Abstract
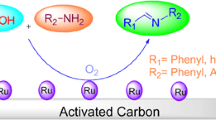
A series of 2-(hetero)arylbenzimidazoles were synthesized by the catalytic condensation of (hetero)aryl aldehydes with 1,2-phenylenediamine derivatives at room temperature in the presence of air as the oxidant. Copper nanoparticles on charcoal was employed as an efficient and mild catalyst for this methodology.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
2-Aryl(heteroaryl)-substituted benzimidazoles have received a considerable amount of attention in diverse areas of chemistry. These compounds exhibit a wide spectrum of biological and pharmacological activities [1–7]. Also, 2-aryl benzimidazole derivatives are fluorescent acid–base indicators [8], dopants for plastic scintillation applications [9] and subunits of polybenzimidazoles as thermally stable polymers [10].
Because of their importance and wide applications, the synthesis of these benzimidazole derivatives has become a focus of synthetic organic chemistry.
Two main synthetic routes could be observed in all of these reported procedures. One route that is common to the 2-aryl benzimidazoles synthesis typically involves direct coupling of a carboxylic acid or carboxylic acid derivatives with an appropriate 1,2-phenylenediamine under the influence of a strong acid such as hydrochloric acid [11] or polyphosphoric acid [12] at high temperature or under microwave irradiation [13]. The other important way involves a two-step procedure that includes the oxidative cyclo-dehydrogenation of Schiff bases, which are often generated from the condensation of o-phenylenediamines and aryl aldehydes. Various oxidative and catalytic reagents have been employed in the second route [14–35].
Albeit several reports [25–33], the direct condensation of o-aryldiamines and aromatic aldehydes at room temperature is less developed because as previously reported [36] this superficially simple reaction is a complex sequence of competing reactions and leads to the formation of a complex mixture of products containing 1,2-disubstituted benzimidazoles and 1,2-disubstituted benzimidazolines as main byproducts. Moreover, most of the catalysts have been used for this purpose are not recoverable and destroyed in the work-up procedure. Therefore, the discovery of such mild and practicable routes for selective synthesis of 2-aryl(heteroaryl)-benzimidazoles continues to attract the attention of researchers.
In the recent years, the use of heterogeneous catalysts has received considerable interest in various disciplines including organic synthesis. Synthetic organic routes followed by using heterogeneous catalysts have advantages over their counterparts in which, used-catalyst can be easily recycled [21]. As a part of our continued efforts to utilize heterogeneous catalysts for developing organic reactions [37–50], herein we report on a new and reused catalyst system based on copper on charcoal (Cu/C). This heterogeneous catalyst system exhibits an excellent catalytic performance for the construction of the 2-arylbenzimidazoles framework. Moreover, Cu/C can be repeatedly used for this transformation and subsequently recovered after the reaction.
2 Results and Discussion
Previously we have reported several practical methods for synthesis of a series of 2-arylbenzimidazoles in high yield at room temperature. The catalyst we used in this project was previously introduced [38], copper nanoparticles on charcoal (Cu/C), as an efficient recyclable heterogeneous catalyst in organic synthesis.
The catalyst was synthesized in two steps. In a general procedure the activated carbon was refluxed with a nitric acid solution for several hours and washed with deionized water until pH 6–7 and then dried in an oven at 110 °C overnight under vacuum. The oxidized activated carbon was refluxed with a solution of CuI under a N2 atmosphere in absolute EtOH, washed with ethanol and finally dried under vacuum in an oven overnight at 110 °C.
In order to ascertain the optimum conditions of the reaction, we optimized various parameters including the solvent and the amount of catalyst for reaction of o-phenylenediamine (1.0 mmol) with benzaldehyde (1.0 mmol) as the model reactants (Scheme 1).
During our optimization studies, various solvents were examined, and it was found that these reactions appeared to be largely dependent on the nature of the solvent. Ethanol appeared as the solvent of choice due to its fast reaction rate, high yield, selectivity, cheapness, and environmental acceptability. The optimal amount of catalyst was found to be 5.0 mol%. A decrease in the amount of catalyst resulted in a significant reduction of the yield while an increased amount of catalyst revealed negligible effect on the efficiency of the reaction (Table 1). The best yield and purity of desired product was obtained in the presence of 5.0 mol% of the catalyst in 10.0 mL of ethanol as the appropriate solvent. The product was isolated by simple washing by ethanol followed by the usual work-up.
Structural assignments of benzimidazole 3a were made by comparison of the 1H- and 13C NMR spectra with those reported previously.
Under the optimized reaction conditions, we obtained exclusively 2-substituted products 3, and either no N-alkylated products 4 were observed or they could be found only in trace amounts in just a few cases (Scheme 2).
To investigate the generality and versatility of this method, the reaction was extended to various structurally diverse aldehydes and o-phenylenediamines derivatives. In all cases, reactions were complete in a reasonable time and 2-arylbenzimidazole derivatives were isolated in good to high yields. The use of this methodology in the reaction of the o-phenylenediamines with different aldehydes produced only one of the possible regioisomers, as expected (Table 2).
As shown in Table 2, the generality and selectivity of this catalysis method is excellent. Electronic variation in the aldehydes or o-phenylenediamines was tolerated and did not change the efficiency of the reaction and afforded the desired benzimidazoles in high yields. Heteroaryl aldehydes, such as 2-pyridinyl- and 2-thiophenylcarboxaldehydes (Table 2, entries 11 and 12), also show good results under these conditions.
We felt that this methodology could then be extended to synthesize azacrown ether-containing benzimidazoles. The azacrown ether, which has a 1,3,5-triazine substituent, containing dialdehydes, at the nitrogen position, gave the corresponding dibenzimidazole in good yield (Table 2, entries 16 and 17). We decided to extend the scope of this methodology to the 4′-formyl-benzo-15-crown-5 as starting material; the reaction proceeded with various o-phenylenediamine derivatives smoothly in good yield. The structures of the products were determined from their spectral (1H NMR, 13C NMR, IR, and mass) analysis.
In order to assess the feasibility of applying this method on a preparative scale, we carried out the coupling of o-phenylenediamine with benzaldehyde in a 30 mmol scale in the presence of the heterogenous catalyst. As expected, the reaction proceeded smoothly, similar to the case in a smaller scale (Table 2, entry 1), and the desired 2-phenylbenzimidazole was obtained in 92% isolated yield in 3 h.
We also studied catalyst recyclability. The Cu/C can be recovered and recycled by simple filtration of the reaction mixture and reused for at least eight consecutive trial runs without significant decrease in the activity (Table 3).
A comparison of the catalytic efficiency of Cu/C with selected previously known catalysts is collected in Table 4 to demonstrate that the present protocol is indeed superior to several of the other protocols.
Most of the listed methodologies suffer from some limitations such as prolonged reaction times, elevated temperatures, or use hazardous materials. For example, preparation of benzimidazole has carried out in CH2Cl2 as a solvent and SOCl2/SiO2 as reagent that both solvent and reagent are hazardous material (Table 4, entry 1).
Additionally, some of protocols require high temperature using previous catalysts (Table 4 entries 2, 3, 4, and 5).
It was also observed that, the preparations of those catalysts and their ligands are very difficult (Table 4, entries 3, 6–11). But the present method shows a new, ligand free, cheap, and easy procedure for preparation of catalyst and introduces a general, simple and efficient synthetic method for preparation of 2-arylbenzimidazoles.
3 Conclusion
As a brief statement, we introduced a general, simple, and efficient synthetic method for preparation of 2-arylbenzimidazoles from phenylenediamines and aromatic aldehydes using Cu/C as catalyst. The mild reaction conditions, excellent yields, large-scale synthesis, easy and quick isolation of products, recyclability of the catalyst, employment of atmospheric air as the oxidant, cost-effectiveness, environmentally friendly, high generality, and good selectivity are the main advantages of this procedure. So we believe that it will find wide application in organic synthesis as well as in industry.
References
Tunçbilek M, Kiper T, Altanlar N (2009) Eur J Med Chem 44:1024
Vitale G, Corona P, Loriga M, Carta A, Paglietti G, La Colla P, Busonera B, Marongiu E, Collu D, Loddo R (2009) Med Chem 5:507
Bressi JC, de Jong R, Wu Y, Jennings AJ, Brown JW, O’Connell S, Tari LW, Skene RJ, Vu P, Navre M, Cao X, Gangloff AR (2010) Bioorg Med Chem Lett 20:3138
Tsukamoto G, Yoshino K, Kohno T, Ohtaka H, Kagaya H, Ito K (1980) J Med Chem 23:734
Mederski WWKR, Dorsch D, Anzali S, Gleitz J, Cezanne B, Tsaklakidis C (2004) Bioorg Med Chem Lett 14:3763
Gungor T, Fouquet A, Teulon JM, Provost D, Cazes M, Cloarec A (1992) J Med Chem 35:4455
Navarrete-Vázquez G, Moreno-Diaz H, Aguirre-Crespo F, León-Rivera I, Villalobos-Molina R, Muñoz-Muñiz O, Estrada-Soto S (2006) Bioorg Med Chem Lett 16:4169
Sabnis RW (2008) Handbook of acid–base indicators. CRC Press (Taylor & Francis Group) Boca Raton, London, New York
Pla-Dalmau A (1995) J Org Chem 60:5468
Ueda M, Sato M, Mochizuki A (1985) Macromolecules 18:2723
Phillips MA (1928) J Chem Soc 1928:2393–2399
Hein DW, Alheim RJ, Leavitt JJ (1957) J Am Chem Soc 79:427
Wang R, Lu X–X, Yu X–Q, Shi L, Sun Y (2007) J Mol Cat A: Chem 266:198
Rosen MD, Simon ZM, Tarantino KT, Zhao LX, Rabinowitz MH (2009) Tetrahedron Lett 50:1219
Ben Alloum A, Bougrin K, Soufiaoui M (2003) Tetrahedron Lett 44:5935
Gogoi P, Konwar D (2006) Tetrahedron Lett 47:79
Coppola GM (2008) Synth Commun 38:3500
Han X, Ma H, Wang Y (2008) Russ J Org Chem 44:863
Khan AT, Parvin T, Choudhury LH (2009) Synth Commun 39:2339
Fazaeli R, Aliyan H (2009) Appl Catal A: General 353:74
Gadekar LS, Arbad BR, Lande MK (2010) Chin Chem Lett 21:1053
Luo X, Zhang Z, Yang Y, Xue F, Xiu N, She Y (2009) Front Chem Eng Chin 3:305
Sharghi H, Asemani O, Hossein Tabaei SM (2008) J Heterocyclic Chem 45:1
Sharghi H, Asemani O, Khalifeh R (2008) Synth Commun 38:1128
Sharghi H, Aberi M, Doroodmand MM (2008) Adv Synth Catal 350:2380
Sharghi H, Beyzavi MH, Doroodmand MM (2008) Eur J Org Chem 24:4126–4138
Sharghi H, Hosseini-Sarvari M, Moeini F (2008) Can J Chem 86:1044
Bahrami K, Khodaei MM, Kavianinia I (2007) Synthesis 4:547–550
Rostamizadeh S, Amani AM, Aryan R, Ghaieni HR, Norouzi L (2009) Monatsh Chem 140:547
Saha D, Saha A, Ranu BC (2009) Green Chem 11:733
Dabhade SK, Bora RO, Farooqui M, Gill CH (2009) Chin Chem Lett 20:893
Rostamizadeh S, Aryan R, Ghaieni HR, Amani AM (2009) J Heterocyclic Chem 46:74
Aliyan H, Fazaeli R, Fazaeli N, Mssah AR, Javaherian naghash H, Alizadeh M, Emami G (2009) Heteroatom Chem 20:202
Bahrami K, Khodaei MM, Naali F (2008) J Org Chem 73:6835
Bahrami K, Khodaei MM, Naali F (2009) Synlett 4:569–572
Smith JG, Ho I (1971) Tetrahedron Lett 38:3541
Sharghi H, Khalifeh R, Doroodmand MM (2009) Adv Synth Catal 351:207
Sharghi H, Beyzavi MH, Safavi A, Doroodmand MM, Khalifeh R (2009) Adv Synth Catal 351:2391
Sharghi H, Jokar M (2007) Heterocycles 71:2721
Sharghi H, Khalifeh R (2008) Can J Chem 86:426
Sharghi H, Khalifeh R (2007) Heterocycles 71:1601
Sharghi H, Hosseini Sarvari M (2003) J Org Chem 68:4096
Sharghi H, Jokar M (2010) Can J Chem 88:14
Sharghi H, Khalifeh R, Salimi Beni A (2010) J Iran Chem Soc 7:275
Sharghi H, Salimi Beni AR (2004) Synthesis 17:2900–2904
Sharghi H, Salimi Beni A, Khalifeh R (2007) Helv Chim Acta 90:1373
Sharghi H, Hosseini-Sarvari M, Moeini F, Khalifeh R, Salimi Beni A (2010) Helv Chim Acta 93:435
Sharghi H, Jokar M, Doroodmand MM, Khalifeh R (2010) Adv Syn Cat 352:3031
Sharghi H, Khalifeh R, Moeini F, Beyzavi MH, Salimi Beni A, Doroodmand MM (2011) J Iran Chem Soc 8:S89
Sharghi H, Jokar H, Doroodmand MM (2011) Adv Syn Cat 353:426
Acknowledgments
We gratefully acknowledge the support of this study by the Shiraz University and Shiraz University of Technology Research Council.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sharghi, H., Khalifeh, R., Mansouri, S.G. et al. Simple, Efficient, and Applicable Route for Synthesis of 2-Aryl(Heteroaryl)-Benzimidazoles at Room Temperature Using Copper Nanoparticles on Activated Carbon as a Reusable Heterogeneous Catalyst. Catal Lett 141, 1845–1850 (2011). https://doi.org/10.1007/s10562-011-0671-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0671-6