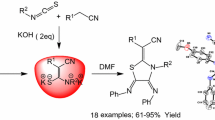
Abstract
The applications of \(N{,}N{,}N^{\prime }{,}N^{\prime }\)-tetrabromobenzene-1,3-disulfonamide [TBBDA], poly(N-bromo-N-ethyl-benzene-1,3-disulfonamide) [PBBS], \(N{,}N{,}N^\prime {,}N^\prime \)-tetrachlorobenzene-1,3-disulfonamide [TCBDA], and poly(N-chloro-N-ethyl-benzene-1,3-disulfonamide) [PCBS] as novel reagents for the preparation of spirocyclopropylbarbiturates from 2-arylidenemalononitriles and barbituric acids are described. In addition, an effective and simple domino procedure for the synthesis of 3-substituted-1,1,2,2-tetracyanocyclopropanes from carbonyl compounds and malononitrile in a one-pot manner is reported. These reactions involve Michael addition, halogenation, and intramolecular ring-closing (MHIRC) reaction sequences.
Graphical Abstract
We have developed an efficient procedure for the synthesis of spirocyclopropylbarbiturates and 3-substituted-1,1,2,2-tetracyanocyclopropanes using TBBDA, PBBS, TCBDA, and PCBS as new reagents

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Barbiturates and spirobarbiturates have attracted intense interest due to their useful biological and pharmacological properties, such as being anticonvulsant, antiepileptic, hypnotic, anti-invasive, antiangiogenic, and anticancer agents [1, 2]. 5-Benzylbarbituric acid derivatives have potential usefulness in the therapy of cancer and AIDS, as well as other pathological and physiological disorders [3].
Spirobarbiturate compounds also play an important role as a basic skeleton for the design of a number of central nervous system (CNS) depressant agents, dihydroorotate dehydrogenase (DHODase), and matrix metalloproteinases (MMPs) inhibitors and the construction of modified oligonucleotides [4, 5].
A number of synthetic approaches to spirocyclopropanes and cyclopropanes have been reported, which include transition metal-catalyzed cyclopropanation of alkenes with diazo compounds, domino aldol/Horner–Wadsworth–Emmons reaction, 1,3-dipolar cycloaddition, Diels–Alder, Simmons–Smith reaction, reaction of alkenes with free carbenes, carbenoids, or ylides, for example, phosphorus, sulfur, arsenic, and henyliodonium ylides [6–10].
Michael-initiated ring closure (MIRC) is one of the most important strategies for the construction of cyclopropane rings [11]. Several methods have been developed for the synthesis of spirodicyanocyclopropyl barbiturates involving the reaction of electron-deficient alkenes with dibromomethylene compounds activated by cyano and ester groups in the presence of LiI or tetrabutylammonium bromide in DMF [12], the reaction of benzylidenemalononitriles with \({ N,N}^{\prime }\)-dialkylbarbituric acids in the presence of bromine and sodium ethoxide in ethanol [13], the combined electrolysis of benzylidenemalononitriles or benzylidenecyanoacetates and barbituric acids in methanol in the presence of sodium bromide [14], and the electrolysis of aldehydes, malononitrile, and barbituric acids in alcohol in the presence of sodium bromide [15].
The methods for 3-substituted-1,1,2,2-tetracyanocyclopropane synthesis have been divided into four main groups: the interaction of 2,2-dibromomalononitrile with carbonyl compounds, the reaction of alkylidenemalononitriles or arylidenemalononitriles with bromomalononitrile, the action of free halogen or active halogen-containing compounds on benzylidenemalononitriles and malononitrile, and the direct transformation of carbonyl compounds and malononitrile for the preparation of 3-substituted-1,1,2,2-tetracyanocyclopropanes by the action of free halogen or active halogen-containing compounds [16–24].
As halogenated organic compounds are particularly significant from the standpoint of biological activity provided a specific site of an organic molecule is substituted with a halogen atom(s), many attempts have been made to develop new methods and reagents for selective halogenation. In this regard, a large number of compounds called N-halo reagents have been widely used in organic transformations and in the chemistry of natural compounds [25]. Some halogenating agents require special equipment and techniques because of their explosive, toxic, unstable, and hygroscopic qualities but N-halo sulfonamides are easy to handle and only half of their halogens are consumed, as in the case of elemental halogen [26].
Results and discussion
Based on the above facts and in continuation of our previous studies on the application of N-halo reagents in organic synthesis [27–30], we now report convenient methods for the cyclopropanation of 2-arylidenemalononitriles 1 with barbituric acids 2 and cyclopropanation of carbonyl compounds 6 with malononitrile 5 using \(N{,}N{,}N^\prime {,}N^\prime \)-tetrabromobenzene-1,3-disulfonamide [TBBDA], poly(N-bromo-N-ethyl-benzene-1,3-disulfonamide) [PBBS], \(N{,}N{,}N^\prime {,}N^\prime \)-tetrachlorobenzene-1,3-disulfonamide [TCBDA], and poly(N-chloro-N-ethyl-benzene-1,3-disulfonamide) [PCBS] (Scheme 1).
The reaction of benzylidenemalononitrile 1g as a model compound with barbituric acid 2 was examined under various reaction conditions (Table 1). First, the effect of various bases was investigated (Table 1, entries 1–7). The best yield of cyclopropane 3g was achieved using NaOAc (Table 1, entry 7). We also found that 1.0 mmol of NaOAc, and 0.25 mmol of TBBDA were sufficient and no improvement in the reaction rate was observed by increasing the amount of them (Table 1, entries 8 and 9). Other halogen sources such as PBBS, TCBDA, and PCBS, were also screened. All of them gave the final product in good to high yields (Table 1, entries 10–12).
Next, solvent effects on the cyclopropanation of benzylidenemalononitrile 1g with barbituric acid 2 were examined by applying the optimized conditions (Table 1, entry 7). In this context, \(\hbox {H}_{2}\hbox {O/EtOH}\) (1:3) is the preferred choice as a solvent system (Table 2, entry 6). When the reaction was carried out at higher temperature (\(50\,^{\circ }\hbox {C}\) or refluxing the entire reaction mixture for 10 min) gave the same results as the room-temperature reaction in \(\hbox {H}_{2}\hbox {O/EtOH}\) (1:3) (Table 2, entries 7 and 8). Therefore, this reaction was most efficient when using benzylidenemalononitrile 1g (1.0 mmol), barbituric acid 2 (1.0 mmol), TBBDA (0.14 g, 0.25 mmol), and NaOAc (1.0 mmol) in \(\hbox {H}_{2}\hbox {O/EtOH}\) (1:3) (2 mL) at room temperature (Table 2, entry 6).
After optimization of the reaction conditions, in order to study the generality of the procedure, various 2-arylidenemalononitriles 1 and barbituric acid or 1,3-dimethylbarbituric acid 2 were submitted to these reaction conditions and provide corresponding spirocyclopropylbarbiturate derivatives 3a–o in good to high yields (Table 3).
Replacing barbituric acid 2 (\(\hbox {R} = \hbox {H}\)) with 1,3-dimethylbarbituric acid 2 (\(\hbox {R} = \hbox {Me}\)) produced the corresponding dimethyl derivatives in a shorter time (Table 3). The reason for longer time in producing the desired products with barbituric acid 2 is probably due to N–H hydrogen atoms which are acidic. The acidity results from the ability of the N to lose hydrogen and the stabilization of the resulting anionic charge of the conjugate base by resonance delocalization [31].
On the other hand, we explored the formation of spirocyclopropylbarbiturates 3 from 5-arylidenebarbituric acids 4 and malononitrile 5 under these reaction conditions (Scheme 2). In our protocol, benzaldehyde and barbituric acid 2 were stirred in water and heated at reflux until the reaction was completed (reaction progress monitored by TLC) [32]. Then, the reaction of 5-benzylidenebarbituric acids 4 with malononitrile 5 under the optimal reaction conditions produced corresponding spirocyclopropylbarbiturates 3 in good to high yields (85–97 %) similar to the results of the reaction between 2-arylidenemalononitriles 1 and barbituric acid 2 under the optimized conditions (Scheme 2).
Since the procedure for the preparation of 2-arylidenemalononitriles 1 has several advantages such as short reaction times, easy work-up, high yields, and pure products compared to the preparation of 5-arylidenebarbituric acids 4, we preferred to carry out synthesis of spirocyclopropylbarbiturates 3 using of 2-arylidenemalononitriles 1 and barbituric acids 2 under the optimized conditions.
Based on these results, a plausible reaction pathway for the cyclopropanation 2-arylidenemalononitriles 1 with barbituric acids 2 using N-halosulfonamides is shown in Scheme 3. First, deprotonation of barbituric acid 2 with the use of an acetate anion in aqueous ethanol gives the barbiturate anion A. Michael addition of the barbiturate anion A to the \(\beta \)-carbon position of 2-arylidenemalononitrile 1 as an \(\alpha ,\beta \)-unsaturated compound afforded intermediate B. The intermediate B should exist in equilibrium with intermediate C by the proton migration possible under the conditions studied. Then, halogenation of the intermediate C with the use of N-halosulfonamides as sources for electrophilic halogens occurs to give the intermediate D. In the presence of base, deprotonation of intermediate D takes place leading to the formation of intermediate E. Intramolecular C-attack of carbanion E to carbon atom containing bromine atom as an electrophile produces spirocyclopropylbarbiturates 3 [14, 33].
To demonstrate the efficiency of the described method in comparison with formerly reported procedures in the literature, we compared the results obtained in the preparation of 2-(2-chlorophenyl)-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile 3h with those of other methods (Table 4). The results clearly indicate that the use of TBBDA is an efficient method for the synthesis of spirocyclopropylbarbiturates. We observed significant decrease of reaction time, product purity, and thus cost efficiency.
In the next stage of our investigation, we decided to test the multicomponent transformation of benzaldehyde, malononitrile 5, and barbituric acid 2 into 4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile 3g. When the reaction was carried out under these conditions, a complex mixture of compounds was found in the end of the reaction (reaction progress was monitored by TLC) and the major product was 3-phenylcyclopropane-1,1,2,2-tetracarbonitrile 7c (49 %). Undoubtedly, the nucleophilic attack of the second molecule of malononitrile 5 to the benzylidenemalononitrile 1 takes place faster than the nucleophilic attack of barbituric acid 2 under the given reaction conditions. Herein, we report an effective and simple domino procedure for cyclopropanation of carbonyl compounds 6 with malononitrile 5 using N-halosulfonamides and sodium acetate in \(\hbox {H}_{2}\hbox {O/EtOH}\) at room temperature (Table 5).
It was shown that the aromatic aldehydes reacted faster than the ketone compounds (Table 5, entries 1–6). Moreover, in most cases, when a carbonyl group conjugated with an aromatic ring or a double bond, the corresponding 3-substituted-1,1,2,2-tetracyanocyclopropanes were achieved in a longer time (Table 5, entries 8, 10–13 and 16). Because of that extra stability, it might not be surprising that conjugated carbonyls are often a little slower to react than regular carbonyls.
A possible mechanism for the cyclopropanation of carbonyl compounds 6 with malononitrile 5 using N-halosulfonamides is shown in Scheme 4. First, the Knoevenagel condensation of the carbonyl compound 6 with malononitrile 5 leads to the formation of alkylidenemalononitrile A. On the other hand, deprotonation of the second molecule of malononitrile 5 occurs to give dicyanocarbanion B in aqueous ethanol with the use of base. Michael addition of dicyanocarbanion B to the \(\beta \)-carbon position of alkylidenemalononitrile A as an \(\alpha \),\(\beta \)-unsaturated compound afforded intermediate C. Thereupon, halogenation of the intermediate C with the use of N-halosulfonamides as sources for electrophilic halogens occurs to give the intermediate D. In the presence of base, deprotonation of intermediate D takes place leading to the formation of intermediate E. Intramolecular C-attack of carbanion E to carbon atom containing bromine atom as an electrophile produces 3-substituted-1,1,2,2-tetracyanocyclopropanes F (Scheme 4, path a). Under these reaction conditions, cyclobutanation of carbonyl compounds 6 with the C-attack of carbanion E to nitrile group did not occur (Scheme 4, path b) [10, 23].
Conclusions
In conclusion, simple and highly efficient methods for the synthesis of spirocyclopropylbarbiturates by cyclopropanation of 2-arylidenemalononitriles 1 with barbituric acids 2 and synthesis of substituted-1,1,2,2-tetracyanocyclopropanes by cyclopropanation of carbonyl compounds 6 with malononitrile 5 using \(N{,}N{,}N^\prime {,}N^\prime \)-tetrabromobenzene-1,3-disulfonamide [TBBDA], poly(N-bromo-N-ethyl-benzene-1,3-disulfonamide) [PBBS], \(N{,}N{,}N^\prime {,}N^\prime \)-tetrachlorobenzene-1,3-disulfonamide [TCBDA], and poly(N-chloro-N-ethyl-benzene-1,3-disulfonamide) [PCBS] were developed. These methods offer several significant advantages, such as being inexpensive reagents, high yield, high atom economy, ease of product isolation, environmental friendliness (non-corrosive reagents) which make them useful, and attractive processes for the rapid synthesis of 4,6,8-trioxo-2-phenyl-5,7-diazaspiro[2.5]octane-1,1-dicarbonitriles, 5,7-dimethyl-4,6,8-trioxo-2-phenyl-5,7-diazaspiro[2.5]octane-1,1-dicarbonitriles and 3-substituted-1,1,2,2-tetracyanocyclopropane derivatives.
Experimental
Melting points were measured with a digital melting point apparatus (Electrothermal) and are uncorrected. Mass spectra were recorded on a Shimadzu QP 1100 BX Mass Spectrometer (University of Tehran, Iran). \(^{1}\hbox {H}\) and \(^{13}\hbox {C}\)-NMR spectra were recorded on Bruker Advance 400 FT NMR spectrometers (undertaken at University of Isfahan, Iran) at 400 and 100 MHz spectrometer in DMSO-\(d_{6}\), respectively. Chemical shift values are reported in parts per million relative to the internal standard of tetramethylsilane (TMS); s (singlet), d (doublet), t (triplet), q (quartet), quin (quintet), sep (septet), m (multiplet); J in Hertz (Hz). Infrared (IR) spectroscopy was performed on a Perkin Elmer GX FT-IR spectrometer in KBr pellets. All starting materials were obtained from commercial sources and used without purification.
General procedure for preparation of 4,6,8-trioxo-2-phenyl-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile and 5,7-dimethyl-4,6,8-trioxo-2-phenyl-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile derivatives 3a–o using N-halosulfonamides
A mixture of 2-arylidenemalononitriles 1 (1 mmol), barbituric acid (0.128 g, 1 mmol) or 1,3-dimethylbarbituric acid 2 (0.156 g, 1 mmol), sodium acetate (0.08 g, 1 mmol), TBBDA (0.14 g, 0.25 mmol) or PBBS (0.2 g) or TCBDA (0.1 g, 0.25 mmol) or PCBS (0.15 g) in \(\hbox {H}_{2}\hbox {O/EtOH}\) (2 mL) (1:3) was placed in a test tube. The mixture was stirred at room temperature for the appropriate time specified in Table 3. After completion of the reaction [monitored by TLC (5:2, n-hexane/acetone)], the precipitate was filtered off, washed with ethanol (\(2 \times 1\) mL), and dried under reduced pressure to isolate pure spirocyclopropylbarbiturates. The filtrate was evaporated, washed with hot water, \(\hbox {CH}_{2}\hbox {Cl}_{2}\) (3 mL) was added, and the precipitated sulfonamide was removed by filtration. The sulfonamide was rehalogenated and used for several times.
General procedure for preparation of 3-substituted-1,1,2,2-tetracyanocyclopropanes 7a–p using N-halosulfonamides
A mixture of carbonyl compound 6 (1 mmol), malononitrile 5 (0.13 g, 2 mmol), sodium acetate (0.08 g, 1 mmol), TBBDA (0.14 g, 0.25 mmol) or PBBS (0.2 g) or TCBDA (0.1 g, 0.25 mmol) or PCBS (0.15 g) in \(\hbox {H}_{2}\hbox {O/EtOH}\) (1 mL) (1:3) was placed in a test tube. The mixture was stirred at room temperature for the appropriate time in Table 5. After completion of the reaction [monitored by TLC (5:3, n-hexane/acetone)], the precipitate was filtered off, washed with ethanol (\(2 \times 1\) mL), and dried under reduced pressure to isolate pure 3-substituted-1,1,2,2-tetracyanocyclopropanes 7. The filtrate was evaporated and washed with hot water, \(\hbox {CH}_{2}\hbox {Cl}_{2}\) (3 mL) was added, and the precipitated sulfonamide was removed by filtration. The sulfonamide was rehalogenated and used for several times.
General procedure for preparation of 2-arylidenemalononitriles 1 using aromatic aldehyde and malononitrile 5
To a 5 mL ethanol solution of aromatic aldehyde (2 mmol) and malononitrile 5 (0.15 g, 2.2 mmol) in a 25-mL round-bottomed flask, 0.5 mL of saturated solution of sodium bicarbonate in water was added. The mixture was magnetically stirred at room temperature for appropriate time (5 min–1 h) monitored by TLC (5:1, n-hexane/acetone). After completion of the reaction, the precipitate was filtered off washed with cold ethanol, and dried to isolate pure 2-arylidenemalononitriles 1 in 75–97 % yields.
Spectral data analysis for compounds
2-(4-Nitrophenyl)-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3a). White solid; Yield: 97 %; M.p: 247–248 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3253, 3125, 2263, 1762, 1723, 1706, 1519, 1398, 1354, 1281, 789, 521 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 4.42 (s, 1H, CH), 7.85 (d, \( J = 8.4\) Hz, 2H, ArH), 8.21 (d, \(J = 8.4\) Hz, 2H, ArH), 11.78 (s, 1H, NH), 11.97 (s, 1H, NH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 22.7, 40.6, 41.3, 110.5, 112.0, 122.9, 131.0, 136.6, 147.2, 149.9, 161.8, 163.7; MS: \(m/z = 325\) (\(\mathrm{M}^+\), 19 %), 282 (24 %), 244 (60 %), 214 (38 %), 193 (17 %), 165 (55 %), 133 (41 %), 89 (95 %), 69 (37 %), 45 (100 %)
2-(4-Chlorophenyl)-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3b). White solid; Yield: 95 %; M.p: 286–289 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3267, 3227, 2262, 1759, 1731, 1711, 1497, 1420, 1360, 1090, 769, 511 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 4.23 (s, 1H, CH), 7.44 (d, \( J = 8.4\) Hz, 2H, ArH), 7.54 (d, \(J = 8.4\) Hz, 2H, ArH), 11.71 (s, 1H, NH), 11.91 (s, 1H, NH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 22.6, 40.4, 41.8, 110.7, 112.2, 127.8, 128.0, 131.3, 132.9, 150.1, 161.8, 164.0; MS: \(m/z = 314\) (\(\hbox {M}^{+}\), 31 %), 313 (98 %), 270 (22 %), 249 (27 %), 227 (38 %), 199 (100 %), 165 (48 %), 123 (29 %), 99 (39 %), 69 (50 %), 43 (91 %).
2-(Naphthalen-2-yl)-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile (3c). White solid; Yield: 92 %; M.p: 258–260 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3279, 3241, 2260, 1761, 1735, 1708, 1522, 1418, 1364, 1196, 768, 504 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 4.43 (s, 1H, CH), 7.56 (m, 2H, ArH), 7.89 (m, 4H, ArH), 8.07 (s, 1H, ArH), 11.74 (s, 1H, NH), 11.97 (s, 1H, NH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 22.6, 40.5, 42.9, 110.9, 112.4, 126.3, 126.5, 126.9, 127.4, 12.7.5, 127.7, 128.4, 132.4, 149.9, 161.8, 164.1; MS: \(m/z = 330\) (\(\hbox {M}^{+}\), 7 %), 294 (19 %), 266 (23 %), 204 (100 %), 180 (79 %), 153 (24 %), 126 (39 %), 89 (13 %), 69 (20 %), 42 (25 %).
2-(2,4-Dichlorophenyl)-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3d). White solid; Yield: 85 %; M.p: 285–287 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3257, 3229, 2262, 1760, 1735, 1716, 1481, 1433, 1355, 1102, 813, 515 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 4.19 (s, 1H, CH), 7.51 (dd, \( J = 8.4\), 2 Hz, 1H, ArH), 7.65 (d, \(J = 8.4\) Hz, 1H, ArH), 7.72 (d, \( J = 2\) Hz, 1H, ArH), 11.90 (s, 1H, NH), 12.12 (s, 1H, NH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 23.3, 40.4, 40.7, 110.4, 111.9, 125.9, 127.1, 128.7, 132.8, 134.1, 134.5, 149.8, 161.7, 163.6; MS: \(m/z = 348\) (\(\hbox {M}^{+}\), 18 %), 313 (100 %), 262 (20 %), 250 (34 %), 239 (44 %), 199 (100 %), 165 (23 %), 123 (16 %), 99 (19 %), 70 (18 %).
2-(4-Bromophenyl)-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3e). White solid; Yield: 89 %; M.p: 270–272 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3273, 3232, 2262, 1759, 1732, 1712, 1491, 1420, 1359, 1012, 768, 510 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 4.21 (s, 1H, CH), 7.47 (d, \( J = 8.4\) Hz, 2H, ArH), 7.56 (d, \(J = 8.4\) Hz, 2H, ArH), 11.72 (s, 1H, NH), 11.92 (s, 1H, NH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 22.5, 40.4, 41.9, 110.7, 112.2, 121.6, 128.2, 130.9, 131.6, 149.9, 161.7, 163.9; MS: \(m/z = 358\) (\(\hbox {M}^{+}\), 44 %), 357 (100 %), 325 (26 %), 290 (36 %), 262 (40 %), 228 (20 %), 192 (17 %), 164 (97 %), 133 (33 %), 91 (49 %).
2-(3-Nitrophenyl)-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3f). White solid; Yield: 94 %; M.p: 271–273 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3420, 3262, 2255, 1758, 1732, 1715, 1540, 1416, 1357, 1034, 821, 511 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 4.37 (s, 1H, CH), 7.69 (t, \( J = 8\) Hz, 1H, ArH), 8.01 (d, \(J = 7.6\) Hz, 1H, ArH), 8.19 (d, \(J = 8.4\) Hz, 1H, ArH), 8.58 (s, 1H, ArH), 11.76 (s, 1H, NH), 11.95 (s, 1H, NH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 22.9, 40.5, 40.8, 110.6, 112.1, 123.0, 124.9, 129.5, 131.2, 136.2, 147.3, 150.0, 161.9, 163.7; MS: \(m/z = 325\) (\(\hbox {M}^{+}\), 5 %), 320 (22 %), 273 (100 %), 239 (60 %), 199 (98 %), 165 (72 %), 152 (72 %), 126 (44 %), 91 (38 %), 56 (64 %).
2-(2,4-Dichlorophenyl)-5,7-dimethyl-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3i). White solid; Yield: 93 %; M.p: 268–270 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)2998, 2251, 1698, 1681, 1592, 1457, 1384, 1301, 1112, 788, 752 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 3.15 (s, 3H, \(\hbox {CH}_{3})\), 3.30 (s, 3H, \(\hbox {CH}_{3})\), 4.34 (s, 1H, CH), 7.52 (dd, \(J = 8.4\), 2 Hz, 1H, ArH), 7.61 (d, \(J = 8.4\) Hz, 1H, ArH), 7.74 (d, \(J = 2\) Hz, 1H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 24.6, 28.6, 29.1, 41.1, 41.6, 110.4, 111.8, 125.7, 127.1, 128.8, 133.0, 134.2, 134.5, 150.5, 160.5, 162.4; MS: \(m/z = 376\) (\(\hbox {M}^{+}\), 6 %), 341 (100 %), 285 (17 %), 262 (19 %), 227 (35 %), 199 (90 %), 187 (27 %), 172 (14 %), 117 (15 %), 56 (30 %).
5,7-Dimethyl-2-(3-nitrophenyl)-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3j). White solid; Yield: 84 %; M.p: 255–257 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3034, 2251, 1750, 1700, 1685, 1536, 1433, 1382, 1343, 1145, 733, 621 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 3.12 (s, 3H, \(\hbox {CH}_{3})\), 3.29 (s, 3H, \(\hbox {CH}_{3})\), 4.51 (s, 1H, CH), 7.70 (t, \(J = 8\) Hz, 1H, ArH), 8.01 (d, \(J = 7.6\) Hz, 1H, ArH), 8.21 (d, \(J = 8\) Hz, 1H, ArH), 8.53 (s, 1H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 23.9, 28.5, 29.0, 41.3, 41.8, 110.5, 112.0, 123.1, 124.6, 129.6, 131.1, 136.0, 147.4, 150.9, 160.7, 162.6; MS: \(m/z = 353\) (\(\hbox {M}^{+}\), 32 %), 326 (14 %), 239 (100 %), 205 (37 %), 187 (63 %), 178 (77 %), 165 (94 %), 140 (39 %), 103 (40 %), 77 (46 %).
5,7-Dimethyl-2-(naphthalen-1-yl)-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3k). White solid; Yield: 87 %; M.p: 283–285 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)2989, 2246, 1752, 1702, 1678, 1509, 1459, 1390, 1297, 1118, 774, 455 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 3.03 (s, 3H, \(\hbox {CH}_{3})\), 3.36 (s, 3H, \(\hbox {CH}_{3})\), 4.76 (s, 1H, CH), 7.65 (m, 5H, ArH), 8.00 (m, 2H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 24.0, 28.5, 29.2, 41.3, 42.8, 110.9, 112.4, 122.5, 124.0, 124.9, 126.1, 127.0, 128.2, 128.9, 129.2, 130.7, 133.1, 150.6, 160.4, 162.7; MS: \(m/z = 358\) (\(\hbox {M}^{+}\), 46 %), 331 (45 %), 293 (45 %), 244 (50 %), 230 (47 %), 215 (74 %), 189 (65 %), 151 (39 %), 69 (100 %), 43 (98 %).
5,7-Dimethyl-4,6,8-trioxo-2-(m-tolyl)-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3l). White solid; Yield: 89 %; M.p: 264–266 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3000, 2249, 1702, 1677, 1459, 1424, 1392, 1299, 1141, 753, 497 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 2.29 (s, 3H, \(\hbox {CH}_{3})\), 3.12 (s, 3H, \(\hbox {CH}_{3})\), 3.27 (s, 3H, \(\hbox {CH}_{3})\), 4.31 (s, 1H, CH), 7.16 (m, 1H, ArH), 7.24 (m, 3H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 20.8, 23.3, 28.5, 28.9, 41.2, 43.9, 110.8, 112.3, 126.1, 128.0, 128.4, 128.8, 129.5, 137.3, 150.8, 160.4, 162.9; MS: \(m/z = 322\) (\(\hbox {M}^{+}\), 26 %), 295 (26 %), 265 (43 %), 238 (18 %), 208 (100 %), 180 (98 %), 166 (57 %), 153 (40 %), 115 (37 %), 56 (24 %).
2-(4-Chloro-3-nitrophenyl)-5,7-dimethyl-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitrile ( 3m). White solid; Yield: 95 %; M.p: 268–270 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3021, 2249, 1702, 1678, 1531, 1460, 1427, 1393, 1300, 1142, 822, 494 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 3.12 (s, 3H, \(\hbox {CH}_{3})\), 3.29 (s, 3H, \(\hbox {CH}_{3})\), 4.49 (s, 1H, CH), 7.85 (d, \(J = 8.4\) Hz, 1H, ArH), 7.91 (d, \(J = 8\) Hz, 1H, ArH), 8.38 (s, 1H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 24.0, 28.5, 29.0, 41.1, 41.2, 110.3, 111.8, 124.8, 126.8, 130.2, 131.3, 134.9, 146.9, 150.8, 160.7, 162.4; MS: \(m/z = 387\) (\(\hbox {M}^{+}\), 40 %), 360 (28 %), 330 (109 %), 273 (100 %), 245 (68 %), 199 (82 %), 165 (45 %), 117 (37 %), 91 (45 %), 56 (59 %).
3-(Thiophen-2-yl)cyclopropane-1,1,2,2-tetracarbonitrile ( 7a). White solid; Yield: 96 %; M.p: 155–157 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3310, 3032, 2260, 2232, 1573, 1429, 1362, 1257, 1147, 1058, 728, 636 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 5.42 (s, 1H, CH), 7.15 (t, \(J = 4\) Hz, 1H, ArH), 7.54 (s, 1H, ArH), 7.70 (d, \(J = 4.4\) Hz, 1H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 24.5, 38.6, 109.9, 111.1, 127.7, 130.4, 130.9; MS: \(m/z = 224\) (\(\hbox {M}^{+}\), 100 %), 197 (92 %), 171 (25 %), 160 (58 %), 147 (9 %), 133 (24 %), 109 (23 %), 96 (95 %), 69 (30 %), 45 (86 %).
3-([1, \(1^{\prime }\) -Biphenyl]-4-yl)cyclopropane-1,1,2,2-tetracar bonitrile ( 7b). White solid; Yield: 98 %; M.p: 224–225 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3063, 3013, 2925, 2263, 1582, 1490, 1406, 1133, 1045, 852, 762, 700 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 5.35 (s, 1H, CH), 7.41 (t, \(J = 3.2\) Hz, 1H, ArH), 7.49 (t, \(J = 7.6\) Hz, 2H, ArH), 7.74 (d, \(J = 7.2\) Hz, 2H, ArH), 7.82 (d, \(J = 8.8\) Hz, 2H, ArH), 7.92 (d, \(J = 8\) Hz, 2H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 23.2, 41.5, 109.5, 111.0, 125.9, 126.8, 127.0, 128.0, 129.0, 130.1, 138.8, 141.5; MS: \(m/z = 294\) (\(\hbox {M}^{+}\), 48 %), 267 (19 %), 230 (44 %), 178 (17 %), 155 (21 %), 133 (11 %), 115 (21 %), 91 (100 %), 65 (28 %), 43 (78 %).
3-Isobutylcyclopropane-1,1,2,2-tetracarbonitrile ( 7d). White solid; Yield: 87 %; M.p: 125–127 \(^{\circ }\)C; IR (KBr): \(\upsilon \)3042, 2964, 2934, 2875, 2265, 1643, 1470, 1390, 1171, 984, 727, 461 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 0.98 (d, \( J = 6.8\) Hz, 6H, 2\(\hbox {CH}_{3})\), 1.66 (t, \(J = 6.8\) Hz, 2H, \(\hbox {CH}_{2}\)), 1.93 (sep, \(J = 2.8\) Hz, 1H, CH), 3.93 (t, \( J = 6.8\) Hz, 1H, CH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 21.8, 22.1, 26.1, 34.9, 38.0, 109.5, 110.7; MS: \(m/z = 198\) (\(\hbox {M}^{+}\), 20 %), 181 (100 %), 167 (35 %), 149 (23 %), 124 (21 %), 105 (33 %), 93 (63 %), 80 (60 %), 69 (72 %), 43 (92 %).
3-Hexylcyclopropane-1,1,2,2-tetracarbonitrile ( 7e). White solid; Yield: 84 %; M.p: 110–112 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)3052, 2956, 2932, 2857, 2261, 1719, 1467, 1379, 1124, 992, 726, 463 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 0.88 (t, \( J = 6.8\) Hz, 3H, \(\hbox {CH}_{3})\), 1.29 (m, 6H, 3\(\hbox {CH}_{2}\)), 1.55 (quin, \(J = 8\) Hz, 2H, \(\hbox {CH}_{2}\)), 1.74 (dd, \(J = 7.2\), 8 Hz, 2H, \(\hbox {CH}_{2}\)), 3.87 (t, \(J = 6.8\) Hz, 1H, CH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 13.8, 21.8, 22.2, 25.7, 26.7, 27.8, 30.7, 39.2, 109.4, 110.8; MS: \(m/z = 226\) (\(\hbox {M}^{+}\), 5 %), 201 (95 %), 198 (74 %), 186 (41 %), 183 (95 %), 152 (53 %), 133 (8 %), 105 (10 %), 77 (100 %), 51 (59 %).
3-Phenethylcyclopropane-1,1,2,2-tetracarbonitrile ( 7f). White solid; Yield: 95 %; M.p: 168–169 \(^{\circ }\)C; IR (KBr): \(\upsilon \)3038, 2961, 2259, 1603, 1496, 1455, 1213, 1130, 998, 753, 701, 489 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 2.00 (t, \(J = 5.6\) Hz, 2H, \(\hbox {CH}_{2}\)), 2.89 (t, \(J = 5.6\) Hz, 2H, \(\hbox {CH}_{2}\)), 3.89 (s, 1H, CH), 7.25 (m, 3H, ArH), 7.27 (m, 2H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 22.6, 29.3, 32.3, 40.2, 109.7, 111.2, 127.1, 129.0, 129.1, 139.7; MS: \(m/z = 246\) (\(\hbox {M}^{+}\), 8 %), 219 (9 %), 192 (6 %), 156 (28 %), 128 (21 %), 107 (5 %), 91 (100 %), 65 (22 %), 50 (9 %).
3-Methyl-3-phenethylcyclopropane-1,1,2,2-tetracarbonitrile ( 7g). White solid; Yield: 94 %; M.p: 146–147 \(^{\circ }\)C; IR (KBr): \(\upsilon \)3067, 3025, 2970, 2255, 1603, 1496, 1458, 1395, 1059, 977, 753, 698, 492 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 1.65 (s, 3H, \(\hbox {CH}_{3})\), 2.02 (dd, \(J = 6.8\), 10 Hz, 2H, \(\hbox {CH}_{2}\)), 2.90 (dd, \(J = 6.8\), 10 Hz, 2H, \(\hbox {CH}_{2}\)), 7.22 (m, 3H, ArH), 7.3 (m, 2H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 16.9, 28.0, 30.7, 35.9, 43.7, 110.4, 110.5, 126.9, 128.6, 129.1, 140.4; MS: \(m/z = 260\) (\(\hbox {M}^{+}\), 49 %), 232 (33 %), 205 (29 %), 178 (52 %), 155 (34 %), 117 (28 %), 112 (39 %), 91 (100 %), 65 (86 %), 41 (29 %).
3-(4-Chlorophenyl)-3-ethylcyclopropane-1,1,2,2-tetracarbonitrile ( 7h). White solid; Yield: 92 %; M.p: 154–156 \(^{\circ }\)C; IR (KBr): \(\upsilon \)3091, 3038, 2980, 2936, 2262, 1595, 1492, 1400, 1095, 1014, 822, 730 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 0.91 (t, \(J = 6.4\) Hz, 3H, \(\hbox {CH}_{3})\), 2.04 (q, \( J = 6.4\) Hz, 2H, \(\hbox {CH}_{2}\)), 7.59 (d, \(J = 6\) Hz, 2H, ArH), 7.98 (d, \(J = 6\) Hz, 2H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 9.4, 28.4, 29.7, 51.1, 110.5, 129.2, 129.3, 132.8, 135.5; MS: \(m/z = 280\) (\(\hbox {M}^{+}\), 19 %), 245 (38 %), 218 (100 %), 191 (21 %), 174 (7 %), 152 (41 %), 117 (92 %), 99 (8 %), 75 (24 %), 50 (16 %).
3-(2-Methoxybenzyl)-3-methylcyclopropane-1,1,2,2-tetracarbonitrile ( 7i). White solid; Yield: 89 %; M.p: 153–154 \(^{\circ }\)C; IR (KBr): \(\upsilon \)3069, 2993, 2944, 2839, 2254, 1603, 1589, 1497, 1249, 1120, 1025, 761 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 1.22 (s, 3H, \(\hbox {CH}_{3})\), 3.16 (s, 2H, \(\hbox {CH}_{2}\)), 3.86 (s, 3H, O\(\hbox {CH}_{3})\), 6.97 (t, \(J = 7.6\) Hz, 1H, ArH), 7.08 (d, \(J = 7.6\) Hz, 1H, ArH), 7.19 (d, \(J = 7.6\) Hz, 1H, ArH), 7.35 (t, \(J = 7.6\) Hz, 1H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 17.5, 28.6, 34.9, 42.5, 55.7, 110.4, 110.5, 111.5, 121.3, 122.3, 130.0, 131.8, 157.8; MS: \(m/z = 276\) (\(\hbox {M}^{+}\), 60 %), 234 (5 %), 197 (10 %), 171 (22 %), 148 (18 %), 132 (24 %), 121 (100 %), 91 (88 %), 78 (29 %), 65 (28 %).
3-Methyl-3-(4-nitrophenyl)cyclopropane-1,1,2,2-tetracarbonitrile ( 7k). White solid; Yield: 84 %; M.p: 238–240 \(^{\circ }\)C; IR (KBr): \(\upsilon \)2924, 2854, 2260, 1604, 1521, 1455, 1354, 1074, 860, 699 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 1.87 (s, 3H, \(\hbox {CH}_{3})\), 8.39 (dd, \(J = 2.8\), 9.2 Hz, 4H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 22.7, 28.0, 46.5, 109.9, 110.0, 124.0, 131.2, 139.3, 148.2; MS: \(m/z = 277\) (\(\hbox {M}^{+}\), 31 %), 250 (59 %), 231 (70 %), 204 (100 %), 177 (47 %), 140 (49 %), 119 (26 %), 102 (43 %), 77 (87 %), 50 (68 %).
3-(4-Fluorophenyl)-3-methylcyclopropane-1,1,2,2-tetracarbonitrile ( 7l). White solid; Yield: 79 %; M.p: 256–257 \(^{\circ }\)C; IR (KBr): \(\upsilon \)3067, 2260, 1602, 1498, 1448, 1389, 1317, 1071, 1054, 983, 761, 699, 565 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 1.84 (s, 3H, \(\hbox {CH}_{3}\)), 7.49 (d, \(J = 7.6\) Hz, 2H, ArH), 7.97 (d, \(J = 7.6\) Hz, 2H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 23.3, 27.9, 47.5, 109.9, 110.2, 128.9, 129.3, 129.7, 132.6; MS: \(m/z = 250\) (\(\hbox {M}^{+}\), 5 %), 243 (94 %), 224 (4 %), 205 (3 %), 186 (100 %), 168 (4 %), 153 (4 %), 130 (6 %), 109 (44 %), 80 (19 %).
Spiro[2.5]oct-4-ene-1,1,2,2-tetracarbonitrile ( 7m). White solid; Yield: 76 %; M.p: 205–207 \(^{\circ }\)C (dec.); IR (KBr): \(\upsilon \)2958, 2926, 2867, 2257, 1621, 1605, 1451, 1301, 1186, 989, 872, 730 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 1.28 (dd, \(J = 9.2\), 12.8 Hz, 1H, CH), 1.55 (d, \(J = 13.6\) Hz, 1H, CH), 1.75 (d, \(J = 12.8\) Hz, 1H, CH), 1.97 (d, \(J = 11.6\) Hz, 1H, CH), 2.25 (quin, \( J = 11.6\) Hz, 1H, CH), 2.63 (t, \(J = 12.8\) Hz, 1H, CH), 5.21 (s, 1H, CH vinyl), 5.23 (s, 1H, CH vinyl); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 21.0, 27.5, 28.3, 31.4, 34.9, 43.8, 109.4, 109.6, 112.6, 112.7; MS: \(m/z = 208\) (\(\hbox {M}^{+}\), 10 %), 199 (21 %), 181 (63 %), 167 (16 %), 145 (29 %), 130 (23 %), 118 (26 %), 104 (32 %), 80 (100 %), 66 (52 %).
6-Benzyl-6-azaspiro[2.5]octane-1,1,2,2-tetracarbonitrile ( 7n). White solid; Yield: 71 %; M.p: 163–164 \(^{\circ }\)C; IR (KBr): \(\upsilon \)3028, 2962, 2805, 2254, 1598, 1493, 1453, 1346, 1314, 1140, 996, 746 \(\hbox {cm}^{-1}\); \(^{1}\hbox {H}\) NMR (400 MHz, DMSO-\(d_{6})\): \(\delta \) 1.98 (m, 2H, \(\hbox {CH}_{2}\)), 2.61 (m, 2H, \(\hbox {CH}_{2}\)), 3.58 (s, 2H, \(\hbox {CH}_{2}\)), 7.33 (s, 5H, ArH); \(^{13}\hbox {C}\) NMR (100 MHz, DMSO-\(d_{6})\): \(\delta \) 26.5, 28.8, 44.5, 49.6, 60.9, 109.5, 127.1, 128.2, 128.8, 137.7; MS: \(m/z = 301\) (\(\hbox {M}^{+}\), 50 %), 236 (7 %), 224 (15 %), 209 (10 %), 172 (34 %), 145 (5 %), 118 (8 %), 91 (100 %), 65 (72 %), 42 (48 %).
References
Maquoi E, Sounni NE, Devy L, Olivier F, Frankenne F, Krell HW, Grams F, Foidart JM, Noel A (2004) Anti-Invasive, antitumoral, and antiangiogenic efficacy of a pyrimidine-2,4,6-trione derivative, an orally active and selective matrix metalloproteinases inhibitor. Clin Cancer Res 10:4038–4047. doi:10.1158/1078-0432.CCR-04-0125
Johns MW (1975) Sleep and hypnotic drugs. Drugs 9:448–478. doi:10.2165/00003495-197509060-00004
Naguib FNM, Levesque DL, Wang EC, Panzica RP, El Kouni MH (1993) 5-Benzylbarbituric acid derivatives, potent and specific inhibitors of uridine phosphorylase. Biochem Pharmacol 46:1273–1283. doi:10.1016/0006-2952(93)90477-E
Fraser W, Suckling CJ, Wood HCS (1990) Latent inhibitors. Part 7: inhibition of oihydro-orotate dehydrogenase by spirocyclopropanobarbiturates. J Chem Soc Perkin Trans 1:3137–3144. doi:10.1039/P19900003137
Renard A, Lhomme J, Kotera M (2002) Synthesis and properties of spiro nucleosides containing the barbituric acid moiety. J Org Chem 67:1302–1307. doi:10.1021/jo016194y
Muthusamy S, Gunanathan C (2003) Reactions of cyclic diazoamides: convenient synthesis of dispirocyclic cyclopropane systems. Synlett 2003:1599–1602. doi:10.1055/s-2003-40996
Krysiak J, Kato T, Gornitzka H, Baceiredo A, Mikolajczyk M, Bertrand G (2001) The first asymmetric cyclopropanation reactions involving a stable carbene. J Org Chem 66:8240–8242. doi:10.1021/jo010586n
Singh VK, DattaGupta A, Sekar G (1997) Catalytic enantioselective cyclopropanation of olefins using carbenoid chemistry. Synthesis 1997:137–149. doi:10.1055/s-1997-1172
Muller P, Allenbach Y, Robert E (2003) Rhodium(II)-catalyzed olefin cyclopropanation with the phenyliodonium ylide derived from Meldrum’s acid. Tetrahedron Asymmetry 14:779–785. doi:10.1016/S0957-4166(03)00029-6
Xin X, Zhang Q, Liang Y, Zhanga R, Dong D (2014) Tandem halogenation/Michael-initiated ringclosing reaction of \(\alpha \), \(\beta \)-unsaturated nitriles and activated methylene compounds: one-pot diastereoselective synthesis of functionalized cyclopropanes. Org Biomol Chem 12:2427–2435. doi:10.1039/c4ob00087k
Little RD, Dawson JR (1980) MIRC (Michael Initiated Ring Closure) reactions formation of three; five, six and seven membered rings. Tetrahedron Lett 21:2609–2612. doi:10.1016/S0040-4039(00)92818-1
Kawai D, Kawasumi K, Miyahara T, Hirashita T, Araki S (2008) Cyclopropanation mediated by lithium iodide of electron-deficient alkenes with activated dibromomethylene compounds. Synlett 2008:2977–2980. doi:10.1055/s-0028-1087341
Elinson MN, Vereshchagin AN, Stepanov NO, Zaimovskaya TA, Merkulova VM, Nikishin GI (2010) The first example of the cascade assembly of a spirocyclopropane structure: direct transformation of benzylidenemalononitriles and \(N{,}N^{\prime }\)-dialkylbarbituric acids into substituted 2-aryl-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitriles. Tetrahedron Lett 51:428–431. doi:10.1016/j.tetlet.2009.11.065
Dorofeeva EO, Elinson MN, Vereshchagin AN, Stepanov NO, Bushmarinov IS, Belyakov PA, Sokolova OO, Nikishin GI (2012) Electrocatalysis in MIRC reaction strategy: facile stereoselective approach to medicinally relevant spirocyclopropylbarbiturates from barbituric acids and activated olefins. RSC Adv 2:4444–4452. doi:10.1039/c2ra20078c
Vereshchagin AN, Elinson MN, Dorofeeva EO, Stepanov NO, Zaimovskaya TA, Nikishin GI (2013) Electrocatalytic and chemical methods in MHIRC reactions: the first example of the multicomponent assembly of medicinally relevant spirocyclopropylbarbiturates from three different molecules. Tetrahedron 69:1945–1952. doi:10.1016/j.tet.2012.12.029
Wideqvist S (1945) 2,2-Dimethyl-1,1,3,3-tetracyanocyclopropane. Arkiv for Kemi 20B:8–14
Hart H, Kim YC (1966) A new synthesis of tetracyanocyclopropanes. J Org Chem 31:2784–2789. doi:10.1021/jo01347a013
Kim YC, Hart H (1969) Synthesis and NMR spectra of 3-aryl-1,1,2,2-tetracyanocyclopropanes. Tetrahedron 25:3869–3877. doi:10.1016/S0040-4020(01)82918-5
Elinson MN, Feducovich SK, Stepanov NO, Vereshchagin AN, Nikishin GI (2008) A new strategy of the chemical route to the cyclopropane structure: direct transformation of benzylidenemalononitriles and malononitrile into 1,1,2,2-tetracyanocyclopropanes. Tetrahedron 64:708–713. doi:10.1016/j.tet.2007.11.027
Vereshchagin AN, Elinson MN, Stepanov NO, Nikishin GI (2009) One-pot cascade assembling of 3-substituted tetracyanocyclopropanes from alkylidenemalononitriles and malononitrile by the only bromine direct action. Mendeleev Commun 19:324–325. doi:10.1016/j.mencom.2009.11.010
Elinson MN, Vereshchagin AN, Stepanov NO, Ilovaisky AI, Vorontsov AY, Nikishin GI (2009) A new type of cascade reaction: direct conversion of carbonyl compounds and malononitrile into substituted tetracyanocyclopropanes. Tetrahedron 65:6057–6062. doi:10.1016/j.tet.2009.05.062
Vereshchagin AN, Elinson MN, Stepanov NO, Nikishin GI (2011) New way to substitute tetracyanocyclopropanes: one-pot cascade assembling of carbonyls and malononitrile by the only bromine direct action. ISRN Org Chem ID 469453:1–5. doi:10.5402/2011/469453
Noroozi Pesyan N, Kimia MA, Jalilzadeha M, Sahin E (2013) A new, fast and easy strategy for one-pot synthesis of full substituted cyclopropanes: direct transformation of aldehydes to 3-aryl-1,1,2,2-tetracyanocyclopropanes. J Chin Chem Soc 60:35–44. doi:10.1002/jccs.201200189
Elinson MN, Feducovich SK, Lizunova TL, Nikishin GI (2000) Electrochemical transformation of malononitrile and carbonyl compounds into functionally substituted cyclopropanes: electrocatalytic variant of the Wideqvist reaction. Tetrahedron 56:3063–3069. doi:10.1016/S0040-4020(00)00195-2
Veisi H, Ghorbani-Vaghei R (2010) Recent progress in the application of \(N\)-halo reagents in the synthesis of heterocyclic compounds. Tetrahedron 66:7445–7463. doi:10.1016/j.tet.2010.07.015
Koval IV (2002) \(N\)-Halo reagents. \(N\)-Halosuccinimides in organic synthesis and in chemistry of natural compounds. Russ J Org Chem 38:301–337. doi:10.1023/A:1016390721218
Ghorbani-Vaghei R, Jalili H (2005) Mild and regioselective bromination of aromatic compounds with \(N{,}N{,}N^\prime {,}N^\prime \)-tetrabromobenzene-1,3-disulfonamide and Poly(\(N{,}N^{\prime }\)-dibromo-\(N\)-ethyl-benzene-1,3-disulfonamide). Synthesis 7:1099–1102. doi:10.1055/s-2005-861851
Ghorbani-Vaghei R, Maghbooli Y, Mahmoodi J, Shahriari A (2015) Poly(\(N{,}N^{\prime }\)-dibromo-\(N\)-ethyl-benzene-1,3-disulfonamide) and \(N{,}N{,}N^\prime {,}N^\prime \)-tetrabromobenzene-1,3-disulfonamide as new efficient reagents for one-pot synthesis of furano and pyrano pyrimidinones (thiones). RSC Adv 5:74336–7434110. doi:10.1039/c5ra16646b
Ghorbani-Vaghei R, Salimi Z, Malaekehpoor SM, Eslami F, Noori S (2014) One-pot synthesis of new derivatives of pyran using \(N\)-halosulfonamide. RSC Adv 4:33582–33586. doi:10.1039/c4ra04929b
Ghorbani-Vaghei R, Shahriari A, Salimi Z, Hajinazari S (2015) Solvent-free synthesis of triazines using \(N\)-halosulfonamides. RSC Adv 5:3665–3669. doi:10.1039/c4ra10892b
Arora R, Shandil A, Deep Kumar Jain A (2011) Synthesis and characterization of some barbituric acid derivatives-5-[(3\(^\prime \)-chloro-4\(^\prime \)-substituted phenyl-2\(^\prime \)-oxo-azetidin-1\(^\prime \)-yl) amino] barbituric acid. IJRPBS 2:1210–1214
Hartman WW, Sheppard OE (1943) Alloxan monohydrate. Org Syn 23:3–5. doi:10.15227/orgsyn.023.0003
Seeliger F, Berger STA, Remennikov GY, Polborn K, Mayr H (2007) Electrophilicity of 5-benzylidene-1,3-dimethylbarbituric and thiobarbituric acids. J Org Chem 72:9170–9180. doi:10.1021/jo071273g
Acknowledgments
We acknowledge with thanks the financial support received from Bu-Ali Sina University, Center of Excellence and Development of Chemical Methods (CEDCM).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghorbani-Vaghei, R., Maghbooli, Y., Shahriari, A. et al. Facile and efficient approach for the synthesis of spirocyclopropylbarbiturates and 3-substituted-1,1,2,2-tetracyanocyclopropanes using N-halosulfonamides. Mol Divers 20, 907–917 (2016). https://doi.org/10.1007/s11030-016-9682-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9682-y