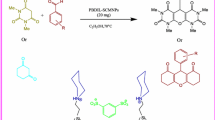
Abstract
Mo(IV) salen complex (2.5 mol%) was found to be a highly efficient catalyst for the one-pot synthesis of 2,4,5-triarylimidazoles via a three-component reaction using benzil or benzoin, aryl aldehydes, and ammonium acetate as a nitrogen source under mild conditions. In order to recover and the reuse of the catalyst, a new Mo(IV) salen–silica nanoparticle as heterogeneous catalyst was prepared by simple and successful immobilization of the catalyst onto silica (3-aminopropyl functionalized silica gel). This procedure can be applied to large-scale conditions with high efficiency. Experimental evidence showed that the catalyst is stable and can be easily recovered and reused for at least five times without significant loss of activity. The nanocatalyst was characterized using FT-IR spectroscopy, scanning electron microscopy, atomic force microscopy, powder X-ray diffraction , transmission electron microscopy, thermogravimetric instrument for analysis of nitrogen adsorption, and inductively coupled plasma spectrometer.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multicomponent reactions (MCRs) have attracted great interest in modern organic synthesis and the pharmaceutical industry because they are one of the best synthesis tools available for the preparation of molecular libraries in a cost and time effective way [1].
Compounds bearing imidazole ring systems are well known for their diverse biological activities and are the core fragment of different natural products [1–3]. Not only they have many beneficial pharmaceutical properties [4, 5], but are also used in photography as photosensitizer compounds [3].
A number of synthetic procedures have been developed for the synthesis of 2,4,5-trisubstituted imidazoles via multicomponent reactions. Generally, these imidazoles have been made via the multicomponent reaction of a 1,2-diketone, \(\alpha \)-hydroxy ketone, or \(\alpha \)-ketomonoxime [6] with ammonium acetate and an aldehyde in the presence of l-proline [1], scolecite [3], polymer-supported zinc chloride [5], \(\hbox {InCl}_{3}\cdot \hbox {3H}_{2}\hbox {O}\) [7], \(\hbox {ZrCl}_{4}\) [8], heteropoly acid supported on silica [9], \(\hbox {NiCl}_{2}\cdot \hbox {6H}_{2}\hbox {O}/\hbox {Al}_{2}\hbox {O}_{3}\) [10], \(\hbox {NaHSO}_{3}\) [11], sulfated tin oxide [12], iodine [13, 14], ceric ammonium nitrate (CAN) [15], \(\hbox {UO}_{2}(\hbox {NO}_{3})_{2}\cdot \hbox {6H}_{2}\hbox {O}\) [16], ionic liquids [17], and DABCO [18]. The major, most common drawbacks of these approaches include the use of elevated temperatures, long reaction times, harsh reaction conditions, and low yields. Therefore, continuing efforts are invested in the search for better catalysts (operational simplicity, economic viability, and reusability) for the synthesis of 2,4,5-trisubstituted imidazoles.
Heterogeneous catalysts are of great importance in green synthesis due to their easy recovery and reusability [19]. In this regard, in recent years significant efforts in catalysis research have been devoted to the identification and application of effective and safe heterogeneous catalysts [5]. In continuation of our previous study in the synthesis and applications of heterogeneous catalysts for the development of heterocyclic compounds [20–25], in this article we report a simple, mild, and efficient one-pot multicomponent method for the synthesis of 2,4,5-triarylimidazoles using Mo-salen complex as a homogeneous catalyst and Mo-salen complex nanoparticles onto silica as a heterogeneous nanocatalyst (2.5 mol%) in EtOH at \(50\; {^\circ }\hbox {C}\). Our methodology offers several advantages including substrate generality, catalyst recyclability, mild reaction conditions, large-scale synthesis, and high yields. This combination of attributes makes our methodology an attractive approach for the synthesis of 2,4,5-triarylimidazoles.
Results and discussion
Catalytic role of metal-salen complexes for the synthesis of 2,4,5-trisubstituted imidazoles
As a part of our continuing efforts in the synthesis of organic compounds using efficient and novel catalysts [20–24], we explored the synthesis of 2,4,5-trisubstituted imidazoles using metallosalens as catalyst. We found that these complexes effectively catalyze the condensation reaction of benzil and ammonium acetate with different aryl aldehydes to form 2,4,5-triarylimidazoles in ethanol under mild conditions (Scheme 1). The condensation of benzil, ammonium acetate, and benzaldehyde was chosen as our model reaction.
The impact of the different metallic ions in metallosalen on the performance of the reaction was examined. The reaction times and yields using various metal-salen complexes are summarized in Table 1 (entries 3–9). It was determined that metal-salen complexes containing Co, Cu, Mn, Fe, V, and Cr ions gave low to moderate yields, and using larger amounts did not improve the output of the reaction. On the other hand, among the metal-incorporated tested, Mo-salen complex was found to improve greatly both reaction rate and yield.
Then, we studied the impact of using different catalytic amounts of Mo-salen in the synthesis of 2,4,5-triphenyl-\(1H\)-imidazole using of benzil (1.0 mmol), ammonium acetate (2.0 mmol), and benzaldehyde (1.0 mmol). The best results were obtained when 2.5 mol% of Mo-salen complex was used since lower catalytic amounts led to lower yields, whereas higher amounts did not improve appreciably both yield and reaction time (Table 1, entries 9–12). To examine the essential role of Mo-salen complex, the reactions were carried out in the absence or presence of ligand (Table 1, entries 1 and 2). In both cases, only traces of the desired product were detected indicating the crucial role the catalyst has in the reaction.
We also studied the temperature effect on the reaction (Scheme 1). The best results were obtained at \(50\; {^\circ }\hbox {C}\) when using ethanol for 1 h in the presence of Mo-salen complex (2.5 mol%) as a catalyst (Table 1).
The choice of solvent is also a very important factor for multicomponent reactions. During our optimization studies, the solvent effect was also examined. The model reaction was carried out using a variety of solvents such as EtOH, MeOH, \(\hbox {CH}_{3}\hbox {CN}\), DMF, \(\hbox {CH}_{2}\hbox {Cl}_{2}, \hbox { CHCl}_{3}\), THF, 1,4-dioxane, and toluene. From these, ethanol was selected because it contributed to faster reaction rates, excellent product yield, and its environmental acceptability.
Using our optimized reaction conditions, we pursued the preparation of 2,4,5-triphenyl-\(1H\)-imidazole this time using benzoin instead of benzil as a starting material (Scheme 2). We found that while 2,4,5-triphenyl-\(1H\)-imidazole was obtained in excellent yield (90 %), the reaction time increased (1.5 h).
A plausible mechanism for the Mo-salen complex catalyzed synthesis of 2,4,5-trisubstituted imidazoles has been proposed [13–18] (Scheme 3). Firstly, ammonium acetate dissociates into ammonia which is required for the initial condensation. Upon formation of diamine intermediate A by the reaction of an aryl aldehyde and ammonia in the presence of Mo-salen complex catalyst. The Mo-salen complex facilitates the formation of a diamine intermediate A which could be attributed to an increase in the electrophilicity of the carbonyl group. The intermediate A, in the presence of Mo-salen complex reacts with the benzil followed by dehydration to afford the imino intermediate B, which in turn rearranges to the 2,4,5-trisubstituted imidazole by a [1,5]-hydrogen shift [7].
We also propose a mechanism for the Mo-salen complex catalyzed reaction for the preparation of 2,4,5-trisubstituted imidazoles when the benzoin was used instead of benzil as a starting material, similar to the reported experiment for the synthesis of 2,4,5-triaryl imidazoles by a ionic liquid [17] at \(100\; {^\circ }\hbox {C}\) and \(\hbox {I}_{2}\) at reflux condition [14]. A solution of benzoin in the presence of Mo-salen complex was stirred in EtOH at \(50\; {^\circ }\hbox {C}\) for several hours in an atmosphere of air. Benzoin was converted to benzil in excellent yield. The formed benzil then reacts with intermediate A followed by dehydration to yield the imino intermediate B, which in turn rearranges to the product by a [1, 5]-hydrogen shift (Scheme 3).
The most significant advantages of heterogeneous catalysts are the possibility of catalyst recycling, ease of separation, and reuse of the catalyst. Since Mo-salen complex was proven to be the best catalyst among the other metal-salen complexes tested, we aimed at preparing a new heterogeneous catalyst via a simple impregnation of Mo-salen complex onto 3-aminopropyl functionalized silica gel.
In industry the reusability of a catalyst is important particularly for large-scale operations. In this regard, the recovery and reusability of the catalyst was tested. The advantage of recovery of catalyst for different uses has been provided by utilizing the Mo-salen complex nanoparticles supported onto silica. The recovered Mo-salen complex onto silica was dried and reused several times without observing a decrease in catalytic performance.
To determine if leaching of the catalyst takes place, the heterogeneous catalyst was stirred in EtOH at \(50\; {^\circ }\hbox {C}\) for 120 min. The catalyst was recovered and the filtrate was used for the preparation of 2,4,5-triphenyl-\(1H\)-imidazole under the optimized conditions for 3 h. Since no product was formed, it was concluded that no leaching took place. Moreover, the ICP results were obtained for the Mo-salen complex after reusing the catalyst for at least five times.
To test this method on a preparative scale, we carried out the synthesis of 2,4,5-triphenyl-\(1H\)-imidazole under the optimized conditions at a 50 mmol scale and found that the desired imidazole product was obtained in excellent yield after 70 min.
Synthesis of different 2,4,5-trisubstituted imidazoles using homogeneous and heterogeneous catalysts
In the next step, we used the optimized conditions using different aromatic aldehydes in the presence of Mo(IV) salen complex and Mo(IV) salen complex nanoparticles onto silica (2.5 mol%) to synthesize different 2,4,5-trisubstituted imidazoles (Scheme 4).
We found in all cases the reactions were clean taking place between 40 and 170 min. Heteroaryl aldehydes, such as 2-thiophenecarboxaldehyde, 2-pyridinylcarboxaldehyde, and 4-pyridinylcarboxaldehyde, (Table 2, entries 9–11), were also well tolerated under these mild conditions. We found that our method is compatible with functional groups such as Me, OMe, Cl, \(\hbox {NO}_{2}\), and OH.
In the presence of Lewis acids, such as \(\hbox {InCl}_{3}\cdot \hbox {3H}_{2}\hbox {O}\) [7] and \(\hbox {ZrCl}_{4}\) [8], the reaction yields and times are comparable to our method, but the reaction either needs higher temperature or a more toxic solvent (Table 3).
Conclusion
We have developed a simple, mild, and efficient one-pot three-component methodology for the preparation of 2,4,5-trisubstituted imidazoles in the presence of Mo-salen complex and Mo-salen complex nanoparticles onto silica (2.5 mol%) in ethanol at 50\({^\circ }\hbox {C}\). The catalyst is stable and can be easily recovered and reused at least five times without significant loss of performance. These attributes make our procedure convenient, mild, economic, green, and efficient for the synthesis of 2,4,5-trisubstituted imidazoles.
Experimental section
Instrumentation, analysis, and starting material
Chemical materials and solvents were purchased from Fluka, Aldrich and Merck. The used aminopropyl silica gel was also purchased from Fluka. NMR spectra were recorded on a Bruker Avance DPX-250 (\(^{1}\)H-NMR 250 MHz and \(^{13}\hbox {C}\) NMR 62.9 MHz) spectrometer using TMS as the internal standard in pure deuterated chloroform and dimethyl sulfoxide. The following NMR spectra abbreviations are used: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and dd = doublet of doublets. IR spectra were obtained by a Shimadzu FT-IR 8300 spectrophotometer. SEM instrumentation is used for Scanning electron micrographs (SEM, XL-30 FEG SEM, Philips, at 20 KV). AFM images are also obtained by an atomic forced microscope (AFM, DME-SPM, version 2.0.0.9). Mass spectra were determined at 70 or 20 ev on a Shimadzu GCMS-QP 1000 EX instruments. Melting points were obtained in open capillary tubes using a Büchi-535 circulating oil melting point apparatus (melting points are uncorrected). Catalysts were characterized using X-ray diffraction (XRD, D8, Advance, Bruker, axs). Thermogravimetry analysis (TG) was carried out using a lab-made TGA instrument. ICP analysis was conducted using a Varian Vista-pro analyzer. The purity of substrates and reaction monitoring were determined by TLC on silica gel PolyGram SILG/UV 254 plates. Column chromatography was performed in glass columns on short columns of silica gel 60 (70–230 mesh). Schiff-base complexes and Schiff-base \(N,N'\)-(salicylaldehyde)ethylenediamine (Salen) were prepared according to the literature [20, 21, 25]. Mo-salen complex was synthesized according to the literature [25].
Immobilization of Mo-salen complexes onto silica
3-Aminopropyl functionalized silica gel (1.0 g) and Mo-salen complex (0.05 g) in 50 mL of dry toluene were mixed. The mixture was stirred under reflux for 4 h. The solid was separated by centrifugation, washed several times with toluene and ethanol, and then dried in vacuum at \(60\,^{\circ }\hbox {C}\).
General procedure for synthesis of 2,4,5-triarylimiazole derivatives in the presence of a homogeneous catalyst
A mixture of benzil or benzoin (1.0 mmol), an aromatic aldehyde (1.0 mmol), ammonium acetate (2.0 mmol), and Mo-salen complex (2.5 mol %) was stirred in EtOH (96 %, 10 mL) at 50 \({^\circ }\hbox {C}\) for the time specified in Table 2. The reaction was monitored by TLC. After reaction completion, the solvent was evaporated to give the crude product, which was purified by silica gel column chromatography employing n-hexane/ethyl acetate (7:1) as eluent.
General procedure for synthesis of 2,4,5-triarylimidazole derivatives in the presence of a heterogeneous catalyst
For each reaction, benzil or benzoin (1.0 mmol), an aryl aldehyde (1.0 mmol), and ammonium acetate (2.0 mmol) was stirred in 10.0 mL of EtOH (96 %) in the presence of heterogeneous catalyst (2.5 mol%) at 50 \({^\circ }\hbox {C}\). The reactions were monitored by TLC using n-hexane/ethyl acetate (7:1). After the completion of the reaction, the reaction mixture was centrifuged and rinsed with EtOH (3 \(\times \) 15 mL). The recovered catalyst was stored for future reaction. The combined filtrate was concentrated to give crude product, which was purified by silica gel column chromatography employing \(n\)-hexane / ethyl acetate (7:1) as eluent.
References
Samai S, Nandi GC, Singh P, Singh MS (2009) L-Proline: an efficient catalyst for the one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron 65:10155–10161. doi:10.1016/j.tet.2009.10.019
Lambardino JG, Wiseman EH (1974) Preparation and antiinflammatory activity of some nonacidic trisubstituted imidazoles. J Med Chem 17:1182–1188. doi:10.1021/jm00257a011
Gadekar LS, Mane SR, Katkar SS, Arbad BR, Lande MK (2009) Scolecite as an efficient heterogeneous catalyst for the synthesis of 2,4,5-triarylimidazoles. Cent Eur J Chem 7:550–554. doi:10.2478/s11532-009-0050-y
Balalaie S, Arabanian A (2000) One-pot synthesis of tetrasubstituted imidazoles catalyzed by zeolite HY and silica gel under microwave irradiation. Green Chem 2:274–276. doi:10.1039/b006201o
Wang L, Cai C (2009) Polymer-supported zinc chloride: a highly active and reusable heterogeneous catalyst for one-pot synthesis of 2,4,5-trisubstituted imidazoles. Monatsh Chem 140:541–546. doi:10.1007/s00706-008-0086-2
Sparks RB, Combs AP (2004) Microwave-assisted synthesis of 2,4,5-triaryl-imidazole; a novel thermally induced N-hydroxyimidazole N–O bond cleavage. Org Lett 6:2473–2475. doi:10.1021/ol049124x
Sharma D, Hazarika P, Konwar D (2008) An efficient and one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by \(\text{ InCl }_{3}.\text{3H }_{2}\text{ O }\). Tetrahedron Lett 49:2216–2220. doi: 10.1016/j.tetlet.2008.02.053
Sharma G, Jyothi Y, Lakshmi P (2006) Efficient room-temperature synthesis of tri- and tetrasubstituted imidazoles catalyzed by \(\text{ ZrCl }_{4}\). Synth Commun 36:2991–3000. doi: 10.1080/00397910600773825
Karimim AR, Alimohammadi Z, Amini MM (2010) Wells-Dawson heteropolyacid supported on silica: a highly efficient catalyst for synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Mol Divers 14:635–641. doi:10.1007/s11030-009-9197-x
Heravi MM, Bakhtiari K, Oskooie HA, Taheri S (2007) Synthesis of 2,4,5-triaryl-imidazoles catalyzed by \(\text{ NiCl }_{2}\cdot \text{6H }_{2}\text{ O }\) under heterogeneous system. J Mol Catal A: Chem 263:279–281. doi:10.1016/j.molcata.2006.08.070
Sangshetti JN, Kokare ND, Kotharkar SA, Shinde DB (2008) Sodium bisulfite as an efficient and inexpensive catalyst for the one-pot synthesis of 2,4,5-triaryl-1\(H\)-imidazoles from benzil or benzoin and aromatic aldehydes. Mont Fur Chem 139:125–127. doi: 10.1007/s00706-007-0766-3
Dake SA, Khedkar MB, Irmale GS, Ukalgaonkar SJ, Thorat VV, Shintre SA, Pawar RP (2012) Sulfated tin oxide: a reusable and highly efficient heterogeneous catalyst for the synthesis of 2,4,5-triaryl-\(1H\)-imidazole derivatives. Synth Commun 42:1509–1520. doi:10.1080/00397911.2010.541744
Parveen A, Ahmed MD, Rafi SK, Shaikh KA, Deshmukh SP, Pawar RP (2007) Efficient synthesis of 2,4,5-triaryl substituted imidazoles under solvent free conditions at room temperature. Arkivoc 16:12–18. doi:10.3998/ark.5550190.0008.g02
Kidwai M, Mothsra P, Bansal V, Goyal R (2006) Efficient elemental iodine catalyzed one-pot synthesis of 2,4,5-triarylimidazoles. Mont Fur Chem 137:1189–1194. doi:10.1007/s00706-006-0518-9
Sangshetti JN, Kakare ND, Kotharkar SA, Shinde DB (2008) Ceric ammonium nitrate catalysed three component one-pot efficient synthesis of 2,4,5-triaryl-1\(H\)-imidazoles. J Chem Sci 120:463–467. doi: 10.1007/s12039-008-0072-6
Satyanarayana VSV, Sivakumar A (2011) An efficient and novel one-pot synthesis of 2,4,5-triaryl-1H-imidazoles catalyzed by \(\text{ UO }_{2}(\text{ NO }_{3})_{2}.\text{6H }_{2}\text{ O }\) under heterogeneous conditions. Chem Pap 65:519–526. doi:10.2478/s11696-011-0028-z.ISSN:0366-6352
Siddiqui SA, Narkhede UC, Palimkar SS, Daniel T, Lahoti RJ, Srinivasan KV (2005) Room temperature ionic liquid promoted improved and rapid synthesis of 2,4,5-triaryl imidazoles from aryl aldehydes and 1,2-diketones or \(\alpha \)-hydroxyketone. Tetrahedron 61:3539–3546. doi: 10.1016/j.tet.2005.01.116
Murthy SN, Madhav B, Nageswar YVD (2010) DABCO as a mild and efficient catalytic system for the synthesis of highly substituted imidazoles via multi-component condensation strategy. Tetrahedron Lett 51:5252–5257. doi:10.1016/j.tetlet.2010.07.128
Surpur MP, Kshirsagar S, Samant SD (2009) Exploitation of the catalytic efficacy of Mg/Al hydrotalcite for the rapid synthesis of 2-aminochromene derivatives via a multicomponent strategy in the presence of microwaves. Tetrahedron Lett 50:719–722. doi:10.1016/j.tetlet.2008.11.114
Sharghi H, Aberi M, Doroodmanda MM (2008) Reusable cobalt(III)-salen complex supported on activated carbon as an efficient heterogeneous catalyst for synthesis of 2-arylbenzimidazole derivatives. Adv Synth Catal 350:2380–2390. doi:10.1002/adsc.200800317
Sharghi H, Aberi M, Doroodmanda MM (2012) One-pot synthesis of 2-arylbenzimidazole, 2-arylbenzothiazole and 2-arylbenzoxazole derivatives using vanadium(IV)-salen complex as homogeneous catalyst and vanadium(IV)-salen complex nanoparticles immobilized onto silica as a heterogeneous nanocatalyst. J Iran Chem Soc 9:189–204. doi:10.1007/s13738-011-0045-4
Sharghi H, Khoshnood A, Doroodmand MM, Khalifeh R (2012) 1,4-Dihydroxyanthraquinone-copper(II) nanoparticles immobilized on silica gel: a highly efficient, copper scavenger and recyclable heterogeneous nanocatalyst for a click approach to the three-component synthesis of 1,2,3-triazole derivatives in water. J Iran Chem Soc 9:231–250. doi:10.1007/s13738-011-0046-3
Sharghi H, Khalifeh R, Mansouri SG, Aberi M, Eskandari MM (2011) Simple, efficient, and applicable route for synthesis of 2-aryl(heteroaryl)-benzimidazoles at room temperature using copper nanoparticles on activated carbon as a reusable heterogeneous catalyst. Catal Lett 141:1845–1850. doi:10.1007/s10562-011-0671-6
Sharghi H, Ebrahimpourmoghaddam S, Doroodmand MM (2013) Facile synthesis of 5-substituted-1\(H\)-tetrazoles and 1-substituted-1\(H\)-tetrazoles catalyzed by recyclable 4\(^{\prime }\)-phenyl-2,2\(^{\prime }\):6\(^{\prime }\),2\(^{\prime \prime }\)-terpyridine copper(II) complex immobilized onto activated multi-walled carbon nanotubes. J Organomet Chem 738:41–48. doi: 10.1016/j.jorganchem.2013.04.013
Sabry DY, Youssef TA, El-Medani SM, Ramadan RM (2003) Reactions of chromium and molybdenum carbonyls with bis-(salicylaldehyde)ethylenediimine schiff-base ligand. J Coord Chem 56:1375–1381. doi:10.1080/00958970310001636471
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharghi, H., Aberi, M. & Doroodmand, M.M. A mild, three-component one-pot synthesis of 2,4,5-trisubstituted imidazoles using Mo(IV) salen complex in homogeneous catalytic system and Mo(IV) salen complex nanoparticles onto silica as a highly active, efficient, and reusable heterogeneous nanocatalyst. Mol Divers 19, 77–85 (2015). https://doi.org/10.1007/s11030-014-9558-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9558-y