandem annulation reaction of o-ethynylanilines 62, aldehydes 1 and malononitrile 2, affording functionalized tricyclic pyranoquinoline derivatives 65 with excellent functional groups in moderate-to-good yields (38–70%) (Scheme 22). The reaction starts with proton-activated highly selective [2 + 2] cycloaddition of C–C triple bond with C=O to afford 2H-oxete ion intermediate A. The ring opening of the resulting intermediate A produces enone B which after subsequent intramolecular Michael addition and aldol condensation with aldehydes 1 results in product 64. The base-promoted [4 + 2] cycloaddition reaction of product 64 with nucleophilic malononitrile 2 affords pyranoquinoline derivatives 65 (Scheme 23).
Almansour et al. [30] reported a four-component reaction for the construction of an inseparable mixture of two diastereomeric 4(H)-pyrans 70 in excellent yields (94–97%). These diastereomers 70 were obtained from the reaction of (R)-1-(1-phenylethyl)tetrahydro-4(1H)-pyridinone 69, aromatic aldehydes 7 and malononitrile 2 in the presence of solid sodium ethoxide under solvent-free conditions (Scheme 24).
Aghbash et al. [31] synthesized derivatives of 2-amino-6-(azidomethyl)-8-oxo-4-aryl-4,8-dihydropyrano[3,2-b]pyran-3-carbonitrile 73 (Scheme 25). The reaction of 71 with thionyl chloride at room temperature followed by the reaction with sodium azide (NaN3) in dry DMF formed 2-(azidomethyl)-5-hydroxy-4H-pyran-4-one 72. The reaction of 72 with Knoevenagel adducts 46 afforded 2-amino-6-(azidomethyl)-8-oxo-4-aryl-4,8-dihydropyrano[3,2-b]pyran-3-carbonitriles 73 in high-to-excellent yields (90–99%).
Rajasekhar et al. [32] developed a new method for the preparation of 2-amino-3-cyano-4H-chromen-4-ylphosphonates 76 in high yields (77–91%). These compounds 76 were synthesized from the reaction of substituted salicylaldehyde 74, malononitrile 2 and trialkyl phosphite 75 in the presence of iodine in water as a catalyst at room temperature (Scheme 26).
Makawana et al. [33] reported the synthesis of a new series of pyrano[4,3-b]pyran 81 and pyrano[3,2-c]chromene 83 derivatives bearing a 2-thiophenoxyquinoline nucleus in good-to-high yields (64–94%). This method was carried out via the reaction of 2-(thiophenoxyquinoline)-3-carbaldehydes 79, 6-methyl-4-hydroxypyran-2-one 80/4-hydroxy-6-(un)-substituted-2H-chromen-2-one 82 with malononitrile 2 at room temperature in the presence of KOH as a basic catalyst (Scheme 27). In this approach, 2-(thiophenoxy)quinoline-3-carbaldehydes 79 was generated by refluxing 2-chloroquinoline-3-carbaldehydes 77 and various thiophenols 78 in the presence of anhydrous potassium carbonate in dry DMF.
Rao et al. [34] described an efficient and convenient procedure for the synthesis of tetrahydrobenzo[b]pyrans 85 in good-to-high yields (86–93%). In their method, tetrahydrobenzo[b]pyrans 85 were produced from the reaction of substituted aromatic aldehydes 7, dimedone 84 and malononitrile 2 in the presence of potassium tertiary butoxide as base in methanol at room temperature (Scheme 28).
Makawana et al. [35] reported the synthesis of fused pyran derivatives bearing 2-morpholinoquinoline nucleus (87–94) from the reaction of 2-morpholinoquinoline-3-carbaldehydes 86 and malononitrile 2 with a variety of C–H-activated compounds. These reactions were conducted in the presence of NaOH as basic catalyst under microwave irradiation (Scheme 29). 2-Morpholinoquinolines 86 were synthesized through the reaction of 2-choloroquinolines 77 with morpholine catalyzed by K2CO3 in DMF.
Organo/organometal/natural catalysts
Olyaei et al. reported the synthesis of a new class of benzo[f]chromene derivatives 97 in good yields (78–85%) (Scheme 30). They introduced a one-pot three-component condensation reaction of 2,3-dihydroxynaphthalene 95, malononitrile 2 and aromatic aldehydes 7 using guanidine hydrochloride 96 as the catalyst under solvent-free conditions [36].
Mansoor et al. [37] described the synthesis of 3,4-dihydropyrano[3,2-c]chromenes 99 and 6-amino-5-cyano-4-phenyl-2-methyl-4H-pyran-3-carboxylic acid ethyl esters 100 in the presence of thiourea dioxide (an efficient, reusable organic catalyst) in aqueous medium. The products 99/100 were obtained through one-pot, three-component reaction of aromatic aldehydes 7, malononitrile 2 and 4-hydroxycoumarin 55/alkyl acetoacetates 98, respectively, in good-to-excellent yields (86–96%) (Scheme 31).
Brahmachari and Banerjee [38] conducted one-pot reaction of aldehydes 1, malononitrile 2 and a variety of C–H-activated acids using urea as an inexpensive organocatalyst in aqueous ethanol at room temperature (Scheme 32). Accordingly, the functionalized 2-amino-3-cyano-4H-pyrans were synthesized in good-to-excellent yields (83–95%).
Wiener et al. [39] prepared Z-amino-3-cyano-4-phenyl-5-oxo-γ-pyrano[3,2-c][I]benzopyrane 112 from the reaction of 4-hydroxycoumarin 55 and benzylidenemalononitrile 46 in the presence of pyridine as a catalyst (Scheme 33).
Zhang et al. [40] reported a four-component reaction of carbonyl compounds 113, hydrazine hydrate 42, malononitrile 2 and β-keto esters 114 catalyzed by meglumine 115 (an amino sugar derived from glucose) in EtOH-H2O at room temperature (Scheme 34). Under these conditions, a series of dihydropyrano[2,3-c]pyrazole derivatives 116 were obtained in high yields (85–95%).
Abdelrazek and Gomha [41] synthesized a series of pyrano[2′,3′:4,5]pyrimido[2,1-b][1, 3, 5]thiadiazine 119 and pyrano[2,3-d][1, 2, 4]triazolo[4,3-a]pyrimidine 120 derivatives in good yields (67–83%). The treatment of 2,4-dichlorobezaldehyde 117, malononitrile 2 and with appropriate active methylene compounds 118 (catalyzed by chitosan in refluxing dioxane) led to products 119 and 120 (Scheme 35).
Huynh et al. [42] synthesized coumarin analogs 125 in good yields (77–80%) by three-component reaction of diketones 124 with malononitrile 2 and acetaldehyde in the presence of N-methylmorpholine in EtOH (Scheme 36). At first, for the synthesis of diketones 124, diethyl-2-oxopropyl-phosphonate 122 reacted with aldehydes 121 in the presence of NaH in THF to generate the conjugated ketones 123. The addition of diethylmalonate to conjugated ketones 123 in the presence of NaOEt in abs EtOH, followed by hydrolysis of the ester in solution of NaOH and subsequent decarboxylation in H2SO4, afforded diketones 124.
Yan et al. [43] synthesized 6-amino-1,3-disubstituted-4-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles 130 in low-to-high yields (14–90%). These compounds 130 were produced from the reaction of 1,3-disubstituted-1H-pyrazol-5(4H)-one 127, various substituted aromatic aldehydes 128 and malononitrile 2 in the presence of N-methylmorpholine in EtOH at room temperature (Scheme 37). 1,3-Disubstituted-1H-pyrazol-5(4H)-one compounds 127 were produced by the reaction of hydrazines 126 with 3-oxo-esters 114 in EtOH.
Hansen et al. [44] reported the synthesis of 2-amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles 132. In two-step protocol B, the aldehydes 1 and malononitrile 2 generated an intermediate condensation products 12, which then reacts with the 1,3-diones 131 to form the final product 132, whereas in one-pot protocol A, three-component reaction of 1,3-diones 131, malononitrile 2 and substituent aldehydes 1 provided the final product 132 in low-to-excellent yields (22–98%). The reactions were carried out in the presence of N-methylmorpholine in EtOH at room temperature (Scheme 38).
Mahdavi et al. [45] synthesized 2-amino-4H-Pyran derivatives 85 in high yields (88–95%) from the three-component reaction of dimedone 84, aromatic aldehydes 7 and malononitrile 2 under the similar conditions (Scheme 39).
Jirandehi and Mirzaiean [46] reported the synthesis of pyrano[3,2-c]pyridines derivatives 134 in moderate-to-excellent yields (65–94%). The one-pot condensation of malononitrile 2, ethyl acetoacetate 133 and aryl aldehydes 7 in the presence of piperazine as a catalyst under solvent-free media conditions by a microwave-assisted process afforded the desired products 134 (Scheme 40).
Jayarajan and Vasuki [47] synthesized skeletally diverse polycyclic/spirocyclic heterocyclic compounds (137, 138) in moderate-to-excellent yields (60–99%) using piperidine as the catalyst in water at room temperature. Piperidine efficiently catalyzed the reaction of malononitrile 2, 4-hydroxy-1,3-dimethyl-1H-pyrazolo[3,4-b]pyridine-6(7H)-one 135 (a novel heterocyclic active methylene compound) and aldehydes 1/isatin derivatives 136 to afford 137/138, respectively (Scheme 41).
Using one-pot multicomponent reaction of ethyl 3-(2,6-dichloro-5-fluoropyridin-3-yl)-3-oxopropanoate 139, malononitrile 2 and substituted aromatic aldehydes 7, Ye et al. [48] were able to prepare polyfunctionalized 4H-pyran derivatives bearing fluorochloro pyridyl moiety 140. These products 140 were isolated in good-to-high yields (81–92%) in the presence of piperidine (Scheme 42).
Ji et al. [49] described the one-pot multicomponent reaction of aldehydes 1, malononitrile 2 with 3-cyanoacetyl indoles 141 in the presence of piperidine under ultrasonic irradiation. As a result, a series of polysubstituted indol-3-yl-substituted pyran derivatives 142 were synthesized in good-to-high yields (74–92%) (Scheme 43).
Murali et al. [50] reported a facile and efficient, three-component synthesis of pyrano[2,3-a]carbazoles 144 in moderate-to-excellent yields (65–95%). The reaction of 2,3,4,9-tetrahydro-1H-carbazol-1-ones 143, malononitrile 2 and aromatic/heteroaromatic aldehydes 7 with catalytic amount of piperidine in refluxing DMF produced the desired products 144 (Scheme 44).
Kemnitzer et al. [51] synthesized the 2-amino-3-cyano-7-(dimethylamino)-4H-chromenes 146 in low-to-high yields (17–93%). Aldehydes 1 were treated with 3-dimethylaminophenol 145 and malononitrile 2 in the presence of piperidine in EtOH at room temperature to afford the corresponding products 146 (Scheme 45).
Cai et al. [7] also reported the synthesis of 2-amino-4-(3-bromo-4,5-dimethoxyphenyl)-3-cyano-4,7-dihydro-7-methyl-pyrano[2,3-e]indoles 150 in moderate yields (66%) as another derivatives of 4-aryl-4H-chromene (Scheme 46). These compounds 150 were synthesized from one-pot reaction of substituted 3-bromo-4,5-dimethoxybenzaldehyde 149 and 4-hydroxy-1-methyl-1H-indole 148 with malononitrile 2 which was catalyzed by piperidine in EtOH at room temperature. 4-Hydroxy-1-methyl-1H-indole 148 was produced by methylation and then deprotection of 4-Hydroxy-1H-indole 147.
Later on, Akbarzadeh et al. [52] reported the synthesis of a new series of 4-aryl-4H-chromenes bearing a 5-arylisoxazol-3-ylmoiety at the C-4 position 156 (Scheme 47). Initially, ethyl 5-arylisoxazole-3-carboxylates 152 was formed from the reaction of ethyl-2,4-dioxo-4-arylbutanoate derivatives 151 with hydroxylamine hydrochloride. Addition of sodium borohydride to 152 produced 5-arylisoxazol-3-ylmethanol derivatives 153. Oxidation of 5-arylisoxazol-3-ylmethanols 153 by MnO2 led to 5-arylisoxazole-3-carboxaldehydes 154 which its reaction with malononitrile 2 and 3-(dimethylamino)phenol 155 in the presence of piperidine in EtOH gave 2-amino-7-dimethylamino-4-(5-arylisoxazol-3-yl)-4H-chromene-3-carbonitriles 156 in low-to-moderate yields (18–45%).
Chen et al. [53] used piperidine as a catalyst for the synthesis of racemic 2-aminopyranopyridine-3-carbonitriles 158 in excellent yields (92–99%). These products 158 were prepared from the reaction of dienones 157 and malononitrile 2 in EtOH at room temperature (Scheme 48).
Ramani et al. [54] reported the synthesis of other derivatives of 4-aryl-4H-chromene-3-carbonitrile 161 from a one-pot reaction of m-substituted benzaldehydes 160, malononitrile 2 and substituted phenols 159. The products 161 were isolated in good yields (70–85%) using piperidine at 60–80 °C in ethanol (Scheme 49).
Once again, piperidine was used as a catalyst in a one-pot three-component synthesis of 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles 132 by Erichsen et al. [5]. The reaction of aldehydes 1 and malononitrile 2 generates an intermediate condensation product 12, which then reacts with the diketones 131 to form 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles 132 in moderate-to-high yields (42–91%) (Scheme 50).
Balalaie et al. [55] described a simple procedure for the synthesis of dihydropyrano[3,2-c]chromene derivatives 112 in moderate-to-high yields (55–92%) by a three-component reaction of aromatic aldehydes 7, malononitrile 2 and 4-hydroxycoumarin 55. These reactions were carried out in the presence of three different catalysts, namely piperidine, triethylamine and sodium carbonate, in aqueous media in H2O: EtOH(1:1) (Scheme 51).
Gaikwad et al. [56] synthesized 2-amino-4-(-4-substituted phenyl)-6-(naphtho[2,1-b]furan-2-yl)-4H-pyran-3-carbonitriles 164 from the reaction of substituted chalcones 163 with malononitrile 2 catalyzed by piperidine (Scheme 52). Initially, 3-(4-hydroxyphenyl)-1(naphtha[2,1-b]furan-2yl) prope-2-en-1-ones 163 was synthesized from Claisen–Schmidt condensation of 2-acetylnaphtho[2,1-b]furan 162 and substituted benzaldehydes 7 in ethanol and aqueous solution of potassium hydroxide. In the next step, the reaction of synthesized 3-(4-hydroxyphenyl)-1(naphtha[2,1-b]furan-2yl)prope-2-en-1-ones 187 with malononitrile 2 in ethanol in the presence of piperidine at room temperature afforded 2-amino-4-(-4-substituted phenyl)-6-(naphtho[2,1-b]furan-2-yl)-4H-pyran-3-carbonitriles 164 in moderate yields (59–67%).
Li et al. [57] developed the synthesis of functionalized 4H-pyrans 166 in moderate-to-excellent yields (62–96%). These compounds 166 were achieved by the reaction of α,β-enones 165 and malononitrile 2 catalyzed by piperidine in ethanol at room temperature (Scheme 53).
Kalaria et al. [2] synthesized 4-(5-(1H-imidazol-1-yl)-3-methyl-1-phenyl-1H-pyrazol-4-yl)-4H-pyran-2-amine derivatives 171 in moderate-to-good yields (66–86%) from the cyclocondensation reaction of 5-(1H-imidazol-1-yl)-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde 169, malononitrile 2 and enolizable ketones/phenols 170 in the presence of piperidine under microwave irradiation (Scheme 54). The starting material 5-(1H-imidazol-1-yl)-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde 169 was prepared by nucleophilic displacement of the chloro group in 5-chloro-3-methyl-1-phenyl-1Hpyrazole-4-carbaldehyde 167 with nitrogen atom of imidazole 168 in refluxing DMF with anhydrous potassium carbonate as a catalyst.
Sashidhara et al. [58] developed the synthesis of a new class 3-aryl coumarin-based aminopyran derivatives 175. Firstly, the Duff reaction on o-substituted phenols 172 in the presence of hexamethylenetetramine (HMTA) and TFA at 120 °C generated aromatic dicarbaldehydes 173. Reaction of the resulting dicarbaldehyde 173 with different substituted phenyl acetic acids in the presence of cyanuric chloride and N-methylmorpholine (NMM) in DMF afforded the corresponding 3-aryl coumarin aldehydes 174. Finally, the multicomponent reaction of 3-aryl coumarin aldehydes 174 with malononitrile 2 and dimedone 84 in DMAP in refluxing EtOH furnished 3-aryl coumarin-based aminopyran derivatives 175 in good yields (Scheme 55).
Kaur et al. [6] used DMAP for the synthesis of 4-aryl/heteroaryl-4H-fused pyrans in moderate-to-high yields (57–88%). The multicomponent reaction of aromatic aldehyde 7, malononitrile 2 and C-H-activated acidic compounds under microwave irradiation afforded the desired products as shown in Scheme 56.
Mungra et al. [59] prepared β-aryloxyquinoline-3-carbaldehydes 184 by Vilsmeiere–Haack reaction of 2-chloro-3-formylquinolines 77 with phenols 183 using anhydrous potassium carbonate as a base in refluxing dimethylformamide. Subsequent one-pot three-component cyclocondensation of β-aryloxyquinoline-3-carbaldehydes 184 with malononitrile 2 and 4-hydroxycoumarins 185 in ethanol which was catalyzed by piperidine afforded pyrano[3,2-c]chromene derivatives 186 in moderate-to-good yields (63–82%) (Scheme 57).
Seydimemet et al. [60] reported a simple approach to the synthesis of coumarin-containing dihydropyrano[2,3-c]pyrazoles 188 via four-component reaction of β-dicarbonyl compound 187, phenylhydrazine 126, aromatic aldehydes 7 and malononitrile 2. The products 188 were obtained in good-to-high yields (78–90%) in the presence of l-proline as organocatalyst in EtOH under ultrasonic irradiation (Scheme 58).
Li et al. [61] also used l-proline in the synthesis of spirooxindole derivatives 191 in good-to-high yields (76–95%) by three-component reaction of isatins 189, malononitrile 2 (cyanoacetic ester) and 1,3-dicarbonyl compounds 190 in water (Scheme 59).
In another l-proline-catalyzed reaction, Poursattar Marjani et al. [62] synthesized 2-amino-4-aroyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles 194 in high yields (86–92%) from the reaction of arylglyoxal monohydrates 192, 1,3-diketones 193 and malononitrile 2 in ethanol (Scheme 60).
Baitha et al. [8] employed imidazole 168 as a catalyst to promote one-pot three-component coupling reaction of 2-ethoxybenzo[d][1, 3]dioxole-5-carbaldehyde 197, malononitrile 2 and different active methylene groups in mixture of EtOH and H2O (1:1) at room temperature. Under these circumstances, a new class of substituted 2-amino-4-(2-ethoxybenzo[d][1, 3]dioxol-5-yl)-4H-pyran-3-carbonitriles (201–206) were produced in high yields (88–92%) (Scheme 61). 2-Ethoxybenzo[d][1, 3]dioxole-5-carbaldehyde 197 was synthesized via treatment of 3,4-dihydroxybenzaldehyde 195 with triethyl orthoformate 196 in the presence of acetic acid in refluxing ethanol.
Kalla et al. [63] described for the first time the use of dibutylamine as a highly efficient organocatalyst in a multicomponent reaction of substituted salicylaldehydes 74, malononitrile 2 and dialkylphosphites 207 in ethanol at ambient temperature. In this process, 2-amino-3-cyano-4H-chromen-4-ylphosphonates 76 were synthesized in good-to-excellent yields (86–96%) (Scheme 62).
Satheesh et al. [64] reported the synthesis of spirooxindoles incorporating 2-aminopyran-3-carbonitrile unit 211 from the reaction of isatilidenes 209 and diethyl 1,3-acetone dicarboxylates 210 using triethylamine as an organic base in ethanol at room temperature. The desired products 211 were isolated in high-to-excellent yields (89–98%) (Scheme 63). A one-pot reaction of diethyl 1,3-acetone dicarboxylate 210, malononitrile 2 and isatin 136 was also conducted which resulted in the same product 211 (R = H, R1 = Et).
Shaabani et al. [65] reported an efficient method for the synthesis of highly functionalized benzo[g]chromene derivatives 212 through addition and subsequent cyclization of 2-hydroxynaphthalene-1,4-dione 38 to the condensation product of the aromatic aldehydes 7 with malononitrile 2 in the presence of a catalytic amount of Et3N. Various aldehydes 7 with electron-withdrawing and electron-donating groups afforded the corresponding benzo[g]chromene derivatives 212 in moderate-to-good yields (65–82%) at room temperature, without any undesirable by-product (Scheme 64).
Larionova et al. [66] reported an original approach for the synthesis of substituted 5H-pyrano[2,3-d]-thieno[3,2-b]pyridines 217 from the substituted thieno[3,2-b]pyridines 216. The synthesis started by the reaction of cyanoacetic acid amides 213 and carbon disulfide to generate monopotassium salt of carbamoylcyanodithioacetic acid 214. Subsequently, regioselective alkylation of compound 214 with ethyl 4-chloroacetoacetate and then consecutive Thorpe–Ziegler and Thorpe–Guareschi reactions in the presence of an excess of KOH in ethanol produced thieno[3,2-b]-pyridine potassium salt 215. Finally, alkylation with alkyl halides afforded compounds 216 (Scheme 65). The three-component reaction malononitrile 2 and aromatic aldehydes 7 with the synthesized thienopyridines 216 in the presence of trimethylamine-produced 5H-pyrano[2,3-d]thieno[3,2-b]pyridines 217 in moderate-to-good yields (58–78%) (Scheme 66).
Litvinov et al. [67] described a new four-component reaction of aldehydes 1, malononitrile 2, ketoesters 114 and hydrazine hydrate 42 (Scheme 67). The reaction was performed in the presence of trimethylamine in refluxing ethanol to afford substituted 6-amino-2H,4H-pyrano[2,3-c]pyrazol-5-carbonitriles 116 in moderate-to-good yields (47–79%).
Preparation of polyalkoxy 4-aryl-4H-chromenes 221 via the three-component domino reaction of polyalkoxybenzaldehydes 218, malononitrile 2 and phenols 220 was reported by Shestopalov et al. [68]. Initially, polyalkoxybenzylidene malononitriles 219 were obtained from the reaction of polyalkoxybenzaldehydes 218 with malononitrile 2 catalyzed by trimethylamine in refluxing EtOH. Michael reaction of polyalkoxybenzylidene malononitriles 219 and phenols 220 and then hetero-Thorpe–Ziegler reaction produced polyalkoxy 4-aryl-4H-chromenes 221 in low-to-good yields (12–82%) (Scheme 68).
Ahmad et al. [69] utilized MCR technique to get pyrano[1, 2]benzothiazines 225 in good-to-excellent yields (72–96%) through the reaction of benzothiazineenolates 224 with malononitrile 2 and various substituted benzaldehydes 7 in the presence of triethylamine as a catalyst (Scheme 69). The mesylation reaction of methyl anthranilate 222 with 223 and then N-benzylation/N-methylation reaction followed by cyclization with sodium hydride provide the required benzothiazineenolates 224.
Abdo and Mohareb [70] reported the synthesis of a series of (2-oxo-2H-chromen-3-yl)-4H-pyran derivatives (227, 229) in moderate-to-good yields (61–77%). These products 227/229 were prepared from a trimethylamine-catalyzed treatment of 3-acetylcoumarin 226/compound 228, malononitrile 2 and various benzaldehydes 7 in ethanol (Scheme 70).
Azzam and Mohareb [71] reported the multicomponent reaction of acetoacetanilide derivatives 230 with aromatic aldehydes 7 and malononitrile 2. Reactions catalyzed by triethylamine were conducted in absolute ethanol which led to 4H-pyran derivatives 231 in moderate yields (58–62%) (Scheme 71).
Mohareb and MegallyAbdo [72] used 3-bromoacetylcoumarin 232 for the synthesis of 2-amino-3-cyano-pyran derivatives 233. 3-(2-Bromoacetyl)-2H-chromen-2-ones 233 were synthesized using three-component reaction of 3-bromoacetylcoumarin 232 (prepared from 3-bromoacetylcoumarin and potassium cyanide) with aromatic aldehydes 7 and malononitrile 2 in the presence of trimethylamine, respectively (Scheme 72).
Padmaja et al. [73] conducted a simple one-pot, three-component reaction of aromatic aldehydes 7, malononitrile 2 and 4-hydroxycarbazoles 234 catalyzed by trimethylamine in ethanol at room temperature. As they reported, pyrano[3,2-c]carbazole derivatives 235 were obtained in high yields (80–90%) under these circumstances (Scheme 73).
Costa et al. [74] reported the synthesis of novel 2-iminochromene dimers 237 in good yields (76–85%) from the combination of salicylaldehyde 236 with 2 equiv of malononitrile 2 in the presence of triethylamine in methanol at room temperature (Scheme 74).
Kalalbandi et al. [75] developed a synthetic route for the synthesis of biscoumarin fused with dihydropyran ring 239 in good-to-high yields (79–93%). The one-pot multicomponent reaction of 4-hydroxy coumarin 55, formyl coumarin 238 and malononitrile 2 was carried out in the presence of catalytic amount of triethylamine in refluxing methanol (Scheme 75).
Schmitt et al. [9] prepared naphthopyran analogs of LY290181 241 in low-to-good yields (15–71%). These compounds 100 were synthesized from a trimethylamine-catalyzed one-pot reaction of malononitrile 2 with benzaldehydes 7 and 1-naphtholes 240 in acetonitrile at room temperature (Scheme 76).
Upadhyay et al. [76] prepared pyrano[3,2-c]quinoline analogs 243 in good-to-high yields (67–93%) from the reaction of 2,4-dihydroxy1-methylquinolin 242, aromatic aldehydes 7, malononitrile 2 which was catalyzed by triethylamine in refluxing absolute ethanol (Scheme 77). Initially, the base-catalyzed Knoevenagel condensation between un(substituted) aromatic aldehydes 7 and malononitrile 2 results into cinnamic nitrile derivative 46. The reaction of prepared cinnamic nitriles 46 in situ with 2,4-dihydroxy-1-methylquinolin 242 and then Michael addition and cyclization produced pyrano[3,2-c]quinolones 243.
Wu et al. [77] synthesized chiral 2-amino-4H-chromenes 246 in high-to-excellent yields (88–97%) with excellent enantioselectivities. These compounds 246 were produced by the reaction of 2-(1-tosylalkyl)phenols 244 and malononitrile 2 under basic conditions catalyzed by asymmetric bifunctional squaramide 245 (Scheme 78).
Ding and Zhao [78] reported the first enantioselective synthesis of 2-amino-8-oxo-tetrahydro-4H-chromene-3-carbonitriles 249 in low-to-moderate yields (12–64%). The tandem Michael addition–cyclization reaction between cyclohexane-1,2-dione 247 and benzylidenemalononitriles 12 in the presence of chiral cinchona alkaloid-derived thiourea organocatalysts 248 produced the desired products 249 (Scheme 79).
Wang et al. [79] reported a DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) mediated three-component reaction of aldehydes 1, malononitrile 2 and α,β-unsaturated ketone derivatives 250 for the synthesis of a series of novel 2-amino-4H-pyran derivatives 251 in moderate-to-excellent yields (65–96%) (Scheme 80).
Esmati et al. [80] successfully accessed novel dihyrobenzo[h]pyrano[3,2-c]chromene derivatives 253 in good-to-high yields (70–90%). The DABCO (1,4-diazabicyclo[2.2.2]octane) mediated three-component reaction of 4-hydroxy-2H-benzo[h]chromen-2-one 252, aromatic aldehydes 7 and malononitrile 2 in ethanol at room temperature resulted into the products 253 (Scheme 81).
Lu et al. [81] synthesized hybrid molecules of phenazine and pyran 256 in good-to-high yields (77–91%) in two steps. In the first step, 2-amino-3-hydroxyphenazine 255 was easily prepared by benzene-1,2-diamine 254 in the presence of FeCl3 in acidic conditions. Subsequently, the resulting 2-amino-3-hydroxyphenazine 255 was reacted with malononitrile 2 and aromatic aldehydes 7 to afford a series of novel pyrano[3,2-a]phenazine derivatives 256 in the presence of DABCO under reflux conditions in ethanol (Scheme 82).
Nikookar et al. [82] synthesized dihydropyrano[3,2-c]quinoline-2-carbonitrile derivatives 260 in good-to-high yields (81–90%). Initially, the reaction of 1-naphthyl amine 257 and malonic acid 258 in the presence of PPA produced benzo[h]quinolin-2(1H)-one 259. Then, the resulting benzo[h]quinolin-2(1H)-one 259 was reacted with various aromatic aldehydes 7 and malononitrile 2 using DABCO in EtOH to obtain dihydropyrano[3,2-c]quinoline-2-carbonitriles 260 (Scheme 83).
Gao and Du [83] succeeded in developing an efficient enantioselective method for preparation of functionalized 2-amino3-cyano-4-(indol-3-yl)-4H-chromenes 264. Friedel–Crafts alkylation of indoles 261 with iminochromenes 262 were carried out in the presence of a thiourea organocatalyst 263 to afford the desired products 264 in moderate-to-good yields (66–87%) (Scheme 84).
Hu et al. [84] described a cascade conjugate addition–intramolecular cyclization pathway for the synthesis of chiral pyran derivatives 267. The reaction of malononitrile 2 with conformationally restricted dienones 265 catalyzed by piperidine-based thiourea-tertiary amine 266 afforded chiral pyran derivatives 267 in low-to-excellent yields (24–99%) with excellent enantioselectivities (Scheme 85).
Cui et al. [85] developed the enantioselective synthesis of 7H-pyrano[2,3-d]thiazoles 270 in good-to-excellent yields (70–94%) from the reaction of malononitrile 2 with a wide range of 5-ylidenethiazol-4-ones 268. The reaction was catalyzed by bifunctional squaramide derived from L-tert-leucine 269 as a chiral organocatalyst (Scheme 86). Proposed mechanism for this [4 + 2] cyclization reaction is shown in Scheme 87.
Chen et al. [86] developed the first enantioselective organocatalytic three-component reaction via a domino Knoevenagel/Michael/cyclization sequence. Cupreine (CPN) 272 as an organocatalyst was used for the synthesis of optically active spiro[4H-pyran-3,3′-oxindoles] 273 via the reaction of malononitrile 2, isatins 162 and 1,3-diones 271 (Scheme 88). In this CPN-catalyzed method, the products 273 were obtained in good-to-excellent yields (72–96%).
Youseftabar-Miri [87] utilized egg shell as a natural, green catalyst for the synthesis of spiro[4H-pyran-oxindole] derivatives 275 in high yields (88–92%) by one-pot multicomponent reaction of 1,3-diketones 274, isatins 162 and malononitrile 2 (Scheme 89).
Bhosale et al. [88] synthesized 2-amino-4H-chromenes 179 in good yields (72–84%) by mixing malononitrile 2, aromatic aldehyde 7 and α-naphthol 178 in lemon juice (as a natural catalyst) using ultrasound waves (Scheme 90).
Hatamjafari [89] demonstrated glutamic acid as an efficient catalyst for the synthesis of 4H-chromenes 85 in good-to-excellent yields (87–95%) via a multicomponent reaction of dimedone 84, aromatic aldehydes 7 and malononitrile 2 (Scheme 91).
Nanoparticle/composite catalysts
Valekar et al. [90] synthesized indeno-pyran derivatives 277 in good-to-high yields (78–94%). Here, CuO nanoparticles catalyzed the one-pot reaction of malononitrile 2, aromatic aldehydes 7 and indane 1,3-dione 276 in water at an ambient temperature (Scheme 92).
An efficient and green protocol for the synthesis of dihydropyrano[2,3-c]chromene derivatives 279 in good-to-high yields (75–91%) was reported by Paul et al. [91]. In this protocol, a one-pot, three-component coupling reaction of aromatic aldehyde 7, malononitrile 2 and 3-hydroxycoumarin 278 using nanostructured ZnO as the catalyst produces the desired products 279 (Scheme 93). In this reaction, when terephthalaldehyde 280 is employed, compound 281 with self-aggregating property is generated in moderate yield (65%) (Scheme 94).
Rajesh et al. [92] introduced a nanocomposite consisting of reduced graphene oxide and zinc oxide nanoparticles (RGO/ZnO). They used this composite as an amphiphilic heterogeneous catalyst in the reaction of substituted indols 282, malononitrile 2 and salicylaldehyde 236 in water to afford various indolyl-4H-chromenes 283 in high-to-excellent yields (90–97%) (Scheme 95).
Ghashang [93] succeeded in developing a ZnAl2O4–Bi2O3 composite nanopowder-catalyzed one-pot synthesis of 2-4H-chromene-3-carbonitriles 285 in good-to-high yields (75–91%). The reaction of disubstituted phenols 284, aromatic aldehydes 7 and malononitrile 2 in refluxing ethanol/water afforded the corresponding products 285 (Scheme 96).
Azarifar et al. [94] reported the synthesis of new pyranopyridine derivatives (2-amino-4-aryl-5-methyl-7-oxo-7,8-dihydro-4H-pyrano[2,3-b]pyridine-3,6-dicarbonitriles) 287 in good-to-excellent yields (83–98%). They carried out a one-pot three-component reaction between aromatic aldehydes 7, malononitrile 2 and 3-cyano-6-hydroxy-4-methylpyridin-2(1H)-one 286 in the presence of guanidinium chloride-functionalized γ-Fe2O3/HAp magnetic nanoparticles under solvent-free conditions to afford the desired products 287 (Scheme 97).
Pourian et al. [95] reported a one-pot synthesis of 4H-pyran derivatives 289 in good-to-high yields (78–92%) by three-component reaction of various aromatic aldehydes 7, malononitrile 2 and 1,3-dicarbonyl compounds 288. These reactions were conducted in the presence of bionanocatalyst Fe3O4@GA@IG under ultrasonic irradiation in refluxing ethanol (Scheme 98).
Azarifar et al. [96] used a Cu(II)-based Lewis acid as a heterogeneous nanocatalyst for the synthesis of new pyranopyridine derivatives 287 in excellent yields (82–98%). Fe3O4@SiO2-acac-2ATP-Cu(II) catalyzed the one-pot three-component reaction of aromatic aldehydes 7, malononitrile 2 and 3-cyano-6-hydroxy-4-methylpyridin-2(1H)-one 286 to afford 4H-pyrano[2,3-b]pyridine-3,6-dicarbonitriles 287 under solvent-free conditions (Scheme 99).
Maleki et al. [97] introduced a green protocol for multicomponent synthesis of 2-amino-3-cyano-4H-pyranes 100 in good-to-excellent yields (83–95%). For this purpose, Fe3O4/PEO/SO3H nanocatalyst was used in the reaction of aromatic aldehydes 7, malononitrile 2 and methyl/ethyl acetoacetate 98 in absolute EtOH at room temperature (Scheme 100).
Eftekhari-Sis et al. [98] reported the synthesis of various chromene and pyran derivatives in moderate-to-excellent yields (61–96%) (Scheme 101) through a three-component reaction. In this method malononitrile 2, substituted benzaldehydes 7 and phenolic or enolic components such as α-naphthol 178, β-naphthol 4, dimedone 84 or kojic acid 71 were reacted in the presence of 2-[(2-pyridylmethyl)amino]acetic acid (PMAA)-functionalized Fe3O4 superparamagnetic nanorods as a catalyst.
Solhy et al. [99] repeated this three-component reaction (between malononitrile 2, α-naphthol 178 and various aldehydes 1) using nanostructured diphosphate Na2CaP2O7 (DIPH) as a basic catalyst. This aqueous green synthesis method successfully synthesized a series of 2-amino-chromenes 179 in good-to-excellent yields (72–94%) (Scheme 102).
Sagar Vijay Kumar et al. [100] synthesized 2-amino-4-(4-hydroxy-3-methoxy-5-(substituted phenyldiazenyl)-chromene-3-carbonitrile 292 in good-to-high yields (79–93%) from the reaction of 1,3-dicarbonyl compounds 274 (substituted phenyl-diazenyl) benzaldehydes 291 and malononitrile 2. These reactions were catalyzed by nano-CeO2 − ZrO2 in water at room temperature (Scheme 103).
Transition metal catalysts
Baghbanian et al. [101] reported a one-pot three-component reaction between aromatic aldehydes 7, malononitrile 2 and 8-hydroxyquenoline 293 in the presence of Ce-Zr/SiO2 in ethanol that affords pyranoquinolines 294 in high yields (90–95%) (Scheme 104).
Mostafa and Khatab [102] obtained 4H-pyran derivatives 295 in moderate-to-high yields (65–90%) under solvent-free conditions. The desired products 295 were obtained through a one-pot reaction between ethyl acetoacetate (EAA) 102, malononitrile 2 and aromatic aldehydes 7 in the presence of silica-supported V2O5 (V2O5/SiO2) as a catalyst at 80 °C (Scheme 105).
An indium trichloride-catalyzed synthesis of pyrano[3,2‐h]quinolines 294 in high yields (85–90%) was reported by the Kumar et al. [103]. The products 294 were produced by the reaction of malononitrile 2, aromatic aldehydes 7 and quinolin-8-ol 293 in ethanol under microwave irradiation (Scheme 106).
Perumal and Shanthi [104] reported another indium trichloride-catalyzed reaction of salicylaldehydes 74, malononitrile 2 and Hantzsch dihydropyridine ester 296 for the synthesis of new 2-amino-3-cyano-chromenes 297 in high yields (82–88%) in aqueous media (Scheme 107). Reaction of salicylaldehydes 74 with malononitrile 2 produced 2-imino-3-cyanocoumarin intermediate A. Hydride transfer from Hantzsch ester 296 to the electrophilic position of this intermediate A formed the final product 297 (Scheme 108). In this protocol, replacing the Hantzsch dihydropyridine ester 296 with indoles 298 under the same conditions led into the construction of novel indolyl chromenes 299 in high yields (80–87%) (Scheme 107).
When Lakshmi et al. [105] examined the cyclic nucleophiles involving 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one 299, oxindole 298 and dimedone 84 under similar reaction conditions. Accordingly, a series of 4-substituted 2-amino-4H-chromenes (300–302) were synthesized in good yields (79–85%) and short reaction times (Scheme 109).
Yamuna and Rajendra Prasad [106] showed that pyrano[2,3-a]carbazoles (304, 305) could be synthesized in high yields (90–95%) with a one-pot multicomponent reaction of benzaldehyde 7/isatin 136 with malononitrile 2 and 1-hydroxycarbazoles 303 in the presence of InCl3 as a catalyst in acetonitrile at 70 °C (Scheme 110).
Heravi and Daraie [107] developed basic alumina-catalyzed synthesis of 6-amino-8-aryl-7-cyano-8H-[1, 3]dioxolo[4,5-g]chromenes 307 in high-to-excellent yields (92–97%). These compounds 307 were obtained from the condensation of 3,4-(methylenedioxy)phenol 306, aromatic aldehydes 7 and malononitrile 2 in refluxing water (Scheme 111).
Pasdar et al. [108] synthesized tetrahydrochromone derivatives 85 by three-component condensation of benzaldehydes 7, malononitrile 2 and dimedone 84 in the presence of potassium alum in refluxing water. The synthesized tetrahydrochromone derivatives 85 were used as ligand in the synthesis of metal complexes (Scheme 112).
Jabbarzare and Ghashang [109] demonstrated that glass–ceramic catalytic system (Na2O–Al2O3–P2O5) can effectively catalyze the three-component coupling reaction, aromatic aldehydes 7, malononitrile 2 and 4-hydroxy-2(3H)-benzoxazolone 308. These reactions afforded 8-amino-6-aryl-1,2-dihydro-2-oxo-6H-pyrano[2,3-e]benzoxazole-7-carbonitrile derivatives 309 in good-to-excellent yields (69–95%) under reflux conditions in aqueous media (Scheme 113).
Phase transfer catalysts
Ballini et al. [110] reported a three-component one-pot reaction among aromatic aldehydes 7, malononitrile 2 and phenols 310 to prepare 2-amino3-cyano-2-chromenes 311 in moderate-to-excellent yields (60–94%). These reactions were carried out in the presence of cetyltrimethylammonium chloride (CTACl) in 110 °C in water (Scheme 114).
Khurana et al. [111] reported the synthesis of 4H-benzo[g]chromenes 312 in high yields (85–92%) through one-pot condensation of aromatic aldehydes 7, malononitrile 2 and 2-hydroxy-1,4-naphthoquinone 38 using catalytic amount of cetyltrimethylammonium bromide (CTAB) in refluxing water under neat conditions. This reaction was also carried out in 1-butyl-3-methyl imidazolium hydroxide ([bmim]OH) ionic liquid as a catalyst (Scheme 115).
Haouchine et al. [112] synthesized imidazo[1,2-a]pyridine derivatives bearing 2-aminonicotinonitrile or 2-aminochromene moiety 317 using 2-(imidazo[1,2-a]pyridin-2-ylmethylene)malononitriles 315 as starting materials (Scheme 116). 2-(Imidazo[1,2-a]pyridin-2-ylmethylene)malononitriles 315 prepared from the reaction of 2-aminopyridine 313 and 1,1,3-trichloroacetone using the Chichibabin method followed by the reaction with malononitrile 2 in water. The resulting 2-(imidazo[1,2-a]pyridin-2-ylmethylene)malononitriles 315 reacted with quinolinol derivatives 316 catalyzed by TBAB in water to afford the imidazo [1,2-a] pyridine derivatives 317 in good-to-excellent yields (68–98%).
Ren et al. [113] used a supramolecular catalyst for the synthesis of 2-amino-4H-chromenes 5 in good-to-excellent yields (82–95%). These compounds 5 were synthesized from the reaction of substituted aldehydes 1, malononitrile 2 and resorcinol 3 catalyzed by amino-appended β-cyclodextrins (ACDs) in water at room temperature (Scheme 117).
Ionic liquids
Fan et al. [114] reported [bmim]BF4 mediated and promoted multicomponent reaction of aldehyde 1, 4-hydroxy-pyridin-2(1H)-one 318 and malononitrile 2 for preparation of pyrano[3,2-c]pyridone derivatives 319 in good yields (80–88%) (Scheme 118).
Fan et al. [114] following this general procedure, several pyrimidine nucleoside-pyrano[3,2-c]pyridone hybrids 321 synthesized from 5-formyl-20-deoxyuridine 320, 4-hydroxy-6-methyl-2-pyranone 80 and malononitrile 2 in [bmim]BF4 in 80 °C (Scheme 119).
Li et al. [115] reported the synthesis of a series of 2-amino-4-aryl-4H,8H-6-methyl-8-oxo-pyrano[3,2-b]pyran derivatives 323 in good-to-excellent yields (70–99%). The Et3N-catalyzed reaction of aromatic aldehydes 7, malononitrile 2 and 5-hydroxy-2-methyl-4H-pyran-4-one 322 in [bmim]BF4 as solvent afforded the desired products 323 (Scheme 120).
Abbaspour-Gilandeh et al. [116] advanced ([BMIm]Cl)-catalyzed synthesis of pyran motifs compounds (36, 325) without using any solvent or additional catalyst. This protocol was carried out by multicomponent condensation of malononitrile 2, aromatic aldehydes 7 and 4-hydroxy-6-methylpyridin-2(1H)-one 324/4-hydroxyquinolin-2(1H)-one 35 (Scheme 121).
Tashrifi et al. [117] also planned and used ionene sulfuric acid ISA as both acidic catalyst and solvent in the synthesis of pyrano[3,2-c]quinolines 36 and pyrano[2,3-d]pyrimidines 327 in high yields (80–92%). The synthesis of these compounds 36/327 was performed by the three-component reaction of 4-hydroxyquinolin-2-one 35/6-hydroxytetrahydropyrimidin-4-one 326 with malononitrile 2 and substituted aromatic aldehydes 7, respectively (Scheme 122).
Mane et al. [118] synthesized 2-amino-5,6,7,8-tetrahydro-7,7-dimethyl-4-(3,4-substituted phenyl)-5-oxo-4H-chromene-3-carbonitrile derivatives 85 in good-to-high yields (84–93%) utilizing triethylamine hydrogen sulfate [Et3NH][HSO4] as ionic liquid catalyst under solvent-free conditions and microwave irradiation. The synthesis was successfully carried out via one-pot multicomponent cyclocondensation reaction of aromatic aldehydes (3, 4-substituted) 7, dimedone 84 and malononitrile 2 (Scheme 123).
Bhupathi et al. [119] prepared a wide range of dihydropyrano[3,2-c]quinolones 243 in high yields (85–93%) in the presence of {(1,8-diazabicyclo[5.4.0]-undec-7-en-8-ium acetate)}[DBU][Ac] 328 under mild reaction conditions. These products 243 were obtained by treatment of 1-methylquinoline-2,4(1H,3H)-dione 242, aromatic aldehydes 7 and malononitrile 2 (Scheme 124).
Rajesh et al. [120] used a biodegradable organocatalyst [TBA][Gly] for the synthesis of 2-amino3-cyano-4-(indol-3-yl)-4H-chromenes 283 in high yields (91–94%). The reaction of malononitrile 2, salicylaldehyde 236 and indoles 282 in the presence of tetrabutylammonium glycinate [TBA][Gly] at 60 °C under solvent-free conditions generated indolyl-4H-chromenes 283 in excellent yield (Scheme 125).
Zeolites
Using nanopowder of natural clinoptilolite (CP) zeolite as a green and reusable catalyst, Baghbanian et al. [101] reported the synthesis of a wide range of 2-amino-3-cyano-4H-chromene derivatives 329. These products 329 were obtained in high-to-excellent yields (85–98%) via reaction of aldehydes 1, malononitrile 2 and a variety of enolizable C–H-activated acidic compounds 328 (Scheme 126).
Reddy et al. [121] applied montmorillonite K10 clay as a green acid catalyst in the multicomponent reaction of ethyl 4-chloro-3-oxobutanoate 330, 5-methyl-1,3,4-thiadiazole-2-thiol 331, hydrazine hydrate 42, malononitrile 2 and arylaldehydes 7 in mixture of solvents (EtOH:H2O). In this method, the new derivatives of dihydropyranopyrazoles 332 were generated in high yields (81–91%) (Scheme 127).
Catalyst-free
Safaei et al. [122] developed a green and versatile method for the synthesis of 4H-pyrans 85 in good-to-high yields (83–92%) under catalyst-free conditions using glycerol as a biodegradable medium. Under this condition, the desired products 85 were obtained from the reaction of aromatic aldehydes 7, dimedone 84 and malononitrile 2 (Scheme 128).
Zonouz et al. [123] developed a four-component reaction of dimethyl acetylenedicarboxylate 60, hydrazine hydrate 42, malononitrile 2 and aromatic aldehydes 7 in water. In their green and facile method, methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates 333 were synthesized in good yields (64–84%) (Scheme 129).
Zhang et al. [124] reported a novel visible-light-promoted three-component reaction of isatins 136, malononitrile 2 and 2-hydroxynaphthalene-1,4-dione 38 in aqueous ethyl lactate at room temperature that affords spirooxindole-pyran derivatives 334 was obtained in good-to-excellent yields (82–95%) (Scheme 130).
Makarem et al. [125] prepared 2-amino-4H-chromenes 5 from the multicomponent condensation of resorcinol 3, malononitrile 2 and various aldehydes 1 in an undivided cell in the presence of NaBr as an electrolyte (Scheme 131).
Based on the proposed mechanism shown in Scheme 132, deprotonation of alcohol 3 and malononitrile 2 at the cathode leads to the formation of an alkoxide A and malononitrile anion, respectively [125]. Next, 2-benzylidenemalononitrile intermediate 12 is generated from the condensation of the aldehyde 1 with malononitrile anion 2 through elimination of hydroxide. Then, phenol 3 C-alkylation and cyclization through nucleophilic attack of the alkoxide on the cyano moiety produces B. Finally, the desired 2-amino-4H-chromenes 5 is produced in good-to-high yields (80–92%) from protonation and rearrangement of B.
Mandha et al. [1] reported a green approach for the construction of substituted pyrano[2,3-c]pyrazoles (305, 335) under non-catalytic conditions (Scheme 133). Initially, the Knoevenagel condensation of aromatic aldehydes 7 with malononitrile 2 in EtOH at room temperature gave intermediate 46. A Michael addition reaction and subsequent intramolecular cyclization of 3-methyl-1H-pyrazol-5(4H)-one 104/5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 299 with intermediate 46 afforded various substituted pyrano[2,3-c]pyrazoles 105/335 in moderate-to-high yields (64–93%).
Mandha et al. [1] with the same method also successfully synthesized spiroindoline- and spirofluorene-substituted pyrano[2,3-c]pyrazoles (338, 341) in high yields (89 and 81%) with isatin 136 and 9-fluorenone 339, respectively (Scheme 134).
Biological activities of the synthesized polysubstituted 2-amino-4H-pyran-3-carbonitriles
Anticancer activity
Kemnitzer et al. [51] tested the synthesized 2-amino-3-cyano-4-aryl-4H-chromenes 146 for anticancer activities against T47D breast cancer cells. 2-Amino-3-cyano-7-(dimethylamino)-4-(3-methoxy-4,5-methylenedioxyphenyl)-4H-chromene 146a induced apoptosis as determined by the flow cytometry analysis assay in multiple human cell lines (e.g., Jurkat, T47D). Compounds 146c and 146e had good activity in the caspase activation assay against T47D breast cancer cells with EC50 values of 19 and 11 nM, respectively. Compound 146c was found to be a potent inhibitor of tubulin polymerization and effectively inhibited the binding of colchicine to tubulin (Scheme 135).
2-Amino-4-(3-bromo-4,5-dimethoxyphenyl)-3-cyano-4,7-dihydro-7-methyl-pyrano[2,3-e]indole 150a was explored by Cai et al. [7] as a novel anticancer agent. Compound 150a also showed low nanomolar or subnanomolar inhibition of cell growth, and inhibited tubulin polymerization. Moreover, structure 150 was recognized as a highly potent apoptosis inducer with an EC50 (loss of half of the microtubuli) value of 2 Nm and a highly potent inhibitor of cell growth with a GI50 value of 0.3 nM in T47D cells (Scheme 136).
Shafiee et al. [52] reported a new series of 4-aryl-4H-chromenes bearing a 5-arylisoxazol-3-yl moiety at the C-4 position 156 as potential anticancer agents. These compounds 156 were tested against a panel of tumor cell lines including MCF-7 (breast cancer), KB (nasopharyngeal epidermoid carcinoma), Hep-G2 (liver carcinoma), MDA-MB-231 (breast cancer) and SKNMC (human neuroblastoma) using the MTT colorimetric assay, and their IC50 values were measured. Doxorubicin, a well-known anticancer drug, was used as the positive standard drug. Compounds 156c, 156j and 156 k were the most potent of the series and displayed good activity against all tested cell lines with IC50 values of 6.5 ± 1.4 to 12.3 ± 0.5 mM. SAR studies showed that 3-methylphenyl-substituted analog 156j was the most potent compound of this series against all tested cell lines (Scheme 137).
Thomas et al. [54] tested the synthesized new 4-aryl-4H-chromene-3-carbonitrile derivatives 161 for anticancer activities in vitro. Compounds 161c–e showed good anticancer activity against MCF-7 cell lines (CTC50 less than 62.5 μg/m) as compared to other derivatives of the series (Scheme 138).
Wang et al. [79] reported the synthesis of a series of novel 2-amino-4H-pyran derivatives 251 in excellent yields (Scheme 139). All derivatives were tested for antitumor activity against three human tumor cell lines, including human colon cancer (HCT116), human cervical cancer (Hela) and non-small cell lung cancer (H1975). At concentrations of 20 μmol/L, compounds 251a, 251b displayed noticeable growth inhibitory activity against the subtotal tested subpanel tumor cell lines; although other compounds exhibited some antitumor activity at concentrations of 10 μmol/L or lower, none of them came up with a profound inhibitory effect.
Esmati et al. [80] tested the novel dihyrobenzo[h]pyrano[3,2-c]chromene derivatives 253 for in vitro cytotoxic activity. Most of the synthesized compounds have no effect on HL-60 and MOLT4 cell lines (the IC50 of these analogs was higher than 50 or 100 mM), and their inhibitory activity levels against tumor cell line are mainly low, i.e., at micromolar concentrations range (Scheme 140).
Schmitt et al. [9] synthesized 2-amino-4-phenyl-4H-naphtho(1,2-b)pyran-3-carbonitriles 241 and observed the best activities for the compounds that bear small substituents at the meta position of the phenyl ring. Sterically more demanding substituents such as benzyl or SF5 probably obstruct naphthopyran–tubulin approach. LY290181 caused a mitotic catastrophe leading to apoptosis in 518A2 melanoma cells, whereas naphthopyrans 241 induced a disruption of the vasculature in the chorioallantoic membrane (CAM) of fertilized chicken eggs as well as in xenograft tumors in mice (Scheme 141).
Mohareb and Abdo [70] evaluated in vitro anticancer activity of 4H-pyrans 227 and 229 against six human cancer cell lines including cells derived from human gastric cancer (NUGC), human colon cancer (DLD1), human liver cancer (HA22T and HEPG2), human breast cancer (MCF), nasopharyngeal carcinoma (HONE1) and normal fibroblast cells (WI38). Among the 4H-pyran derivatives 227, compounds 227a, 227c were the most potent. The compound 227c exhibited high potency toward six cancer cell lines, while compound 227a analog was only potent against four cancer cell lines, namely NUGC, DLD1, HA22T and MCF with IC50 values of 48, 59, 122 and 480 nM, respectively. Similarly, among the 4H-pyran derivatives 229, the highest activities were found for compound 229c with remarkable activity against six human cancer cell lines (Scheme 142).
Azzam et al. [71] reported 4H-pyran derivatives 231 and 342 with antitumor activities. These compounds 231 and 342 were tested for antitumor activity against six human cancer and normal cell lines including cells derived from human gastric cancer (NUGC), human colon cancer (DLD1), human liver cancer (HA22T and HEPG2), human breast cancer (MCF) and nasopharyngeal carcinoma (HONE1) as well as a normal fibroblast cells (WI38). The results showed that compounds 231a, 231b, 231f and most of 342 analogs had optimal cytotoxic effect against cancer cell lines as they had IC50 < 550 nM. Compounds 231f and 342a–d showed no toxicity against shrimp larvae (Scheme 143).
Alla et al. synthesized substituted pyrano[2,3-c]pyrazoles 105 and evaluated their cytotoxic activity against MCF-7 (breast cancer cell line) by MTT assay with Taxol as a standard Ref. [1]. Among all synthesized compounds, compound 105i was the most effective one with IC50 = 1.630 lg/mL (Scheme 144).
Shestopalov et al. [68] evaluated the synthesized 4-aryl-4H-chromenes 221 in a phenotypic sea urchin embryo assay for antimitotic- and microtubule-destabilizing activity. Compounds 221a-e exhibited strong cytotoxicity in the NCI60 human tumor cell line. The results suggest that synthetically feasible polyalkoxy-substituted 4H-chromenes 221 may prove to be advantageous for further design as anticancer agents (Scheme 145).
Mohareb et al. [72] synthesized 2-amino-3-cyano-pyran derivatives 233 and evaluated them for anticancer activity against six different human cancer cell lines: human liver cancer (HA22T and HEPG2), human gastric cancer (NUGC), human colon cancer (DLD1), nasopharyngeal carcinoma (HONE1), human breast cancer (MCF) and normal fibroblast cells (WI38). The best results were obtained with 4-chlorophenyl and furan substituents as they were active against most cancer cell lines. Compound 233a with IC50 of 29 nM was almost equipotent to the standard CHS 828 (IC50 = 25 nM) against human gastric cancer NUGC, and 233b with IC50 = 89 nM revealed the highest cytotoxicity among the four derivatives against MCF. The other derivatives of the series were less potent (Scheme 146).
Lu et al. [81] indicated that their synthesized novel pyrano[3,2-a]phenazines 256 exhibited cytotoxicity against HCT116, MCF7, HepG2 and A549 cancer cell lines in vitro. In particular, compounds 256a and 256b were found to have an excellent antiproliferative activity against the HepG2 cancer cell line. In particular, compound 256a showed more potent than positive control drug both in vivo and in vitro (Scheme 147).
Padmaja et al. [73] reported the synthesis and biological evaluation of novel pyrano[3,2-c]carbazole derivatives 235 as antitumor agents inducing apoptosis via tubulin polymerization inhibition. The antiproliferative activity of these compounds was tested against various cancer cell lines such as MDA-MB-231, K562, A549 and HeLa. Compounds 235a, 235c, 235g and 235i showed superior antiproliferative activity over other derivatives with IC50 values ranging from 0.43 to 8.05 μM and significantly induced apoptosis by inhibiting tubulin polymerization (Scheme 148).
Upadhyay et al. [76] evaluated the synthesized pyrano[3,2-c]quinolines 243 for their anti-inflammatory and cytotoxic activity at inhibiting TNF-α production in human peripheral blood mononuclear cells (hPBMC) assay. The results showed that compounds 243c, 243f, 243i and 243j were most active as both anti-inflammatory and anticancer. The structure–activity relationship suggests that the 3-substitution on the aryl ring at C4 position of the pyrano[3,2-c]quinolone structural motif is essential for both TNF-α and IL-6 inhibition and anticancer activity as well (Scheme 149).
Kalla et al. [63] synthesized 2-amino-3-cyano-4H-chromen-4-ylphosphonates 76. All synthesized compounds showed moderate activity at 20 and 40 mM concentrations, while compounds 76a and 76b showed remarkable activity against the A549 and KB cell lines (Scheme 150).
Yan et al. [43] successfully produced a series of 6-amino-1,3-disubstituted-4-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile compounds 130 and tested their Ral inhibitory potential in vitro and in vivo on human lung cancer cell line H2122. Ral GTPase is an enzyme that has two isoforms, RalA and RalB, which interact with a variety of downstream effectors and play distinct key roles in both normal and neoplastic cell physiology including regulation of vesicular trafficking, migration and invasion, tumor formation, metastasis and gene expression. Among the tested derivatives, compounds 130a (BQU057) and 130b (BQU082) displayed a dose-dependent effect on RalA and RalB activity in H2122 spheroids. Indeed, similar to a parent drug, e.g., RBC8 or RBC10, the two compounds 130a and 130b were observed in the mouse xenograft model in a good distributed manner (Scheme 151).
Antimicrobial activity
Mungra et al. [59] developed a new class of β-aryloxyquinolines and their pyrano[3,2-c]chromene derivatives 186 incorporating a validated molecular target and evaluated their antimicrobial activities. Compounds 186f, 186l and 186q displayed excellent in vitro antimicrobial activity against a representative panel of pathogenic strains specifically Bacillus subtilis, Clostridium tetani, Streptococcus pneumoniae, Escherichia coli, Salmonella typhi, Vibrio cholera, Aspergillus fumigatus and Candida albicans, while compound 186p displayed more potent antifungal activity than the standard drug griseofulvin. Also, compound 186f was a promising antimicrobial member, and majority of the compounds 186 possessed better antimicrobial activity compared to the standard bactericidal ampicillin (Scheme 152).
Makawana et al. [35] evaluated antimicrobial activity of a novel series of fused pyran derivatives which bear 2-morpholinoquinoline nucleus 87–94, against three Gram-positive bacteria (Streptococcus pneumoniae, Clostridium tetani and Bacillus subtilis), three Gram-negative bacteria (Salmonella typhi, Vibrio cholerae and Escherichia coli) and two fungi (Aspergillus fumigatus and Candida albicans) based on minimal inhibitory concentrations (MIC) values. Among all compounds 87–94, the best results were obtained with compounds that are shown in Scheme 153.
Baitha et al. [8] synthesized a new class of substituted 2-amino-4-(2-ethoxybenzo[d][1, 3]dioxol-5-yl)-4H-pyran-3-carbonitriles 201–206 as antifungal and antibacterial agents. Among the tested compounds, 201a, 202b and 205e were found to be potent antibacterial agents at the MIC of 100 μg/mL, while compounds 203c, 204d and 206f were moderately active at the MIC of 100 μg/mL against all tested bacteria. The compounds 201a, 203c and 205e showed one to two orders of magnitude more antifungal activity almost against all tested fungi as compared with the standard miconazole at the same level of concentration (MIC of 10 μg/mL). Compounds 202b, 203d and 206f also were moderately active at the MIC of 100 ppm; 100 mg/L (Scheme 154).
Mandha et al. [1] synthesized substituted pyrano[2,3-c]pyrazoles 105 and then evaluated them for antibacterial activity against two Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus), two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) with ciprofloxacin as a standard. Of all compounds 105 tested, compound 105i with 3-phenoxyphenyl substitution was found to be the most potent compound (Scheme 155).
Kidwai et al. [13] synthesized 2-amino-6-benzothiazol-2-ylsulfanyl-chromenes 14 and screened their antibacterial activity in vitro, against standard strains of P aeruginosa, E. coli, S. aureus and S. epidermidis. They used QSAR analysis for the synthesized compounds to find a statistically reliable model for explaining their antibacterial activities. One of the main conclusions was that compounds 14h and 14c showed maximum and minimum MIC against Escherichia coli, respectively, where the lower MIC of 14c was attributed to the presence of indole ring. For Staphylococcus aureus, 14e/14i had the maximum/minimum MIC, respectively. The higher MIC of 14e is due to the presence of a strong electron-withdrawing NO2 group which lowers electron density in chromene ring. Similarly for Staphylococcus epidermidis, 14g/14b had the highest/lowest MIC, respectively (Scheme 156).
Kalaria et al. [2] designed and synthesized 4-(5-(1H-imidazol-1-yl)-3-methyl-1-phenyl-1Hpyrazol-4-yl)-4H-pyran-2-amines 171. The biological activity of these compounds was attributed to imidazole and pyrazole nuclei, which are present in the synthesized 171. They screened the final motifs for their preliminary in vitro antibacterial activity against a panel of pathogenic strains of bacteria (C. tetani/B. subtilis) and fungi (C. albicans). For antifungal and antibacterial activity, compounds 171q and 171 s showed excellent activity compared to standards griseofulvin and ampicillin, respectively (Scheme 157).
Li et al. [57] synthesized of functionalized 4H-pyrans 166 and evaluated them for in vitro antibacterial activity against three ATCC-bacterial strains Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Enterococcus faecalis (ATCC 29212). The MIC values of these compounds were between 16 and 128 μg/mL and exhibited their promising antibacterial activity. Among these functionalized 4H-pyrans 166, compounds 166k, 166 m, 166q and 166r have shown antibacterial activity against the three ATCC-bacterial strains. In general, compounds with substitutions such as meta-chlorobenzene, naphthalene or thiophene displayed strong antibacterial activity at relatively low concentrations (Scheme 158).
Makawana et al. [33] synthesized a new series of pyrano[3,2-c]chromene derivatives 83 bearing a 2-thiophenoxyquinoline nucleus and evaluated them as antimicrobial agents using the broth microdilution MIC method against three Gram-positive bacteria (Streptococcus pneumoniae, Clostridium tetani and Bacillus subtilis), three Gram-negative bacteria (Escherichia coli, Salmonella typhi and Vibrio cholerae) and two fungi (Aspergillus fumigatus and Candida albicans). Among all compounds 83, 83e against B. subtilis, 83o against E. coli and 83p against S. pneumoniae proved to be most efficient antimicrobial members. Compounds 83b and 83 g, bearing methyl group either at the 6th position of the quinoline ring or at the 4th position of the thiophenol ring showed remarkable activity against most of the species tested (Scheme 159).
Costa et al. [74] synthesized novel 2-iminochromene dimers and evaluated their antifungal activity. Among all 2-iminochromene dimers 237, compound 237a presented significant antifungal activity, when tested on four Aspergillus species A. alliaceus, A. carbonarius, A. niger and A. ochraceus at concentrations of 2 mM. The analogous structure 237b showed a poor antifungal activity that was attributed to its instability in dilute solution (Scheme 160).
Kalalbandi et al. [75] developed a synthetic route for the synthesis of biscoumarin fused with dihydropyran ring 239. All the newly synthesized compounds were screened for their antibacterial and antifungal activities in vitro against Enteroccocus faecalis (MTCC 3382), Staphylococcus aureus (MTCC 3160), Pseudomonas aeruginosa (MTCC 1034) and Escherichia coli (MTCC 1089). Majority of the compounds exhibited promising antimicrobial activities. In comparison with ciprofloxacin, compound 239 was found to be more potent against S. aureus and P. Aeruginosa (Scheme 161).
Reddy et al. [121] generated thiadiazole-attached pyranopyrazole derivatives 332. The synthesized compounds 332 were tested for their antimicrobial activity against six medically significant bacterial and fungal species: Gram-positive/Gram-negative bacteria, Staphylococcus aureus, Bacillus subtilis/Proteus vulgaris, Escherichia coli and fungi strains (Aspergillus flavus, Aspergillus niger). All synthesized compounds except two possess higher to low antibacterial property against whole bacteria used in this screening. Compound 332f showed outstanding activity against all six pathogens due to the presence of nitro substituent, while the compounds 332j and 332 k had no effect on the bacterial strains (Scheme 162).
Kidwai et al. [11] reported green synthesis of substituted 2-amino-4H-chromenes 6 and benzo[e]chromenes 5. It was shown that all synthesized compounds 5, 6 possess antibacterial activity as tested in vitro against standard strains of Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Staphylococcus aureus (ATCC 25923) (Scheme 163).
Rajasekhar et al. [32] prepared 2-amino-3-cyano-4H-chromen-4-ylphosphonates 76 and screened their antibacterial and antifungal activity by disk diffusion method against the growth of Staphylococcus aureus (ATCC 25923) (Gram positive) and Escherichia coli (ATCC 25922) (Gram negative) in comparison with penicillin as reference compound. All the compounds 76 showed moderate-to-good activity against both strains. Antifungal activity of the synthesized compounds was also screened against Aspergillus niger (ATCC 16404) and Helminthosporium oryzae (ATCC 11000) species in comparison with fungicide griseofulvin. All the compounds 76 showed good activity against both fungi (Scheme 164).
Azarifar et al. [94] developed synthesis and biological evaluation of new pyranopyridine derivatives 287. These compounds 287 were evaluated for antifungal capacity against the fungus Fusarium oxysporum, and most of them showed excellent antifungal activity against this fungus (Scheme 165).
Mahdavi et al. [45] synthesized 2-amino-4H-pyrans 85 and tested their antimycobacterial and antifungal activities. The results demonstrated that the majority of the synthesized compounds 85 were active against M. bovis and poorly active against C.albicans. The compounds 85a and 85f showed the highest antimycobacterial activity. It was concluded that the position of substituents in the aromatic ring and their electron-donating capacity affected the activity of compounds 85 (Scheme 166).
Rao et al. [34] synthesized tetrahydrobenzo[b]pyrans 85 and examined their antibacterial/antifungal activities against five pathogenic bacterial strains (Gram-negative bacteria: Escerichia coli, Pseudomonas aeruginosa and Gram-positive bacteria: S. ureas, B. substills)/the organism of Aspergillus niger and Candida ablicans in comparison with standard drugs amoxicillin/ketoconazole, respectively. Among all the tested compounds 85, the compounds having halogens showed excellent activity. Generally, compounds possessing electron-donating groups showed moderate activity compared to those with electron-withdrawing groups (Scheme 167).
Gaikwad et al. [56] synthesized 2-amino-4-(-4-substituted phenyl)-6-(naphtho[2,1-b]furan-2-yl)-4H-pyran-3-carbonitriles 164 and evaluated their in vitro antimicrobial activity by cup-plate method against two bacteria (Salmonua typhi and Staphylococcus aureus) and two fungi (Aspergillus niger and Candida albicans). All the compounds 164 showed significant antibacterial and antifungal activities at 20 mg/ml concentration levels compared to penicillin and griseofulvin as standards, respectively. Among, compounds 164b,c and 164e were more potent on the bacterial strain, whereas all compounds showed maximum antifungal activity (Scheme 168).
Rbaa et al. [16] evaluated and screened new pyran derivatives based on 8-hydroxyquinoline 22 in vitro by the disk diffusion technique against Gram-positive and Gram-negative bacterial strains (E. coli (ATCC35218), S. aureus (ATCC29213), V. parahaemolyticus (ATCC17802) and P. aeruginosa (ATCC27853)). All the compounds 22 displayed a potential antibacterial activity against all the tested four Gram bacteria. The two products 22b, 22c showed antibacterial activity against the Gram-positive and Gram-negative strains compared to the standard antibiotic penicillin G. Among the tested compounds 22, compound 22a showed no effect against the strain and compound 22d showed the most important antibacterial activity at MIC values comparable to the control (penicillin G). The molecules with electron-withdrawing substituents (acid function, nitro, etc.) have shown a lower activity than those having electron-donating substituents (O-alkyl, O-aryl, chlorophenyl, etc.) (Scheme 169).
Hatamjafari et al. [89] screened the synthesized 4H-chromenes 85 for antimicrobial activity. The majority of the compounds exhibited significant activity against selected bacteria (V. cholerae, E. coli, B. subtillus, S. aureus) and fungi (Chrysosporium sp., Trichoderma sp., A. niger, A. parasitica). For antibacterial activity, V. cholera, B. subtillus, S. aureus, Trichoderma sp. and E. coli were highly vulnerable to compounds 85c–g compared with ciprofloxacin as standard, at 100 µgmL−1. For antifungal activity, Chrysosporium sp. and Trichoderma sp. were highly active for compounds 85d, 85e compared with clomatrimazole as standard at 100 µgmL−1, respectively (Scheme 170).
Pasdar et al. [108] synthesized 2-amino-7,7-dimethyl-5-oxo-4-methylbenzen5,6,7,8-tetrahydro-4H-chromone-3-carbonitriles 85 and evaluated in vitro antibacterial effect of this compound both ligand and Cu (II), Ni (II), Co (II) and Zn (II) complexes by disk diffusion and micro-broth dilution methods. The results showed that the complexes have higher antibacterial activity in comparison with the ligand. Among, the most effective complex was the Cu complex with MIC value of 62.5 μg/mL against E. coli and 125 μg/mL against S. aureus (Scheme 171).
Antidepressant-like activity
Sashidhara et al. [58] synthesized and evaluated a new series of coumarin–aminopyran hybrids 175. Among all synthesized coumarin-based aminopyran derivatives 175, compound 175a at a very low dose of 0.5 mg/kg caused a reduction in immobility time comparable to the standard drugs fluoxetine (FXT) and imipramine (IMI). This confirms that these prototypes exhibited antidepressant-type activity and may be a potential antidepressant drug for the treatment of mental depression (Scheme 172).
Ahmad et al. [69] reported the synthesis and in vitro biological evaluation of pyranobenzothiazine derivatives 225 for the inhibition of monoamine oxidases (A and B). Most of the tested pyranobenzothiazines 225 inhibited MAO-A and MAO-B isozymes with an IC50 values in the lower micromolar range. Monoamine oxidase involved in a number of psychiatric and neurological diseases and monoamine oxidase inhibitors (MAOIs) are best known as powerful antidepressants.
Among the tested compounds 225, compound 225d and 225q are the selective inhibitors of monoamine oxidase A; however, the selective and potent inhibitors of monoamine oxidase B included compounds 225 h and 225r. Moreover, compound 225 l as a dual inhibitor showed more inhibitory activity toward both the isozymes (Scheme 173).
Enzyme inhibitory activity
Xanthine oxidase inhibitory activity
Xanthine oxidase is a type of enzyme that generates reactive oxygen species and leads to many diseases such as gout and symptoms of other diseases such as oxidative damage to the tissue. The selective inhibition of xanthine oxidase would be an appropriate treatment for these diseases [126, 127].
Kaur et al. [6] reported the synthesis and biological evaluation of 4-aryl/heteroaryl-4H-fused pyrans 99 for xanthine oxidase inhibition. All of the synthesized compounds 99 were screened for in vitro xanthine oxidase inhibition and compound 99a was the most potent one, exhibiting significant inhibition against the enzyme with an IC50 value of 0.59 lM. Enzyme kinetic study showed that the compound 99a was a mixed-type inhibitor. The docking study of 99a confirmed that S-enantiomer of 99a fits well in the binding site, while R-enantiomer was not able to get into the cavity (Scheme 174).
EAAT1 inhibitory activity
The excitatory amino acid transporters (EAATs) are glutamate transporters that remove glutamate from the synaptic cleft and extrasynaptic site via glutamate reuptake into glial cells and neurons. Thereby, the transporters play a central role in regulating the synaptic as well as extrasynaptic concentration of glutamate below levels of neurotoxicity.
Huynh et al. [42] synthesized and evaluated a series of coumarin-based fluorescent analogs of UCPH-101/102 as subtype-selective inhibitors at EAAT1. Among synthesized compounds 125, the analogs UCPH-101 (IC50 = 0.66 lM) and UCPH-102 (IC50 = 0.43 lM) were the most potent inhibitors of the series, whereas 125a and 125b inhibited EAAT1 with IC50 values in the medium micromolar range (17 lM and 14 lM, respectively) (Scheme 175).
Erichsen et al. [5], in another similar work, designed, synthesized and evaluated the analogs of 2-amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (UCPH-101). The SAR studies showed that potency was largely influenced by the chemical nature of the R1 substituent. The presence of an aromatic ring in the 7-position (R1) and an alkyl or aryl substituent in the 4-position (R2) of the parental skeleton was mandatory and essential for the inhibitory activity of the analogs 132 at EAAT1. Accordingly, compounds 132b, 132h, 132i and 132j showed high inhibitory activity at EAAT1 (Scheme 176).
In a complementary contribution, they made further investigations on the structure–activity requirements of the (EAAT1) inhibitor, 2-amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4Hchromene-3-carbonitrile (UCPH-101) [44]. Separation of the four stereoisomers of analog 132i was unequivocally confirmed that stereochemistry at C4 was mandatory for EAAT1 inhibitory activity, but the stereochemistry at C7 was not vital. Accordingly, only diastereomers with R configuration at C4 and both of the C7 diastereomers were active as EAAT1 inhibitors (Scheme 177).
Antidiabetic activity
Nikookar et al. [82] synthesized dihydropyrano[3,2-c]quinoline derivatives 260 and evaluated in vitro α-glucosidase inhibitory activities. All synthesized compounds 260 displayed excellent activity in the range of 10.3 ± 0.3 mM–172.5 ± 0.8 mM against the yeast α-glucosidase enzyme compared to the standard drug acarbose (IC50 = 750.0 ± 1.5 mM). Among, compounds 260e and 260d displayed the most potent α-glucosidase inhibitory activity (IC50 = 10.3 ± 0.3 and 15.7 ± 0.5 mM, respectively) (Scheme 178).
Antioxidant activity
Azarifar et al. [94] developed synthesis and biological evaluation of new pyranopyridine derivatives 287. The obtained pyranopyridine derivatives 287 were evaluated as antioxidants by using a 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay. Most of the synthesized compounds 287 showed excellent antioxidant bioactivity with an IC50 value of 0.140 ± 0.003 to 0.751 ± 0.008 mg mL−1 (Scheme 179).
Thomas et al. [54] reported the synthesis of 4-aryl-4H-chromene-3-carbonitrile derivatives 161, and in vitro antioxidant activity of all the synthesized compounds was assessed by nitric oxide and hydrogen peroxide free radical scavenging methods using ascorbic acid and butylated hydroxytoluene as references. All the synthesized compounds 161 showed significant-to-moderate activities in both the methods. Among, the analog 161e (IC50 30.0 μg/mL) exhibited higher nitric oxide-scavenging activity, while compounds 161c (IC50 = 26.8 μg/mL) and 161e (IC50 = 24.2 μg/mL) showed maximum hydrogen peroxide-scavenging activity compared to ascorbic acid and butylated hydroxytoluene as standard references (Scheme 180).
Rajasekhar et al. [32] prepared 2-amino-3-cyano-4H-chromen-4-ylphosphonates 76 and screened their antioxidant activity. All the synthesized compounds showed a good activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH), nitric oxide (NO) scavenging power. Among the synthesized compounds 76, 76e exhibited the highest activity (Scheme 181).
Antitubercular activity
Chen et al. [53] synthesized racemic 2-aminopyranopyridine-3-carbonitriles 158 and investigated their antitubercular activities against autoluminescent M. tuberculosis H37Ra and standard strain M. tuberculosis H37Rv. Isoniazid and rifampicin were used as the positive controls. The synthesized racemic compounds 158 did not show obvious antitubercular activities (MIC > 10 μg/mL) (Scheme 182).
Mungra et al. [59] synthesized a new class of β-aryloxyquinolines and their pyrano[3,2-c]chromene derivatives 186 and evaluated their antitubercular activities. Majority of the compounds possessed poor antitubercular activity. Compound 186f showed better antitubercular activity against Mycobacterium tuberculosis H37Rv in vitro (250 mg/ml) (Scheme 183).
Kalaria et al. [2] screened in vitro antituberculosis activity of synthesized 4-(5-(1H-imidazol-1-yl)-3-methyl-1-phenyl-1Hpyrazol-4-yl)-4H-pyran-2-amines 171 against Mycobacterium tuberculosis H37Rv. Among tested compounds, compound 171o exhibited good better antituberculosis activity compared with rifampicin and isoniazid as the standard drugs (95% inhibition at 250 µg/mL concentration and 92% inhibition at 20, 100 µg/mL concentration). Accordingly, compounds 171o may become new class of antitubercular agents in the future (Scheme 184).
Antimalarial activity
Kalaria et al. [2] screened antimalarial activity of novel 5-imidazopyrazole nucleus-bearing fused pyran derivatives 171 against Plasmodium falciparum. Almost one-third of the synthesized compounds have shown excellent activity against strains of P. falciparum as compared to quinine (IC50 = 0.268) as the reference drug; compound 171 g was found to possess moderate activity (IC50 = 0.034) compared to chloroquine (Scheme 185).
Conclusions
In this review, the existing methods for the synthesis of 2-amino-3-cyano-4H-chromenes and their fused heterocyclic analogs were classified according to the type of catalyst used in the pertinent reactions. The wide range of potential pharmacological applications and biological activities of these compounds were also addressed in a separate section with focus on recently published articles.
In summary, the synthesis of 2-amino-3-cyano-4H-chromenes could be facilitated with various conventional and advanced catalysts, and even in the absence of any catalysts. In addition, these compounds are good candidates for a wide range of biological applications including anticancer, antimicrobial, antifungal, antidepressant-like, enzyme inhibition, antitubercular, antimalarial, antioxidant and antidiabetic activities.
References
Mandha SR, Siliveri S, Alla M, Bommena VR, Bommineni MR, Balasubramanian S (2012) Eco-friendly synthesis and biological evaluation of substituted pyrano [2,3-c] pyrazoles. Bioorg Med Chem Lett 22:5272–5278. https://doi.org/10.1016/j.bmcl.2012.06.055
Kalaria PN, Satasia SP, Raval DK (2014) Synthesis, characterization and biological screening of novel 5-imidazopyrazole incorporated fused pyran motifs under microwave irradiation. New J Chem 38:1512–1521. https://doi.org/10.1039/C3NJ01327H
Kemnitzer W, Drewe J, Jiang S, Zhang H, Zhao J, Crogan-Grundy C, Xu L, Lamothe S, Gourdeau H, Denis R, Tseng B (2007) Discovery of 4-aryl-4 H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high-throughput screening assay. 3. Structure-activity relationships of fused rings at the 7, 8-positions. J Med Chem 50:2858–2864. https://doi.org/10.1021/jm070216c
Zhang G, Zhang Y, Yan J, Chen R, Wang S, Ma Y, Wang R (2012) One-pot enantioselective synthesis of functionalized pyranocoumarins and 2-amino-4 H-chromenes: discovery of a type of potent antibacterial agent. J Org Chem 77:878–888. https://doi.org/10.1021/jo202020m
Erichsen MN, Huynh TH, Abrahamsen B, Bastlund JF, Bundgaard C, Monrad O, Bekker-Jensen A, Nielsen CW, Frydenvang K, Jensen AA, Bunch L (2010) Structure-activity relationship study of first selective inhibitor of excitatory amino acid transporter subtype 1: 2-amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5, 6, 7, 8-tetrahydro-4 H-chromene-3-carbonitrile (UCPH-101). J Med Chem 53:7180–7191. https://doi.org/10.1021/jm1009154
Kaur R, Naaz F, Sharma S, Mehndiratta S, Gupta MK, Bedi PM, Nepali K (2015) Screening of a library of 4-aryl/heteroaryl-4H-fused pyrans for xanthine oxidase inhibition: synthesis, biological evaluation and docking studies. Med Chem Res 24:3334–3349. https://doi.org/10.1007/s00044-015-1382-0
Kemnitzer W, Drewe J, Jiang S, Zhang H, Crogan-Grundy C, Labreque D, Bubenick M, Attardo G, Denis R, Lamothe S, Gourdeau H, Tseng B, Kasibhatla S, Cai SX (2008) Discovery of 4-aryl-4 H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high throughput screening assay. 4. Structure–activity relationships of N-alkyl substituted pyrrole fused at the 7, 8-positions. J Med Chem 51:417–423. https://doi.org/10.1021/jm7010657
Baitha A, Gopinathan A, Krishnan K, Dabholkar VV (2018) Synthesis of 2-amino-4-(2-ethoxybenzo [d][1,3] dioxol-5-yl)-4H-pyran-3-carbonitrile derivatives and their biological evaluation. J Heterocycl Chem 55:1189–1192. https://doi.org/10.1002/jhet.3152
Schmitt F, Gold M, Rothemund M, Andronache I, Biersack B, Schobert R, Mueller T (2019) New naphthopyran analogues of LY290181 as potential tumor vascular-disrupting agents. Eur J Med Chem 163:160–168. https://doi.org/10.1016/j.ejmech.2018.11.055
Sonsona I, Marqués-López E, Herrera R (2015) Enantioselective organocatalyzed synthesis of 2-amino-3-cyano-4H-chromene derivatives. Symmetry 7:1519–1535. https://doi.org/10.3390/sym7031519
Kidwai M, Saxena S, Khan MK, Thukral SS (2005) Aqua mediated synthesis of substituted 2-amino-4H-chromenes and in vitro study as antibacterial agents. Bioorg Med Chem Lett 15:4295–4298. https://doi.org/10.1016/j.bmcl.2005.06.041
Dinh Thanh N, Son Hai D, Thi Ngoc Bich V, Thi Thu Hien P, Thi Ky Duyen N, Thi Mai N, Thi Dung T, Van Thi Kim H, Ngoc Toan V, Huy NH, Van Thi Thanh T (2019) Synthesis and structure of some substituted 2-amino-4-aryl-7-propargyloxy-4 H-chromene-3-carbonitriles. Synth Commun 49:102–117. https://doi.org/10.1080/00397911.2018.1543779
Kidwai M, Poddar R, Bhardwaj S, Singh S, Luthra PM (2010) Aqua mediated synthesis of 2-amino-6-benzothiazol-2-ylsulfanyl-chromenes and its in vitro study, explanation of the structure–activity relationships (SARs) as antibacterial agent. Eur J Med Chem 45:5031–5038. https://doi.org/10.1016/j.ejmech.2010.08.010
Karimi-Jaberi Z, Pooladian B (2012) A facile synthesis of new 2-amino-4H-pyran-3-carbonitriles by a one-pot reaction of α, α′-bis (arylidene) cycloalkanones and malononitrile in the presence of K2CO3. Sci World J 2012:1–5
Karami B, Khodabakhshi S, Eskandari K (2012) A one-pot, three-component synthesis of new pyrano [2,3-h] coumarin derivatives. Tetrahedron Lett 53:1445–1446. https://doi.org/10.1016/j.tetlet.2012.01.024
Rbaa M, Bazdi O, Hichar A, Lakhrissi Y, Ounine K, Lakhrissi B (2018) Synthesis, characterization and biological activity of new pyran derivatives of 8-hydroxyquinoline. Eurasian J Anal Chem 13:19–30
Mohan TJ, Bahulayan D (2017) Design, synthesis and fluorescence property evaluation of blue emitting triazole-linked chromene peptidomimetics. Mol Divers 21:585–596. https://doi.org/10.1007/s11030-017-9744-9
Yao C, Feng X, Wang C, Jiang B, Yu C, Wang X, Li T, Tu S (2011) Solvent-free three-component synthesis of 7-aryl-1, 1-dioxothieno [3,2-b] pyran derivatives catalyzed by ammonium acetate. J Heterocycl Chem 48:1111–1116. https://doi.org/10.1002/jhet.696
Damavandi S (2011) Base-catalyzed three-component synthesis of 2-amino-4,5-dihydro-4-arylpyrano [3,2-b] indole-3-carbonitriles. Heterocycl Commun 17:125–127. https://doi.org/10.1515/hc.2011.032
Lei M, Ma L, Hu L (2011) A green, efficient, and rapid procedure for the synthesis of 2-amino-3-cyano-1,4,5,6-tetrahydropyrano [3,2-c] quinolin-5-one derivatives catalyzed by ammonium acetate. Tetrahedron Lett 52:2597–2600. https://doi.org/10.1016/j.tetlet.2011.03.061
Ghadari R, Hajishaabanha F, Aghaei M, Shaabani A, Ng SW (2012) A facile three-and four-component procedure toward the synthesis of functionalized pyrano-and benzo [f] quinoxaline derivatives. Mol Divers 16:453–461. https://doi.org/10.1007/s11030-012-9379-9
Gein VL, Zamaraeva TM, Slepukhin PA (2014) A novel four-component synthesis of ethyl 6-amino-4-aryl-5-cyano-2, 4-dihydropyrano [2,3-c] pyrazole-3-carboxylates. Tetrahedron Lett 55:4525–4528. https://doi.org/10.1016/j.tetlet.2014.06.077
Wang SL, Wu FY, Cheng C, Zhang G, Liu YP, Jiang B, Shi F, Tu SJ (2011) Multicomponent synthesis of poly-substituted benzo [a] pyrano [2,3-c] phenazine derivatives under microwave heating. ACS Comb Sci 13:135–139. https://doi.org/10.1021/co1000376
Shestopalov AM, Larionova NA, Fedorov AE, Rodinovskaya LA, Mortikov VY, Zubarev AA, Bushmarinov IS (2013) Synthesis of isomeric isothiazolo [4′,3′: 4,5]-and isothiazolo [4′,5′: 4,5] thieno [3,2-b] pyrano [2,3-d] pyridines by combination of domino reactions. ACS Comb Sci 15:541–545. https://doi.org/10.1021/co400066y
Khodabakhshi S, Karami B, Eskandari K, Farahi M (2014) Synthesis of new 4-aroyl-pyrano [c] chromenes via a one-pot, three-component reaction based on aryl glyoxals. Tetrahedron Lett 55:3753–3755. https://doi.org/10.1016/j.tetlet.2014.05.072
Han G, Du J, Chen L, Zhao L (2014) Synthesis and characterization of 11-amino-3-methoxy-8-substituted-12-aryl-8,9-dihydro-7H-chromeno [2,3-b] quinolin-10 (12H)-one derivatives. J Heterocycl Chem 51:1094–1099. https://doi.org/10.1002/jhet.2010
Molla A, Hussain S (2014) Borax catalyzed domino reactions: synthesis of highly functionalised pyridines, dienes, anilines and dihydropyrano [3,2-c] chromenes. RSC Adv 4:29750–29758. https://doi.org/10.1039/C4RA03627A
El-Ablak FZ, Abu-Elenein NS, Sofan MA (2016) Synthesis of new pyrrolo heterocycles (i): novel synthesis of pyrano [2,3-c] pyrrole, isoindoline, pyrrolo [3,4-b] pyridine, and pyrrolo [3,4-d] pyrimidine derivatives. J Heterocycl Chem 53:1999–2006. https://doi.org/10.1002/jhet.2520
Wang X, Liu M, Chen Z (2016) Brønsted-acid catalyzed cascade annulations toward the fused pyranoquinoline derivatives. Tetrahedron 72:4423–4426. https://doi.org/10.1016/j.tet.2016.06.004
Almansour AI, Kumar RS, Arumugam N, Sriram D (2012) A solvent free, four-component synthesis and 1, 3-dipolar cycloaddition of 4(H)-pyrans with nitrile oxides: Synthesis and discovery of antimycobacterial activity of enantiomerically pure 1,2,4-oxadiazoles. Eur J Med Chem 53:416–423. https://doi.org/10.1016/j.ejmech.2012.04.021
Aghbash KO, Pesyan NN, Notash B (2018) The clean synthesis and confirmatory structural characterization of new 2-amino-4, 8-dihydropyrano [3,2-b] pyran-3-cyano based on Kojic acid. Monatshefte Chem Chem Mon 149:2059–2067. https://doi.org/10.1007/s00706-018-2254-3
Rajasekhar M, Rao KUM, Sundar CS, Reddy NB, Nayak SK, Chemical Reddy C S, Bulletin Pharmaceutical (2012) Green synthesis and bioactivity of 2-amino-4h-chromen-4-yl-phosphonates. Chem Pharm Bull 60:854–858. https://doi.org/10.1248/cpb.c12-00160
Makawana JA, Patel MP, Patel RG (2012) Synthesis and antimicrobial evaluation of new pyrano [4,3-b] pyran and pyrano [3,2-c] chromene derivatives bearing a 2-thiophenoxyquinoline nucleus. Arch Pharm 345:314–322. https://doi.org/10.1002/ardp.201100203
Rao NK, Rao TN, Parvatamma B, Devi KP, Setty SC (2018) Multi component one pot synthesis and characterization of derivatives of 2-amino-7, 7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile and study of anti-microbial activity. Bull Chem Soc Ethiop 32:133–138. https://doi.org/10.4314/bcse.v32i1.12
Makawana JA, Mungra DC, Patel MP, Patel RG (2011) Microwave assisted synthesis and antimicrobial evaluation of new fused pyran derivatives bearing 2-morpholinoquinoline nucleus. Bioorg Med Chem Lett 21:6166–6169. https://doi.org/10.1016/j.bmcl.2011.07.123
Olyaei A, Shahsavari MS, Sadeghpour M (2018) Organocatalytic approach toward the green one-pot synthesis of novel benzo [f] chromenes and 12H-benzo [5,6] chromeno [2,3-b] pyridines. Res Chem Intermed 44:943–956. https://doi.org/10.1007/s11164-017-3145-7
Mansoor SS, Logaiya K, Aswin K, Sudhan PN (2015) An appropriate one-pot synthesis of 3, 4-dihydropyrano [c] chromenes and 6-amino-5-cyano-4-aryl-2-methyl-4H-pyrans with thiourea dioxide as an efficient, reusable organic catalyst in aqueous medium. J Taibah Univ Sci 9:213–226. https://doi.org/10.1016/j.jtusci.2014.09.008
Brahmachari G, Banerjee B (2013) Facile and one-pot access to diverse and densely functionalized 2-amino-3-cyano-4H-pyrans and pyran-annulated heterocyclic scaffolds via an eco-friendly multicomponent reaction at room temperature using urea as a novel organo-catalyst. ACS Sustain Chem Eng 2:411–422. https://doi.org/10.1021/sc400312n
Wiener C, Schroeder CH, West BD, Link KP (1962) Studies on the 4-hydroxycoumarins. XVIII. 1a 3-[α-(acetamidomethyl) benzyl]-4-hydroxycoumarin and related products1b. J Org Chem 27:3086–3088. https://doi.org/10.1021/jo01056a024
Guo RY, An ZM, Mo LP, Yang ST, Liu HX, Wang SX, Zhang ZH (2013) Meglumine promoted one-pot, four-component synthesis of pyranopyrazole derivatives. Tetrahedron 69:9931–9938. https://doi.org/10.1016/j.tet.2013.09.082
Gomha SM, Abdelrazek FM (2016) A facile three-component one-pot synthesis of some novel tricyclic hetero-ring systems. J Heterocycl Chem 53:1892–1896. https://doi.org/10.1002/jhet.2503
Huynh TH, Abrahamsen B, Madsen KK, Gonzalez-Franquesa A, Jensen AA, Bunch L (2012) Design, synthesis and pharmacological characterization of coumarin-based fluorescent analogs of excitatory amino acid transporter subtype 1 selective inhibitors, UCPH-101 and UCPH-102. Bioorg Med Chem 20:6831–6839. https://doi.org/10.1016/j.bmc.2012.09.049
Yan C, Theodorescu D, Miller B, Kumar A, Kumar V, Ross D, Wempe MF (2016) Synthesis of novel Ral inhibitors: an in vitro and in vivo study. Bioorg Med Chem Lett 26:5815–5818. https://doi.org/10.1016/j.bmcl.2016.10.021
Hansen SW, Erichsen MN, Huynh TH, Ruiz JA, Haym I, Bjørn-Yoshimoto WE, Abrahamsen B, Hansen J, Storgaard M, Eriksen AL, Jensen AA (2016) New Insight into the structure–activity relationships of the selective excitatory amino acid transporter subtype 1 (EAAT1) inhibitors UCPH-101 and UCPH-102. Chem Med Chem 11:382–402. https://doi.org/10.1002/cmdc.201500525
Mahdavi SM, Habibi A, Dolati H, Shahcheragh SM, Sardari S, Azerang P (2018) Synthesis and antimicrobial evaluation of 4H-pyrans and schiff bases fused 4H-pyran derivatives as inhibitors of mycobacterium bovis (BCG). Iran J Pharm Res 17:1229–1239
Jirandehi HF, Mirzaiean M (2012) Synthesis of pyrano [3,2-c] pyridines derivatives. Asian J Chem 24:3168–3170
Jayarajan R, Vasuki G (2012) Building libraries of skeletally diverse scaffolds from novel heterocyclic active methylene compound through multi-component reactions. Tetrahedron Lett 53:3044–3048. https://doi.org/10.1016/j.tetlet.2012.04.013
Ye Z, Xu R, Shao X, Xu X, Li Z (2010) One-pot synthesis of polyfunctionalized 4H-pyran derivatives bearing fluorochloro pyridyl moiety. Tetrahedron Lett 51:4991–4994. https://doi.org/10.1016/j.tetlet.2010.07.065
Chen T, Xu XP, Ji SJ (2013) Facile and efficient synthesis of indol-3-yl substituted pyran derivatives via one-pot multicomponent reactions under ultrasonic irradiation. J Heterocycl Chem 50:244–2051. https://doi.org/10.1002/jhet.983
Murali K, Arya KR, Prasad KJ (2015) Design and synthesis of pyrano [2,3-a] carbazoles by multicomponent reaction. Synth Commun 45:586–598. https://doi.org/10.1080/00397911.2014.956368
Kemnitzer W, Drewe J, Jiang S, Zhang H, Wang Y, Zhao J, Jia S, Herich J, Labreque D, Storer R, Meerovitch K (2004) Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high-throughput screening assay. 1. Structure–activity relationships of the 4-aryl group. J Med Chem 47:6299–6310. https://doi.org/10.1021/jm049640t
Akbarzadeh T, Rafinejad A, Mollaghasem JM, Safavi M, Fallah-Tafti A, Pordeli M, Ardestani SK, Shafiee A, Foroumadi A (2012) 2-Amino-3-cyano-4-(5-arylisoxazol-3-yl)-4H-chromenes: synthesis and in vitro cytotoxic activity. Arch Pharm 345:386–392. https://doi.org/10.1002/ardp.201100345
Chen C, Lu M, Liu Z, Wan J, Tu Z, Zhang T, Yan M (2013) Synthesis and evaluation of 2-amino-4H-pyran-3-carbonitrile derivatives as antitubercular agents. Open J Med Chem 3:128–135. https://doi.org/10.4236/ojmc.2013.34015
Thomas N, Zachariah SM, Ramani P (2016) 4-Aryl-4H-chromene-3-carbonitrile derivates: synthesis and preliminary anti-breast cancer studies. J Heterocycl Chem 53:1778–1782. https://doi.org/10.1002/jhet.2483
Balalaie S, Khazaie A, Ashouriha M (2013) One-pot synthesis of dihydropyrano [2,3-c] chromenes via a three-component reaction in aqueous media. Comb Chem High Throughput Screening 16:845–850. https://doi.org/10.2174/1386207311301010005
Gaikwad SS, Kishan SL, Suryawanshi VS, Kulkarni DR (2012) Synthesis and Biological activity of some 2-amino-4(-4-substituted phenyl)-6-(naphtho[2,1-b]furan-2yl)-4H-pyran-3-carbonitrile derivatives. Int J Basic Appl Res 3:80–83
Li JL, Li Q, Yang KC, Li Y, Zhou L, Han B, Peng C, Gou XJ (2016) A practical green chemistry approach to synthesize fused bicyclic 4H-pyranes via an amine catalysed 1,4-addition and cyclization cascade. RSC Adv 6:38875–38879. https://doi.org/10.1039/C6RA06441H
Sashidhara KV, Modukuri RK, Singh S, Rao KB, Teja GA, Gupta S, Shukla S (2015) Design and synthesis of new series of coumarin–aminopyran derivatives possessing potential anti-depressant-like activity. Bioorg Med Chem Lett 25:337–341. https://doi.org/10.1016/j.bmcl.2014.11.036
Mungra DC, Patel MP, Rajani DP, Patel RG (2011) Synthesis and identification of β-aryloxyquinolines and their pyrano [3,2-c] chromene derivatives as a new class of antimicrobial and antituberculosis agents. Eur J Med Chem 46:4192–4200. https://doi.org/10.1016/j.ejmech.2011.06.022
Seydimemet M, Ablajan K, Hamdulla M, Li W, Omar A, Obul M (2016) l-Proline catalyzed four-component one-pot synthesis of coumarin-containing dihydropyrano [2,3-c] pyrazoles under ultrasonic irradiation. Tetrahedron 72:7599–7605. https://doi.org/10.1016/j.tet.2016.10.016
Li Y, Chen H, Shi C, Shi D, Ji S (2010) Efficient one-pot synthesis of spirooxindole derivatives catalyzed by l-proline in aqueous medium. J Comb Chem 12:231–237. https://doi.org/10.1021/cc9001185
Poursattar Marjani A, Ebrahimi Saatluo B, Nouri F (2018) An efficient synthesis of 4H-chromene derivatives by a one-pot, three-component reaction. Iran J Chem Chem Eng (IJCCE) 37:149–157
Kalla RM, Choi JS, Yoo JW, Byeon SJ, Heo MS, Kim I (2014) Synthesis of 2-amino-3-cyano-4H-chromen-4-ylphosphonates and their anticancer properties. Eur J Med Chem 76:61–66. https://doi.org/10.1016/j.ejmech.2014.02.025
Satheesh M, Balachandran AL, Devi PR, Deepthi A (2018) An expedient synthesis of spirooxindoles incorporating 2-amino pyran-3-carbonitrile unit employing dialkyl acetone-1, 3-dicarboxylates. Synth Commun 48:582–587. https://doi.org/10.1080/00397911.2017.1416143
Shaabani A, Ghadari R, Ghasemi S, Pedarpour M, Rezayan AH, Sarvary A, Ng SW (2009) Novel one-pot three-and pseudo-five-component reactions: synthesis of functionalized benzo [g]-and dihydropyrano [2,3-g] chromene derivatives. J Comb Chem 11:956–959. https://doi.org/10.1021/cc900101w
Larionova NA, Zubarev AA, Rodinovskaya LA, Shestopalov AM (2013) New method for the synthesis of substituted thieno [3,2-b] pyridines and 5H-pyrano [2,3-d] thieno [3,2-b] pyridines derived from them. Russ Chem Bull 62:1304–1306. https://doi.org/10.1007/s11172-013-0182-2
Litvinov YM, Shestopalov AA, Rodinovskaya LA, Shestopalov AM (2009) New convenient four-component synthesis of 6-amino-2, 4-dihydropyrano [2,3-c] pyrazol-5-carbonitriles and one-pot synthesis of 6′-aminospiro [(3H)-indol-3,4′-pyrano [2,3-c] pyrazol]-(1H)-2-on-5′-carbonitriles. J Comb Chem 11:914–919. https://doi.org/10.1021/cc900076j
Shestopalov AM, Litvinov YM, Rodinovskaya LA, Malyshev OR, Semenova MN, Semenov VV (2012) Polyalkoxy substituted 4H-chromenes: synthesis by domino reaction and anticancer activity. ACS Comb Sci 14:484–490. https://doi.org/10.1021/co300062e
Ahmad S, Jalil S, Zaib S, Aslam S, Ahmad M, Rasul A, Arshad MN, Sultan S, Hameed A, Asiri AM, Iqbal J (2019) Synthesis, X-ray crystal and monoamine oxidase inhibitory activity of 4, 6-dihydrobenzo [c] pyrano [2,3-e][1,2] thiazine 5,5-dioxides: In vitro studies and docking analysis. Eur J Pharm Sci 131:9–22. https://doi.org/10.1016/j.ejps.2019.02.007
Mohareb RM, Abdo NY (2015) Synthesis and cytotoxic evaluation of pyran, dihydropyridine and thiophene derivatives of 3-acetylcoumarin. Chem Pharm Bull 63:678–687. https://doi.org/10.1248/cpb.c15-00115
Azzam RA, Mohareb RM (2015) Multicomponent reactions of acetoacetanilide derivatives with aromatic aldehydes and cyanomethylene reagents to produce 4H-pyran and 1,4-dihydropyridine derivatives with antitumor activities. Chem Pharm Bull 63:1055–1064. https://doi.org/10.1248/cpb.c15-00685
Mohareb R, MegallyAbdo N (2015) Uses of 3-(2-bromoacetyl)-2H-chromen-2-one in the synthesis of heterocyclic compounds incorporating coumarin: synthesis, characterization and cytotoxicity. Molecules 20:11535–11553. https://doi.org/10.3390/molecules200611535
Padmaja P, Rao GK, Indrasena A, Reddy BV, Patel N, Shaik AB, Reddy N, Dubey PK, Bhadra MP (2015) Synthesis and biological evaluation of novel pyrano [3,2-c] carbazole derivatives as anti-tumor agents inducing apoptosis via tubulin polymerization inhibition. Org Biomol Chem 13:1404–1414. https://doi.org/10.1039/C4OB02015D
Costa M, Areias F, Abrunhosa L, Venâncio A, Proença F (2008) The condensation of salicylaldehydes and malononitrile revisited: synthesis of new dimeric chromene derivatives. J Org Chem 73:1954–1962. https://doi.org/10.1021/jo702552f
Kalalbandi VK, Bijjaragi SC, Seetharamappa J (2018) multicomponent synthesis and antimicrobial activity of dihydropyran-bis coumarins. ChemistrySelect 3:3925–3929. https://doi.org/10.1002/slct.201800335
Upadhyay KD, Dodia NM, Khunt RC, Chaniara RS, Shah AK (2018) Synthesis and biological screening of pyrano [3,2-c] quinoline analogues as anti-inflammatory and anticancer agents. ACS Med Chem Lett 9:283–288. https://doi.org/10.1021/acsmedchemlett.7b00545
Wu B, Gao X, Yan Z, Huang WX, Zhou YG (2015) Enantioselective synthesis of functionalized 2-amino-4H-chromenes via the o-quinone methides generated from 2-(1-tosylalkyl) phenols. Tetrahedron Lett 56:4334–4338. https://doi.org/10.1016/j.tetlet.2015.05.076
Ding D, Zhao CG (2010) Organocatalyzed synthesis of 2-amino-8-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles. Tetrahedron Lett 51:1322–1325. https://doi.org/10.1016/j.tetlet.2009.12.139
Wang DC, Xie YM, Fan C, Yao S, Song H (2014) Efficient and mild cyclization procedures for the synthesis of novel 2-amino-4H-pyran derivatives with potential antitumor activity. Chin Chem Lett 25:1011–1013. https://doi.org/10.1016/j.cclet.2014.04.026
Esmati N, Foroughian M, Saeedi M, Mahdavi M, Khoshneviszadeh M, Firuzi O, Tanideh N, Miri R, Edraki N, Shafiee A, Foroumadi A (2015) Synthesis and cytotoxic activity of some novel dihyrobenzo [h] pyrano [3,2-c] chromene derivatives. J Heterocycl Chem 52:97–104. https://doi.org/10.1002/jhet.1991
Lu Y, Yan Y, Wang L, Wang X, Gao J, Xi T, Wang Z, Jiang F (2017) Design, facile synthesis and biological evaluations of novel pyrano [3,2-a] phenazine hybrid molecules as antitumor agents. Eur J Med Chem 127:928–943. https://doi.org/10.1016/j.ejmech.2016.10.068
Nikookar H, Mohammadi-Khanaposhtani M, Imanparast S, Faramarzi MA, Ranjbar PR, Mahdavi M, Larijani B (2018) Design, synthesis and in vitro α-glucosidase inhibition of novel dihydropyrano [3,2-c] quinoline derivatives as potential anti-diabetic agents. Bioorg Chem 77:280–286. https://doi.org/10.1016/j.bioorg.2018.01.025
Gao Y, Du DM (2013) Facile synthesis of chiral 2-amino-4-(indol-3-yl)-4H-chromene derivatives using thiourea as the catalyst. Tetrahedron Asymmetry 24:1312–1317. https://doi.org/10.1016/j.tetasy.2013.08.018
Hu ZP, Lou CL, Wang JJ, Chen CX, Yan M (2011) Organocatalytic conjugate addition of malononitrile to conformationally restricted dienones. J Org Chem 76:3797–3804. https://doi.org/10.1021/jo200112r
Cui L, Wang Y, Zhou Z (2016) Enantioselective synthesis of 7H-pyrano [2,3-d] thiazoles via squaramide-catalyzed [2 + 4] annulation of malononitrile and 5-ylidenethiazol-4-ones. Tetrahedron Asymmetry 27:1056–1061. https://doi.org/10.1016/j.tetasy.2016.08.014
Chen WB, Wu ZJ, Pei QL, Cun LF, Zhang XM, Yuan WC (2010) Highly enantioselective construction of spiro [4H-pyran-3,3′-oxindoles] through a domino Knoevenagel/Michael/cyclization sequence catalyzed by cupreine. Org Lett 12:3132–3135. https://doi.org/10.1021/ol1009224
Youseftabar-Miri L (2019) A clean and efficient synthesis of spiro [4H-pyran-oxindole] derivatives catalyzed by egg shell. Iran Chem Commun 7:142–152
Bhosale HD, Shisodia SU, Ingle RD, Kendrekar PS, Shisodia AU, Kótai L, Pawar RP (2018) An expeditious and green approach for the synthesis of 2-amino-4H-chromenes using a catalyst of natural origin. Eur Chem Bull 7:120–122. https://doi.org/10.17628/ecb.2018.7.120-122
Hatamjafari F (2016) Glutamic acid as an environmentally friendly catalyst for one-pot synthesis of 4H-chromene derivatives and biological activity. J Chem Health Risks 6:133–142
Valekar NJ, Patil PP, Gore AH, Kolekar GB, Deshmukh MB, Anbhule PV (2015) Sequence selective michael addition for synthesis of indeno-pyridine and indeno-pyran derivatives in one-pot reaction using CuO nanoparticles in water. J Heterocycl Chem 52:1669–1676. https://doi.org/10.1002/jhet.2228
Paul S, Bhattacharyya P, Das AR (2011) One-pot synthesis of dihydropyrano [2,3-c] chromenes via a three component coupling of aromatic aldehydes, malononitrile, and 3-hydroxycoumarin catalyzed by nano-structured ZnO in water: a green protocol. Tetrahedron Lett 52:4636–4641. https://doi.org/10.1016/j.tetlet.2011.06.101
Rajesh UC, Wang J, Prescott S, Tsuzuki T, Rawat DS (2014) RGO/ZnO nanocomposite: an efficient, sustainable, heterogeneous, amphiphilic catalyst for synthesis of 3-substituted indoles in water. ACS Sustain Chem Eng 3:9–18. https://doi.org/10.1021/sc500594w
Ghashang M (2016) ZnAl2O4–Bi2O3 composite nano-powder as an efficient catalyst for the multi-component, one-pot, aqueous media preparation of novel 4H-chromene-3-carbonitriles. Res Chem Intermed 42:4191–4205. https://doi.org/10.1007/s11164-015-2269-x
Azarifar D, Ghaemi M, Golbaghi M, Karamian R, Asadbegy M (2016) Synthesis and biological evaluation of new pyranopyridine derivatives catalyzed by guanidinium chloride-functionalized γ-Fe2O3/HAp magnetic nanoparticles. RSC Adv 6:92028–92039. https://doi.org/10.1039/C6RA15781E
Pourian E, Javanshir S, Dolatkhah Z, Molaei S, Maleki A (2018) Ultrasonic-assisted preparation, characterization, and use of novel biocompatible core/shell Fe3O4@GA@isinglass in the synthesis of 1, 4-dihydropyridine and 4 H-pyran derivatives. ACS Omega 3:5012–5020. https://doi.org/10.1021/acsomega.8b00379
Azarifar D, Mahmoudi-GomYek S, Ghaemi M (2018) Immobilized Cu (II) Schiff base complex supported on Fe3O4 magnetic nanoparticles: a highly efficient and reusable new catalyst for the synthesis of pyranopyridine derivatives. Appl Organomet Chem 32:e4541. https://doi.org/10.1002/aoc.4541
Maleki A, Azizi M, Emdadi Z (2018) A novel poly (ethyleneoxide)-based magnetic nanocomposite catalyst for highly efficient multicomponent synthesis of pyran derivatives. Green Chem Lett Rev 11:573–582. https://doi.org/10.1080/17518253.2018.1547795
Eftekhari-Sis B, Sarvari Karajabad M, Haqverdi S (2017) Pyridylmethylaminoacetic acid functionalized Fe3O4 magnetic nanorods as an efficient catalyst for the synthesis of 2-aminochromene and 2-aminopyran derivatives. Sci Iran 24:3022–3031
Solhy A, Elmakssoudi A, Tahir R, Karkouri M, Larzek M, Bousmina M, Zahouily M (2010) Clean chemical synthesis of 2-amino-chromenes in water catalyzed by nanostructured diphosphate Na2CaP2O7. Green Chem 12:2261–2267. https://doi.org/10.1039/C0GC00387E
Sagar Vijay Kumar P, Suresh L, Vinodkumar T, Reddy BM, Chandramouli GV (2016) Zirconium doped ceria nanoparticles: an efficient and reusable catalyst for a green multicomponent synthesis of novel Phenyldiazenyl–chromene derivatives using aqueous medium. ACS Sustain Chem Eng 4:2376–2386. https://doi.org/10.1021/acssuschemeng.6b00056
Baghbanian SM, Rezaei N, Tashakkorian H (2013) Nanozeolite clinoptilolite as a highly efficient heterogeneous catalyst for the synthesis of various 2-amino-4 H-chromene derivatives in aqueous media. Green Chem 15:3446–3458. https://doi.org/10.1039/C3GC41302K
Mostafa EA, Khatab TK (2018) Silica supported V2O5 as a catalyst promoted the synthesis of 4h-pyrans through multicomponent reaction under solvent free conditions. Org Chem Indian J 14:1–6
Kumar GS, Zeller M, Frasso MA, Prasad KJ (2015) InCl3 promoted synthesis of pyrano [3,2-h] quinolines via microwave irradiation. J Heterocycl Chem 52:926–930. https://doi.org/10.1002/jhet.2067
Shanthi G, Perumal PT (2007) An eco-friendly synthesis of 2-aminochromenes and indolyl chromenes catalyzed by InCl3 in aqueous media. Tetrahedron Lett 48:6785–6789. https://doi.org/10.1016/j.tetlet.2007.07.102
Lakshmi NV, Kiruthika SE, Perumal PT (2011) A rapid and efficient access to 4-substituted 2-amino-4H-chromenes catalyzed by InCl3. Synlett 2011:1389–1394
Yamuna E, Rajendra Prasad KJ (2014) InCl3-assisted synthesis of pyrano [2,3-a] carbazoles via multicomponent reaction. Synth Commun 44:2656–2661. https://doi.org/10.1080/00397911.2014.910526
Heravi MM, Daraie M (2014) Heterogeneous catalytic three-component one-pot synthesis of novel 8H-[1,3] dioxolo [4,5-g] chromenes by basic alumina in water. Monatshefte Chem Chem Mon 145:1479–1482. https://doi.org/10.1007/s00706-014-1201-1
Pasdar H, Foroughifar N, Hedayati Saghavaz B (2015) Investigation into the antibacterial activity of metal complexes derived from substituted chromone in comparison with tetracycline, and cephradine as standard drugs against Escherichia coli and Staphylococcus aureus. J Med Microbiol Infect Dis 3:75–79
Jabbarzarea S, Ghashangb M (2015) Na2O–Al2O3–P2O5 glass-ceramic system: efficient catalyst for the aqueous media preparation of pyrano [2,3-e] benzoxazole derivatives. Lett Org Chem 12:713–719. https://doi.org/10.1002/chin.201611070
Ballini R, Bosica G, Conforti ML, Maggi R, Mazzacani A, Righi P, Sartori G (2001) Three-component process for the synthesis of 2-amino-2-chromenes in aqueous media. Tetrahedron 57:1395–1398. https://doi.org/10.1016/S0040-4020(00)01121-2
Khurana JM, Magoo D, Chaudhary A (2012) Efficient and green approaches for the synthesis of 4 H-Benzo [g] chromenes in water, under neat conditions, and using task-specific ionic liquid. Synth Commun 42:3211–3219. https://doi.org/10.1080/00397911.2011.580069
Haouchine AL, Kabri Y, Bakhta S, Curti C, Nedjar-Kolli B, Vanelle P (2018) Simple synthesis of imidazo [1,2-A] pyridine derivatives bearing 2-aminonicotinonitrile or 2-aminochromene moiety. Synth Commun 48:2159–2168. https://doi.org/10.1080/00397911.2018.1479759
Ren Y, Yang B, Liao X (2016) The amino side chains do matter: chemoselectivity in the one-pot three-component synthesis of 2-amino-4 H-chromenes by supramolecular catalysis with amino-appended β-cyclodextrins (ACDs) in water. Catal Sci Technol 6:4283–4293. https://doi.org/10.1039/C5CY01888A
Fan X, Feng D, Qu Y, Zhang X, Wang J, Loiseau PM, Andrei G, Snoeck R, De Clercq E (2010) Practical and efficient synthesis of pyrano [3,2-c] pyridone, pyrano [4,3-b] pyran and their hybrids with nucleoside as potential antiviral and antileishmanial agents. Bioorg Med Chem Lett 20:809–813. https://doi.org/10.1016/j.bmcl.2009.12.102
Li Y, Zhao B, Du B, Jiang Q, Wang X, Cao C (2013) Efficient and mild one-pot three-component reaction to synthesize pyrano [3,2-b] pyran derivatives in ionic liquid. Tetrahedron Lett 54:227–230. https://doi.org/10.1016/j.tetlet.2012.11.006
Abbaspour-Gilandeh E, Azimi SC, Rad-Moghadam K, Mohammadi-Barkchai A (2013) A green, efficient, and rapid procedure for the synthesis of pyrano [3,2-c] quinoline and pyrano [3,2-c] pyridone derivatives catalyzed by [BMIm] Cl. Iran J Catal 3:15–20
Tashrifi Z, Rad-Moghadam K, Mehrdad M (2017) Catalytic performance of a new Brønsted acidic oligo (ionic liquid) in efficient synthesis of pyrano [3,2-c] quinolines and pyrano [2,3-d] pyrimidines. J Mol Liq 248:278–285. https://doi.org/10.1016/j.molliq.2017.10.065
Mane VU, Chavan SM, Choudhari BR, Mane DV (2019) Microwave assisted synthesis of tetrahydrobenzo[b]pyrans via one pot multicomponent reaction using [Et3NH][HSO4] as ionic liquid catalyst. J Pharm Chem Biol Sci 6:311–319
Bhupathi R, Madhu B, Devi BR, Reddy CV, Dubey PK (2016) DBU acetate mediated: one-pot multi component syntheses of dihydropyrano [3,2-c] quinolones. J Heterocycl Chem 53:1911–1916. https://doi.org/10.1002/jhet.2506
Rajesh UC, Kholiya R, Thakur A, Rawat DS (2015) [TBA][Gly] ionic liquid promoted multi-component synthesis of 3-substituted indoles and indolyl-4H-chromenes. Tetrahedron Lett 56:1790–1793. https://doi.org/10.1016/j.tetlet.2015.02.058
Reddy GM, Garcia JR, Zyryanov GV, Sravya G, Reddy NB (2019) Pyranopyrazoles as efficient antimicrobial agents: green, one pot and multicomponent approach. Bioorg Chem 82:324–331. https://doi.org/10.1016/j.bioorg.2018.09.035
Safaei HR, Shekouhy M, Rahmanpur S, Shirinfeshan A (2012) Glycerol as a biodegradable and reusable promoting medium for the catalyst-free one-pot three component synthesis of 4H-pyrans. Green Chem 14:1696–1704. https://doi.org/10.1039/C2GC35135H
Zonouz AM, Eskandari I, Khavasi HR (2012) A green and convenient approach for the synthesis of methyl 6-amino-5-cyano-4-aryl-2, 4-dihydropyrano [2,3-c] pyrazole-3-carboxylates via a one-pot, multi-component reaction in water. Tetrahedron Lett 53:5519–5522. https://doi.org/10.1016/j.tetlet.2012.08.010
Zhang M, Fu QY, Gao G, He HY, Zhang Y, Wu YS, Zhang ZH (2017) Catalyst-free, visible-light promoted one-pot synthesis of spirooxindole-pyran derivatives in aqueous ethyl lactate. ACS Sustain Chem Eng 5:6175–6182
Makarem S, Mohammadi AA, Fakhari AR (2008) Tetrahedron Lett 49:7194–7196. https://doi.org/10.1021/acssuschemeng.7b01102
Sharma S, Sharma K, Ojha R, Kumar D, Singh G, Nepali K, Bedi PM (2014) Microwave assisted synthesis of naphthopyrans catalysed by silica supported fluoroboric acid as a new class of non purine xanthine oxidase inhibitors. Bioorg Med Chem Lett 24:495–500. https://doi.org/10.1016/j.bmcl.2013.12.031
Pacher PA, Nivorozhkin A, Szabó C (2006) Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58:87–114. https://doi.org/10.1124/pr.58.1.6
Acknowledgements
This research was supported by a grant from Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tashrifi, Z., Mohammadi-Khanaposhtani, M., Hamedifar, H. et al. Synthesis and pharmacological properties of polysubstituted 2-amino-4H-pyran-3-carbonitrile derivatives. Mol Divers 24, 1385–1431 (2020). https://doi.org/10.1007/s11030-019-09994-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09994-9