Abstract
Various mono and bis-1,6-dihydropyrazine-2,3-dicarbonitrile derivatives were efficiently synthesized by reacting 2,3-diaminomaleonitrile (DAMN), isocyanides and ketones in the presence of a catalytic amount of \(N,N{,}N^{\prime }{,}N^{\prime }\)-tetrabromobenzene-1,3-disulfonamide [TBBDA] and poly(\(N\)-bromo-\(N\)-ethylbenzene-1,3-disulfonamide) [PBBS] in EtOH/H\(_{2}\)O at ambient temperature.
Graphic abstract \(N,N,N^{\prime },N^{\prime }\) -Tetrabromobenzene-1,3-disulfonamide and poly( \(N\)-bromo-\(N\)-ethylbenzene-1,3-disulfonamide) as new and efficient catalysts for the synthesis of highly substituted 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chemistry of heterocyclic compounds has attracted much attention in recent times due to its increasing importance in the field of pharmaceuticals and industrial chemicals. A number of compounds based on nitrogen-containing heterocycles show antimicrobial activity and have been developed for clinical usage such as Echinomycin, Levomycin, and Actinoleutin [1–3]. Multi-component reactions (MCRs) are significant tools for the rapid and efficient synthesis of a wide variety of organic molecules [4]. These reactions have been investigated extensively in organic and diverse-oriented synthesis, primarily due to their ability to generate complex molecular functionalities from simple starting materials via one-pot reaction [5, 6]. Dihydropyrazine derivatives are an important class of heterocycles being the core fragment of different natural products and biological systems. The biological and physical roles of dihydropyrazines (DHPs) in DNA cleavage [7], growth inhibition of Escherichia coli [8], and cyclooxygenase inhibitory activity [9] are well documented.
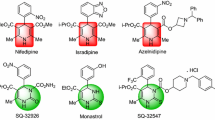
Several methods have been developed for the synthesis of DHPs involving condensation of 1,2-diamines with \(\alpha \)-diketones [10–12], 1,4-addition of 1,2-diamines to diazenylbutenes [13] and carbenoid N–H insertion [14]. Thus, the synthesis of dihydropyrazines is an important and useful area in organic chemistry. Whereas the derivatives of pyrazine are an excellent scaffold for drug development, they exhibit a wide variety of biological and medicinal activities. For instance, the pyrazine derivatives such as compound A and B (Fig. 1) were tested for growth inhibitory activity against human cancer cell lines, such as MCF-7 (breast cancer), NCI-H460 (non-small-cell lung cancer), and SF-268 (glioma) [15].
The great potential of isocyanides for the development of multicomponent reactions lies in the diversity of bond forming processes available, functional group tolerance, and the high levels of chemo-, regio and stereoselectivity often observed. Moreover, there is virtually no restriction on the nature of the nucleophiles and electrophiles in isocyanide multicomponent reactions (IMCRs). Multicomponent reactions involving isocyanides have emerged as valuable tools for the preparation of structurally diverse chemical libraries of drug-like heterocyclic compounds [16–23].
Results and discussion
In a continuation of our interest in the application of \(N\!{,}N\!{,}N^{\prime }\!\!{,}N^{\prime }\)-tetrabromobenzene-1,3-disulfonamide [TBBDA] and poly(\(N\)-bromo-\(N\)-ethylbenzene-1,3-disulfonamide) [PBBS] [24] (Scheme 1) in organic synthesis [25–37], we report here a facile and improved protocol for preparation of 1,6-dihydropyrazine-2,3-dicarbonitrile, from isocyanide, 2,3-diaminomaleonitrile and various aliphatic, alicyclic, and aromatic ketones in the presence of TBBDA and PBBS as catalysts in EtOH/H\(_{2}\)O at ambient conditions (Scheme 2).
The advantages of TBBDA and PBBS are as follows:
-
1.
The preparation of TBBDA and PBBS is easy.
-
2.
TBBDA and PBBS are stable under atmospheric conditions (room temperature and oxygen) for two months.
-
3.
After completion of the reaction, the catalysts are recovered and can be reused several times without decreasing the yield.
Synthesis of organic molecules via green, mild, and simple procedures is currently receiving considerable attention. Also, reducing or eliminating the use and generation of hazardous substances is a goal of green chemistry. In this context, EtOH-H\(_{2}\)O is the preferred choice as a solvent. Reactions in aqueous media are generally environmentally safe, devoid of any carcinogenic effects, simple to handle, cheaper to operate, and especially important in industry [38, 39]. Initially, we decided to explore the role of our catalysts in various solvents for the preparation of 5-(cyclohexylamino)-6-benzyl-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile as a model compound (Table 2, entry 6). In the absence of catalysts, no product was observed, even after prolonged reaction times. Since the synthesis of 5-(cyclohexylamino)-6-benzyl-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile failed in the absence of catalyst, the effect of catalysts was also investigated in various conditions, and the results are presented in Table 1. We found that the reaction was rapid and gave excellent yields when catalyzed by \(N,N,N^{\prime },N^{\prime }\)-tetrabromobenzene-1,3-disulfonamide [TBBDA] (60 min, 98 %, Table 1, entry 12) and poly(\(N\)-bromo-\(N\)-ethylbenzene-1,3-disulfonamide) [PBBS] (65 min, 90 %, Table 1, entry 12).
Our preliminary examination shows that TBBDA and PBBS are reusable catalytic reagents. Thus, after the produce of 5-(cyclohexylamino)-6-benzyl-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile in first run with TBBDA, which gave the corresponding product in 98 % isolated yield (Table 1, entry 12), the \(N,N,N^{\prime },N^{\prime }\)-tetrabromobenzene-1,3-disulfonamide (TBBDA) catalyst was subjected to a second reaction from which it gave product in 90 % yield; the average chemical yield for five consecutive runs was 50 %. In this curve (Scheme3), we show that repetition has reduced gradually. The data shows a good agreement with the function of \(y= -11.6x+112.8\) with a regression coefficient of \(R^{2}=0.955\).
To test the generality and versatility of this new procedure in the synthesis of pyrazines, we examined a number of aliphatic and aromatic ketones and cyclohexyl and tert-butyl isocyanide under optimized conditions (Table 2).
Mechanistically, it is likely that TBBDA or PBBS releases Br\(^{+}\) in situ, which acts as an electrophilic species and the mechanism shown in Scheme 4 is proposed for the synthesis of the 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives. In summary, we have developed a new and facile protocol for the synthesis of new aliphatic and aromatic 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives, from the reaction of ketones, 2,3-diaminomaleonitrile (DAMN) and isocyanide compounds using TBBDA and PBBS at room temperature. Initially, the reaction involves a Br\(^{+}\) transfer from the catalysts to an amino group to form (\(Z)\)-2-amino-\(N\)-bromo-1,2-dicyanoethenaminium (A). Then, reaction of A with ketone followed by elimination of H\(_{2}\)O produced imine intermediate (D). After a nucleophilic attack by isocyanide, intramolecular cyclization (E) and imine-enamine tautomerization of intermediate (F) 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives (G) are obtained.
Conclusions
In summary, we have developed a new and facile protocol for the synthesis of mono and bis-1,6-dihydropyrazine-2,3-dicarbonitrile derivatives starting from simple and readily available substrates (mono and bis-aliphatic, alicyclic and aromatic ketones, 2,3-diaminomaleonitrile, cyclohexyl isocyanide and tert-butyl isocyanide), using stable and inexpensive \(N\)-halo catalysts (TBBDA and PBBS) at room temperature. This reaction can be regarded as a new approach for the preparation of potential pharmaceutically relevant, highly substituted 1,6-dihydropyrazine-2,3 dicarbonitrile derivatives.
Experimental
All commercially available chemicals were obtained from Merck and Fluka companies, and used without further purification unless otherwise stated. Nuclear magnetic resonance, \(^{1}\)H and \(^{13}\)C NMR spectra were recorded on a Bruker Avance 300 FT NMR spectrometer. Infrared (IR) spectroscopy was conducted on a Perkin Elmer GX FT-IR spectrometer. Mass spectra were recorded on a Shimadzu QP 1100 BX Mass spectrometer. Elemental analyses (CHN) were performed with a Heraeus CHN-Rapid analyzer.
Typical procedure for the synthesis of 5-(cyclohexylamino)-6-benzyle-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile
To a solution of DAMN (1 mmol, 0.108 g), benzylacetone (1 mmol, 0.148, g), and cyclohexyl isocyanide (1 mmol, 0.109 g) in 5 mL of EtOH/H\(_{2}\)O (4:1) TBBDA (0.109 mmol, 0.06 g) or PBBS (0.12 g) was added. The resulting mixture was stirred for 60 min at ambient temperature. After completion of the reaction, as indicated by TLC (ethyl acetate/\(n\)-hexane, 3:2), the solid products were precipitated by addition of crushed ice (10 g), filtered off and washed with water. Then, the filtered solid was added to cold dichloromethane (30 mL) and stirred for 10 min at ambient temperature. The catalyst was removed by simple filtration. Removal of the solvent under reduced pressure gave the desired crude product. The crude product was recrystallized from ethyl acetate/\(n\)-hexane (1:5) to give the pure product (98 %) as a yellow powder.
Typical procedure for the synthesis of bis-5-(cyclohexylamino)-6,\(6^{\prime }\)-cyclohexyl-1,6-dihydropyrazine-2,3-dicarbonitrile
To a solution of DAMN (0.216 g, 2 mmol), cyclohexane-1,4-dione (0.112 g, 1 mmol), cyclohexyl isocyanide (0.218 g, 2 mmol) in 5 mL of EtOH/H\(_{2}\)O (4:1), was added TBBDA (0.12 g, 0.218 mmol) or PBBS (0.24 g). The resulting mixture was stirred for 90 min at ambient temperature. After completion of the reaction, as indicated by TLC (ethyl acetate/\(n\)-hexane, 3:2), the solid product was precipitated by addition of crushed ice (10 g), filtered off and washed with water. Then, the filtered solid was added to cold dichloromethane (30 mL) and stirred for 10 min at ambient temperature. The catalyst was removed by simple filtration. Removal of the solvent under reduced pressure gave the desired crude product. The crude product was recrystallized from ethyl acetate/\(n\)-hexane (1:5) to give the pure product (95 %) as a white powder.
The spectral (IR,\(^{1}\) H NMR, \(^{13}\) C NMR, Ms, CHN) data for the selected compounds are presented below
Table 2, entry 1: \({bis-5-(cyclohexylamino)-6,6^{\prime }}-cyclohexyl-1,6-dihydropyrazine-2,3-dicarbonitrile\). (95 %); mp 290–292 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3450, 3344 (N–H), 2932, 2855 (C–H), 2214 (C\(\equiv \)N), 1564, 1456; \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 1.06–1.52 (28H, m, cyclohexyl), 3.71 (2H, m, CH–NH), 6.49 (2H, br s, CH–NH), 7.02 (2H, br s, NH); \(\delta \)c (75 MHz, DMSO-\(d _{6})\); 25.21, 25.64, 26.36, 50.54, 50.68, 108.05, 112.84, 114.99, 117.89, 152.64; MS, \(m/z\) ( %): 510 (M\(^{+}\), 4), 296 (14), 215 (16), 159 (16), 83 (23), 55 (49), 41 (55), 29 (9), 18 (4); [found C, 66.18; H, 6.72; N, 27.50. C\(_{28}\)H\(_{34}\)N\(_{10}\) requires C, 65.86; H, 6.71; N, 27.43 %].
Table 2, entry 2: \(bis-5-(tert-butylamino)-6,6^{\prime }-cyclohexyl-1,6-dihydropyrazine-2,3-dicarbonitrile\). (98 %); mp 296–298 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu \quad _{max}\), cm\(^{-1})\) 3460, 3384, 3355 (N–H), 2943, 2934 (C–H), 2221 (C\(\equiv \)N), 1541, 1536; \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 1.33 (18H, s, tert-butyl), 1.54-1.64 (8H, m, cyclohexyl), 5.68 (2H, br s,CH–NH), 7.09 (2H, br s, CH–NH); \(\delta \)c (75 MHz, DMSO-\(d _{6})\); 26.49, 28.63, 50.42, 53.17, 108.36, 111.86, 114.87, 117.83, 151.47; MS, \(m/z\) (%): 458 (M\(^{+}\), 3), 148 (14), 57 (43), 41 (78), 39 (100), 27 (53), 15 (42); [found C, 62.97; H, 6.60; N, 30.50. C\(_{24}\)H\(_{30}\)N\(_{10}\) requires C, 62.86; H, 6.59; N, 30.54 %].
Table 2, entry 3: \(bis(5-(cyclohexylamino)-6-methyl-6,6^{\prime }-(ethane-1,2-diyl)1,6-dihydropyrazine-2,3-dicarbonitrile)\). (85 %); mp 180–182 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu \quad _{max}\), cm\(^{-1})\) 3451, 3340 (N–H), 2933, 2855 (C–H), 2214 (C\(\equiv \)N), 1564, 1566; \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 0.95 (6H, s, 2CH\(_{3})\), 1.16–1.65 (20H, m, cyclohexyl), 1.8–2.1 (4H, m, –CH\(_{2}\)CH\(_{2}\)–), 3.40, 3.64 (2H, m, CH–NH), 7.03, 7.3 (2H, br s, CH–NH); \(\delta \)c (75. MHz, DMSO-\(d _{6})\); 25.12, 25.59, 25.69, 31.67, 32.46, 36.46, 40.77, 48.83, 50.24, 50.34, 60.85, 71.34, 108.78, 114.33, 115.71, 118.64, 154.62, 171.49; MS, \(m/z\) (%): 512 (M\(^{+}\), 4), 296 (100), 215 (14), 55 (49), 41 (55), 18 (3); [found C, 65.90; H, 7.18; N, 27.38. C\(_{28}\)H\(_{36}\)N\(_{10}\) requires C, 65.60; H, 7.08; N, 27.32 %].
Table 2, entry 4: \(bis(5-(tert-butylamino)-6-methyl-6,6^{\prime }-(ethane-1,2-diyl)1,6-dihydropyrazine-2,3-dicarbonitrile)\). (95 %); mp 251–253 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3363, 3305 (N–H), 2932, 2854 (C–H), 2211(C\(\equiv \)N), 1658, 1626; \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 0.87, 1.66 (6H, ss, 2CH\(_{3})\), 1.18, 1.29 (18H, ss, 2 tert-butyl), 1.8-2.1 (4H, m, –CH\(_{2}\)CH\(_{2-})\), 6.51 (2H, br s, NH), 6.76 (2H, br s, NH); \(\delta \)c (75 MHz, DMSO-\(d _{6})\); 22.37, 24.78, 28.61, 28.65, 32.66, 36.51, 51.14, 52.62, 60.69, 71.95 108.76, 113.56, 115.75, 118.75, 153.61, 171.99; MS, \(m/z\) (%): 460 (M\(^{+}\), 5), 296 (85), 214 (55), 54 (35), 41 (100), 18 (15); [found C, 62.81; H, 7.04; N, 30.29. C\(_{24}\)H\(_{32}\)N\(_{10}\): C, 62.59; H, 7.00; N, 30.41 %].
Table 2, entry 5: \( bis(5-(tert-butylamino)-6-methyl-6,6^{\prime }-methylene-1,6-dihydropyrazine-2,3-dicarbonitrile)\). (85 %), mp 282–284 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu \quad _{max}\), cm\(^{-1})\) 3413, 3324 (N–H), 2976, 2933 (C–H), 2212 (C\(\equiv \)N), 1565, 1542; \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 0.88, 1.65 (6H, ss, 2CH\(_{3})\), 1.14, 1.22 (18H, ss, 2tert-butyl), 1.81 (2H, s, –CH\(_{2}\)–), 6.50 (2H, br s, NH), 6.70 (2H, br s, NH); \(\delta \)c (75 MHz, DMSO-\(d _{6})\); 22.37, 24.78, 28.61, 32.66, 36.51, 51.14, 52.62, 60.69, 71.95 108.76, 113.56, 115.75, 118.75, 153.61, 171.99; MS, \(m/z\) (%): 446 (M\(^{+})\), [found C, 62.11; H, 6.74; N, 31.39. C\(_{23}\)H\(_{30}\)N\(_{10}\) requires C, 61.86; H, 6.77; N, 31.37 %].
Table 2, entry 6: 5-(cyclohexylamino)-6-benzyl-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile. (98 %); mp 186–188 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu \quad _{max}\), cm\(^{-1})\) 3354, 3300 (N–H), 2926, 2854 (C–H), 2212 (C\(\equiv \)N), 1564, 1541; \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 1.30 (3H, s, CH\(_{3})\), 1.58–1.90 (12H, m, CH\(_{2}\), cyclohexyl), 2.45 (2H, t, CH\(_{2}\)-Ph), 3.70 (1H, m, CH–NH), 6.90 (1H, br s, NH), 7.01 (1H, br s, NH), 7.14–7.23 (5H, m, Ph); \(\delta \)c (75 MHz, DMSO-\(d _{6})\); 23.70, 25.22, 25.71, 29.53, 31.99, 32.08, 49.94, 53.12, 53.20, 109.53, 110.66, 114.83, 118.54, 126.30, 128.60, 128.83, 141.79, 154.76; MS, \(m/z\) (%): 347 (M\(^{+}\), 3), 243 (5), 103 (54), 91 (100), 77 (57), 41 (63), 39 (62) 15 (3); [found C, 72.77; H, 7.16; N, 19.92. C\(_{21}\)H\(_{25}\)N\(_{5}\) requires C, 72.59; H, 7.25; N, 20.16 %].
Table 2, entry 7: 5-(tert-butylamino)-6-benzyl-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile). (98 %); mp 150–152 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu \quad _{max}\), cm\(^{-1})\) 3414, 3349 (N–H), 2960, 2954 (C–H), 2210 (C\(\equiv \)N), 1544, 1495 (C=N); \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 1.26 (3H, s, CH\(_{3})\), 1.28 (9H, s, tert-butyl), 1.84 (2H, t, -CH \(_{2}\)CH\(_{2}\)-Ph), 2.17 (2H, t-CH\(_{2}\) CH \(_{2}\)-Ph), 6.30 (1H, s, NH), 6.81 (1H, s, NH) 7.26 (5H, m, Ph); \(\delta \)c (75 MHz, DMSO-\(d _{6})\); 1.32 (1H, s), 1.34(9H, s), 1.59 (2H, t), 1.66 (2H, t), 6.33 (1H, br s), 7.13 (1H, br s) 7.16-7.27 (5H, s); 23.3, 28.6, 29.4, 52.6, 52.8, 108.9, 110.6, 114.7, 118.5, 126.3, 128.8, 141.9, 154.04; MS, \(m/z\) (%): 321 (M\(^{+}\), 1), 160 (12), 91 (22), 57 (36), 41 (100), 39 (72), 15 (38); [found: C, 71.47; H, 7.24; N, 21.62. C\(_{19}\)H\(_{23}\)N\(_{5}\) requires C, 71.00; H, 7.21; N, 21.79 %].
Table 2, entry 8: 5-(cyclohexylamino)-6-(4-methoxyphenyl)-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile) (94 %); mp 166–168 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3450, 3380 (N–H), 2938, 2848 (C–H), 2210 (C\(\equiv \)N), 1554, 1546 (C=N); \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 1.01-1.89 (10H, m, cyclohexyl), 1.60 (3H, s, CH\(_{3})\), 3.45 (1H, m, CH–NH), 3.75 (3H, s, –OCH\(_{3})\), 6.58 (1H, br s), 6.9–7.25 (4H, m, ph), 7.31 (1H, br s); \(\delta \)c (75 MHz, DMSO-\(d\)6); 21.70, 25.22, 28.71, 29.53, 35.99, 49.94, 53.12, 53.20, 55.34, 109.53, 110.66, 114.83, 118.54, 126.30, 128.60, 128.83, 141.79, 164.76; MS, \(m/z\) (%): 349 (M\(^{+}\) 5), 296 (50), 252 (2), 91 (50), 57 (60), 41 (85), 39 (49), 15 (15); [found C, 69.05; H, 6.54; N, 20.13. C\(_{20}\)H\(_{23}\)N\(_{5}\)O requires C, 68.74; H, 6.63; N, 20.04 %].
Table 2, entry 9: 5-(tert-butylamino)-6-(4-methoxyphenyl)-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile). (85 %); mp 146–148 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3440, 3350 (N–H), 2928, 2858 (C–H), 2215 (C\(\equiv \)N), 1564, 1546 (C=N); \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 1.38 (9H, s, tert-butyl), 1.63 (3H, s, CH\(_{3})\), 3.7 (3H, s, –OCH\(_{3})\), 6.51 (1H, br s), 6.8 (2H, d), 7.13 (2H, d), 7.90 (1H, br s); \(\delta \)c (75 MHz, DMSO-\(d\)6); 25.6, 28.5, 53.1, 54.8, 54.9, 55.5, 110.22, 111.1, 114.2, 114.6, 111, 126.6, 134.5, 152.5, 159.1; MS, \(m/z\) (%): 323 (\(\mathrm{M}^{+}\), 2), 296 (48) 57 (100), 41 (97), 39 (49), 29 (36), 15 (13); [found: C, 66.35; H, 6.56; N, 21.63. C\(_{18}\mathrm{H}_{21}\mathrm{N}_{5}\)O requires C, 66.85; H, 6.55; N, 21.66 %].
Table 2, entry 10: 5-(tert-butylamino)-6-(4-hydroxyphenyl)-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile). (96 %); mp 187–189 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3364, 3351 (N–H), 2954, 2853 (C–H), 2211(C\(\equiv \)N), 1669, 1564 (C=N); \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 1.36 (9H, s, tert-butyl), 1.59 (3H, s, CH\(_{3})\), 6.42 (1H, br s, NH), 6.68–7.01 (4H, d, Ar), 7.82 (1H, br s, NH), 9.46 (1H, s, OH); \(\delta \)c (75 MHz, DMSO-\(d\)6); 21.5, 24.9, 24.8, 34.4, 48.4, 53.3, 55.8, 104.2, 114.2, 114.4, 128.4, 132.2, 139.5, 158.6, 164.1; MS, \(m/z\) (%): 309 (\(\mathrm{M}^{+}\), 5), 296 (35), 215 (16), 56 (50), 41 (55), 39 (15), 18, (5); [found C, 66.26; H, 6.30; N, 22.60. C\(_{17}\mathrm{H}_{19}\mathrm{N}_{5}\)O requires C, 66.00; H, 6.19; N, 22.64 %].
Table 2, entry 11: 5-(tert-butylamino)-6-cyclohexyl-1,6-dihydropyrazine-2,3-dicarbonitrile). (98 %); mp 202–204 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3405, 3320 (N–H), 2933, 2860 (C–H), 2213 (C\(\equiv \)N), 1563, 1542 (C=N); \(\delta _{H}\) (300 MHz, DMSO-d\(_{6})\) 1.36 (9H, s, tert-butyl), 1.4–1.6 (10H, m, cyclohexyl), 6.13 (1H, br s), 6.8 (1H, br s); \(\delta \)c (75 MHz, DMSO-\(d\)6); 20.4, 24.9, 28.5, 29.6, 51.7, 52.6, 108.6, 111.3, 115.07, 118.27, 153.7; MS, \(m/z\) (%): 271 (M\(^{+}\), 16), 215 (50), 172, (65), 57 (92), 41 (92), 29 (56), 14 (100); [found C, 66.88; H, 7.81; N, 25.77. C\(_{15}\mathrm{H}_{21}\mathrm{N}_{5}\) requires C, 66.39; H, 7.80; N, 25.81 %].
Table 2, entry 12: 5-(tert-butylamino)-6-N-benzylpiperidin-1,6-dihydropyrazine-2,3-dicarbonitrile). (45 %); mp 260–262 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3410, 3350 (N–H), 2936, 2864 (C–H), 2225, 2219 (C\(\equiv \)N), 1560, 1526 (C=N); \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 1.28 (9H, s, tert-butyl), 1.84 (2H, t, CH\(_{2}\)–C) 2.17 (2H, t, CH \(_{2}\)–C), 3.43 (2H, s, –NCH \(_{2}\)Ph), 2.54 (2H, t, CH\(_{2}\)–N), 3.43 (2H, t, CH\(_{2}\)–N), 6.30 (1H, br s), 6.81 (1H, br s) 7.26 (5H, m, Ph); \(\delta \)c (75 MHz, DMSO-\(d\)6); 28.4, 29.4, 47.7, 50.16, 52.75, 62.54, 107.9, 111.7, 115.1, 118.1, 127.3, 128.6, 129.3, 138.8, 152.4; MS, \(m/z\) (%): 362 (M\(^{+}\), 1), 91 (88), 57 (67), 41 (100), 39 (60), 15 (18); [found C, 69.66; H, 7.20; N, 23.24 C\(_{21}\mathrm{H}_{26}\mathrm{N}_{6}\) requires C, 69.58; H, 7.23; N, 23.19 %].
Table 2, entry 13: 5-tert-butylamino)-6-cyclooctyl-1,6-dihydropyrazine-2,3-dicarbonitrile. (95 %); mp 250–252 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3415, 3398 (N–H), 2922, 2854(C–H), 2225, 2210 (C\(\equiv \)N), 1564, 1526 (C=N); \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 1.3 (9H, s, tert-butyl), 1.40-1.51 (10H, m, cyclooctyl), 1.82 (4H, t, cyclooctyl), 5.81 (1H, br s, NH), 6.95 (1H, br s, NH); \(\delta \)c (75 MHz, DMSO-\(d\)6); 21.1, 24.2, 27.8, 28.5, 29.4, 52.8, 54.7, 109.05, 110.9, 114.9, 118.2, 153.8; MS, \(m/z\) (%): 299 (M\(^{+}\) 10), 215 (60), 172 (50), 57 (58), 41 (100), 29 (25), 14 (15); [found C, 68.20; H, 8.38; N, 23.35. C\(_{17}\mathrm{H}_{25}\mathrm{N}_{5}\) requires C, 68.19; H, 8.42; N, 23.39 %].
Table 2, entry 14: 5-(cyclohexylamino)-6-isobutyl-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile. (94 %); mp 180–182 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3351 (N–H), 2933, 2856 (C–H), 2213 (C\(\equiv \)N) 1584, 1544 (C=N); \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 0.83–0.84 (6H, d, 2CH\(_{3})\), 1.07–1.21 (3H, m, CH\(_{2}\)–CH), 1.32 (3H, s, CH\(_{3})\), 1.38–1.76 (10H, m, cyclohexyl), 3.67 (1H, m, CH–NH), 6.89 (1H, br s, NH), 7.10 (1H, br s, NH); \(\delta \)c (75 MHz, DMSO-\(d\)6); 24.9, 25.2, 25.24, 25.7, 31.8, 32.1, 49.9, 53.05, 53.12, 109.53, 110.28, 110.38, 114.83, 118.59, 155.1, 155.2; MS, \(m/z\) (%): 299 (M\(^{+})\), [found C, 68.63; H, 8.69; N, 23.25. C\(_{17}\mathrm{H}_{25}\mathrm{N}_{5}\) requires C, 68.19; H, 8.42; N, 23.39 %].
Table 2, entry 15: 5-(tert-butylamino)-6-isobutyl-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile. (90 %); mp 210–212 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3412, 3320 (N–H), 2930, 2844 (C–H), 2225, 2216 (C\(\equiv \)N), 1543, 1551 (C=N); \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 0.84–0.86 (6H, d, 2CH\(_{3})\), 1.13–1.17 (2H, d, –CH\(_{2}\)–), 1.3 (9H, s, tert-butyl), 1.33 (3H, s, –CH\(_{3})\), 1.64 (1H, m, –CH(CH\(_{3}) _{2})\), 6.19 (1H, br s, NH), 7.12 (1H, br s, NH); \(\delta \)c (75 MHz, DMSO-\(d\)6); 23.4, 23.5, 24.4, 25.01, 28.5, 43.8, 52.56, 52.8, 108.9, 110.3, 114.7, 118.5, 154.3; MS, \(m/z\) (%): 273 (M\(^{+}\), 2), 160 (12), 57 (12), 41 (100), 39 (68), 27 (16), 15 (48); [found C, 65.66; H, 8.42; N, 25.67. C\(_{15}\mathrm{H}_{23}\mathrm{N}_{5 }\)requires C, 65.90; H, 8.48; N, 25.62 %].
Table 2, entry 16: 5-(tert-butylamino)-6-ethyl-6-methyl-1,6-dihydropyrazine-2,3-dicarbonitrile. (98 %); mp 175–177 \({^{\circ }}\mathrm{C}\); IR (KBr) (\(\nu _{max}\), cm\(^{-1})\) 3394, 3334 (N–H), 2969, 2924 (C–H), 2225 (C\(\equiv \)N), 1549, 1541 (C=N); \(\delta _{H}\) (300 MHz, DMSO-\(d _{6})\) 0.75 (3H, t, CH \(_{3}\)–CH\(_{2})\), 1.26 (3H, s, CH\(_{3})\), 1.31 (9H, s, tert-butyl), 1.55 (2H, q, CH\(_{2}\)–), 6.17 (1H, br s), 7.1 (1H, br s); \(\delta \)c (75 MHz, DMSO-\(d\)6); 22.6, 28.6, 28.9, 52.6, 53.02, 53.1, 109.14, 110.52, 114.81, 118.53, 154.07, 154.14; MS, \(m/z\) (%): 246 (M\(^{+}\), 2), 159 (17), 56 (15), 42 (27), 41 (85), 29 (84), 27 (100), 15 (85); [found C, 63.76; H, 7.85; N, 28.70. C\(_{13}\mathrm{H}_{19}\mathrm{N}_{5 }\)requires C, 63.65; H, 7.81; N, 28.55 %].
References
Ohkanda J, Katoh A (1998) $N$-hydroxyamide-containing heterocycles-synthesis, reactivities, and iron (III) chelating properties. Rev Heteroatom Chem 18:87–118
Dell A, William DH, Morris HR, Smith GA, Feeney J, Roberts GCK (1975) Structure revision of the antibiotic echinomycin. J Am Chem Soc 97:2497–2502. doi:10.1021/ja00842a029
Bailly C, Echepare S, Gago F, Waring MJ (1999) Recognition elements that determine affinity and sequence-specific binding to DNA of 2QN, a biosynthetic bis-quinoline analogue of echinomycin. Anti Cancer Drug Des 14:291–303
Habibi A, Shikhhosseini-Lori E, Shockravi A (2009) Synthesis of novel furo-pyran derivatives via reaction between an isocyanide and alkylidene substituted Meldrum’s acid. Tetrahedron Lett 50:1075–1078. doi:10.1016/j.tetlet.2008.12.082
Domling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chem Int Ed 39:3168–3210. doi:10.1002/15213773(20000915)39:18%3c3168
Domling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89. doi:10.1021/cr0505728
Yamaguchi T, Matsumoto S, Watanabe K (1998) Generation of free radicals from Dihydropyrazines with DNA strand-breakage activity. Tetrahedron Lett 39: 8311–8312. doi:10.1016/S0040-4039(98)01859-0
Takeda O, Takechi S, Katoh T, Yamaguchi T (2005) Effects of phenyl derivatives of dihydropyrazines with ability to generate radical species on Escherichia coli. Biol Pharm Bull 28:1161–1164. doi:10.1248/bpb.30.1663
Singh SK, Saibaba V, Ravikumar V, Rudrawar SV, Daga P, Rao CS, Akhila V, Hegde P, Rao YK (2004) Synthesis and biologicalevaluation of 2,3-diarylpyrazines and quinoxalines as selective COX-2 inhibitors. Bioorg Med Chem 12:1881–1893. doi:10.1016/j.bmc.2004.01.033
Brown DJ (2004) Quinoxalines supplements II. In: Taylor EC, Wipf P (eds) chemistry of heterocyclic compounds. Wiley, Hoboken
Bhosale RS, Sarda SR, Andhapure SS, Jadhav WN, Bhusare SR, Pawar RP (2005) An efficient protocol for the synthesis of quinoxalinederivatives at room temperature using molecular iodine as thecatalyst. Tetrahedron Lett 46:7183–7186. doi:10.1016/j.tetlet.2005.08.080
More SV, Sastry MNV, Yao C-F (2006) Cerium (IV) ammonium nitrate (CAN) as a catalyst in tap water: a simple, proficient and green approach for the synthesis of quinoxalines. Green Chem 8:91–95. doi:10.1039/B510677J
Aparicio D, Attanasi OA, Filippone P, Ignacio R, Lillini S, Mantellini F, Palacios F, de los Santos JM (2006) Straightforward access to pyrazines, piperazinones, and quinoxalines by reactions of 1,2-diaza-1,3-butadienes with 1,2-diamines under solution, solvent-free, or solid-phase conditions. J Org Chem 71:5897–5905. doi:10.1021/jo060450v
Zhang X, Sui Z (2006) Application of carbenoid N-Hinsertion in the synthesis of the tricyclic 1,4-dihydropyrazines. Tetrahedron Lett 47:5953–5955. doi:10.1016/j.tetlet.2006.06.053
Dubinina GG, Platonov MO, Golovach SM, Borysco PO, Tolmachov AO, Volovenko YM (2006) Novel 5,7-disubstituted 6-amino-5H-pyrrolo[3,2-b]pyrazine-2,3- dicarbonitriles, the promising protein kinase inhibitors with antiproliferative activity. Eur J Med Chem 41:727–737. doi:10.1016/j.ejmech.2006.03.019
Li J, Liu Y, Li C, Jia X (2009) CAN-catalyzed syntheses of 3,4-dihydroquinoxalin-2-amine derivatives based on isocyanides. Tetrahedron Lett 50:6502–6505. doi:10.1016/j.tetlet.2009.09.022
Fujiwara S-I, Asanuma Y, Shin-ike T, Kambe N (2007) Copper(I)-catalyzed highly efficient synthesis of benzoselenazoles and benzotellurazoles. J Org Chem 12:8087–8090. doi:10.1021/jo7013164 (ISSN: 0022-3263)
Ugi I, Domling A, Werner B (2000) Since 1995 the new chemistry of multicomponent reactions and their libraries, including their heterocyclic chemistry. J Heterocycl Chem 37:647–658. doi:10.1002/jhet.5570370322
Krasavin M, Parchinsky V (2008) Expedient Entry into 1,4- dihydroquinoxalines and quinoxalines via a novel variant of isocyanide-based MCR. Synlett 5:645–648. doi:10.1055/s-2008-1032106
Krasavin M, Shkavrov S, Parchinsky V, Bukhryakov K (2009) Imidazo[1,2-a]quinoxalines accessed via two sequential isocyanide-based multicomponent reactions. J Org Chem 74: 2627–2629. doi:10.1021/jo900050k
Nikulnikov M, Tsirulnikov S, Kysil V, Ivachtchenko A, Krasavin M (2009) tert-Butyl isocyanide as convertible reagent in Ugi reaction: Microwave-assisted preparation of 5,6-dihydropyrazolo[1,5-a]pyrazine-4,7-diones. Synlett 2:260–262. doi:10.1002/chin.200922162
Krasavin M, Tsirulnikov S, Nikulnikov M, Kysil V, Ivachtchenko A (2008) Poorly reactive 5-piperazin-1-yl-1,3,4-thiadiazol-2-amines rendered as valid substrates for Groebke-Blackburn type multi-component reaction with aldehydes and isocyanides using TMSCl as a promoter. Tetrahedron Lett 49:5241–5243. doi:10.1016/j.tetlet.2008.06.113
Kysil V, Tkachenko S, Khvat A, Williams C, Tsirulnikov S, Churakova M, Ivachtchenko A (2007) TMSCl-promoted isocyanide-based MCR of ethylenediamines: an efficient assembling of 2-aminopyrazine core. Tetrahedron Lett 48(36):6239–6244. doi:10.1016/j.tetlet.2007.07.044
Ghorbani-Vaghei R, Jalili H (2005) Mild and regioselective bromination of aromatic compounds with $N, N, N^{\prime }, N^{\prime }$-tetrabromobenzene- 1,3-disulfonylamide and poly($N$- bromobenzene-1,3- disulfonylamide). Synthesis 7:1099–1102. doi:10.1055/s-2005-861851
Ghorbani-Vaghei R, Shahbazee E, Veisi H (2005) $N, N^{\prime }$-Diiodo-$N, N^{\prime }$-1,2-ethanediy toluenesulfonamide) as a reagent for conversion of aldehydes to methyl esters. Mendeleev Commun 15:207–208. doi:10.1070/MC2005v015n05ABEH002091
Ghorbani-Vaghei R, Zolfigol MA, Chegeny M, Veisi H (2006) Poly($N$-bromobenzene- 1,3-disulfonamide) and $N, N, N^{\prime }, N^{\prime }$-tetrabromobenzene-1,3-disulfonamide as novel catalytic reagents for silylation of alcohols, phenols, and thiols using hexamethyldisilazane. Tetrahedron Lett 47:4505–4508. doi:10.1016/j.tetlet.2006.03.157
Ghorbani-Vaghei R, Shahbazee E (2005) Facile and mild deprotection of semicarbazones under solvent-free conditions with $N, N, N^{\prime }, N^{\prime }$-tetrabromo-benzene-1,3-disulfonylamide. J Braz Chem Soc 16:647–649
Zolfigol MA, Ghorbani-Vaghei R, Mallakpour S, Chehardoli G, Ghorbani Choghamani A, Yazdi Hosain A (2006) Simple, convenient and heterogeneous method for conversion of urazoles to triazolinediones using $N, N, N^{\prime }, N^{\prime }$-tetrabromobenzene-1,3-disulfonylamide or trichloromelamine under mild and heterogeneous conditions. Synthesis 10:1631–1634. doi:10.1055/s-2006-926446
Ghorbani-Vaghei R, Akbari-Dadamahaleh S (2009) Poly($N$-bromo-$N$-ethylbenzene-1,3 disulfonamide) and $N, N, N^{\prime }, N^{\prime }$-tetrabromobenzene-1,3-disulfonamide as efficient reagents for synthesis of quinolines. Tetrahedron Lett 50:1055–1058. doi:10.1016/j.tetlet.2008.12.076
Ghorbani-Vaghei R (2003) Mild and regioselective iodination of aromatic compounds with $N, N^{\prime }$-diiodo-$N, N^{\prime }$-1,2-ethanediyl bis ($p$-toluenesulphonamide). Tetrahedron Lett 44:7529–7532. doi:10.1016/j.tetlet.2003.08.019
Ghorbani-Vaghei R, Chegini M, Veisi H, Karimi-Tabar M (2009) Poly($N, N^{\prime }$-dibromo- $N$-ethyl-benzene-1,3-disulfonamide) $N, N, N^{\prime }, N^{\prime }$-tetrabromobenzene-1,3 disulfonamide and novel poly($N, N^{\prime }$-dibromo-$N$-phenylbenzene-1,3-disulfonamide) as powerful reagents for benzylic bromination. Tetrahedron Lett 50:1861–1865. doi:10.1016/j.tetlet.2009.02.007
Ghorbani-Vaghei R, Veisi H, Keypour H, Dehghani-Firouzabadi A (2010) A practical and efficient synthesis of bis(indolyl) methanes in water, and synthesis of di-, tri- and tetra(bis- indolyl)methanes under thermal conditions catalyzed by oxalic acid dihydrate. Mol Divers 14:87–96. doi:10.1007/s11030-009-9150-z
Ghorbani-Vaghei R, Toghraei-Semiromi Z, Karimi-Nami R (2011) One-pot synthesis of 4H-chromene and dihydropyrano[3,2-c]chromene derivatives in hydroalcoholic media. J Braz Chem Soc 22:905–909
Ghorbani-Vaghei R, Karimi-Nami R, Toghraei-Semiromi Z, Amiri M, Ghavidel M (2011) One-pot synthesis of aliphatic and aromatic 2H-indazolo[2,1-b]phthalazine-triones catalyzed by $N$-halosulfonamides under solvent-free conditions. Tetrahedron 67:1930–1937. doi:10.1016/j.tet.2011.01.024
Ghorbani-Vaghei R, Shahbazi H, Veisi H (2012) Mild bromination of unreactive aromatic compounds. Tetrahedron Lett 53: 2325–2327. doi:10.1016/j.tetlet.2012.02.101
Ghorbani-Vaghei R, Veisi H (2010) The application of poly($N, N^{\prime }$-dibromo-$N$-ethyl- benzene-1,3-disulfonamide) and N, N, N’, N’-tetrabromobenzene-1,3-disulfonamide as catalysts for one-pot synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles and 1,5- benzodiazepines, and new reagents for synthesis of benzimidazoles. Mol Divers 14:249–256. doi:10.1007/s11030-009-9169-1
Veisi H, Ghorbani-Vaghei R (2010) Recent progress in the application of $N$-halo reagents in the synthesis of heterocyclic compounds. Tetrahedron 66:7445–7463. doi:10.1016/j.tet.2010.07.015
Grieco PA (1998) Organic synthesis in water. Blackie Academic and Professional, London
Li C-J (2005) Organic reactions in aqueous media with a focus on C-C bond formations: a decade update. Chem Rev 105:3095–3165. doi:10.1021/cr030009u
Acknowledgments
We are thankful to Bu-Ali Sina University, Center of Excellence in development of environmentally friendly methods for chemical synthesis (CEDEFMCS) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbani-Vaghei, R., Amiri, M., Karimi-Nami, R. et al. \(N,N,N^{\prime },N^{\prime }\)-Tetrabromobenzene-1,3-disulfonamide and poly(\(N\)-bromo-\(N\)-ethylbenzene-1,3-disulfonamide) as new and efficient catalysts for the synthesis of highly substituted 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives. Mol Divers 17, 251–259 (2013). https://doi.org/10.1007/s11030-013-9427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-013-9427-0