Abstract
Ischemic stroke is one of the most common and undertreated cerebral diseases with high mortality and disability rate. Various intrinsic and extrinsic factors regulate the onset, severity, and progression of ischemic stroke. As an integral part of the neuronal glia system, astrocytes provide many housekeeping functions in nervous system, and perform multiple functions both beneficial and detrimental for neuronal survival after ischemic stroke. In addition, the small GTPase Rho and its downstream Rho kinase (ROCK) are associated with various neuronal functions such as dendrite development, migration and axonal extension, and numerous central nervous system (CNS) diseases. The aim of this review is to summarize the role of RhoA/ROCK signaling pathway and astrocytes on neurological function after ischemic stroke. We also discuss the interaction of RhoA/ROCK signaling pathway and astrocytes on the tissue repair after brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is one of main causes responsible for disability and death over the world. The tissue plasminogen activator (t-PA) is the only FDA-approved therapeutic drug for acute ischemic stroke. But only a small proportion of brain ischemia patients are suitable for t-PA treatment for the high risk of secondary impairments (Malone et al. 2019). Consequently, there is a pressing need to explore the intrinsic and extrinsic factors which regulate the onset, severity, and progression of ischemic stroke.
The brain is surprisingly plastic after stroke, and pathological processes of cerebral ischemia can increase neurogenesis in the adult brain (Liepert et al. 2000; Rossini et al. 2003). Neuroblasts from the sub-ventricular zone proliferate and migrate to the damaged regions (Jin et al. 2003) and then differentiate (Arvidsson et al. 2002), which is called neurogenesis (Spadafora et al. 2010). Neurogenesis has become a hot topic of interest in research since it was shown that the brain maintains the capability to generate new neurons (Codega et al. 2014; Thier et al. 2019). Besides neurogenesis, the recovering brain also experiences angiogenesis, which is also regarded as a treatment strategy for stroke (Brea et al. 2009). After stroke, intercellular signaling among the neuron, glial and brain endothelial cell mediates angiogenesis (Shibuya 2009), and this leads to a new concept of neurovascular unit, which has generally been accepted and considered as an important therapeutic strategy in the stroke (Sa-Pereira et al. 2012). Therefore, both neurogenesis and angiogenesis are critical events contributing to neuronal rehabilitation and survival (Du et al. 2019).
In the brain, astrocytes are the most abundant glial cells, the astrocytic inflammatory response to stroke may exacerbate the ischemic injury, but astrocytes also provide neuroprotective potential by releasing neurotrophins and limiting lesion extension via anti-excitotoxicity effects (Liu and Chopp 2016), which caused increasing number of researches that concentrate on the effect of brain astrocytes after stroke in recent years. In addition, accumulated evidences have revealed that astrocytes were involved in the retinal angiogenesis under hypoxic conditions (Rattner et al. 2019; Scott et al. 2010) or during development (Hirota et al. 2011; Stenzel et al. 2011). In conclusion, astrocytes perform multiple functions both beneficial and detrimental for neuronal survival during the acute phase of an ischemic stroke.
The small G protein RhoA (Ras homolog gene family) and its downstream effector Rho-dependent kinase (ROCK) are ubiquitous expression in astrocytes and neurons, and are involved in the regulation of the cytoskeleton via downstream regulation of myosin, actin, and so on. The effects of RhoA/ROCK on cellular functions of astrocytes and neurons under normal and pathological conditions have been intensively studied (Maldonado et al. 2017; Tonges et al. 2011; Wen et al. 2019).Thus, the key focus of this review was to discuss the functions of RhoA/ROCK pathway in astrocyte-mediated events during stroke and recovery.
RhoA/ROCK pathway in astrocyte‐mediated angiogenesis
Angiogenesis occurs more frequently in ischemic/hypoxic tissues, and is formed by new capillaries from pre-existing blood vessels (Liu et al. 2018). The hedgehog gene (Hh) was first discovered in the fruit fly, drosophila melanogaster (Nusslein-Volhard and Wieschaus 1980). There are three Hh family members in mammals, namely sonic hedgehog (Shh), desert hedgehog (Dhh), and indian hedgehog (Ihh). Among them, Shh is a soluble extracellular protein and the best studied ligand of the hedgehog signaling pathway. As a morphogen and mitogen, Shh is involved in cell migration and proliferation in a variety types of cells in individual development (Chen et al. 2016; He et al. 2017) and tissue repair after injury (Kawagishi et al. 2018; Peng et al. 2015). Furthermore, Shh is considered as an indirect angiogenic factor that can up-regulate two families of angiogenic growth factors, angiopoietins and vascular endothelial growth factor (VEGF) (Pola et al. 2001). More importantly, exogenous Shh has been found to directly promote the tube formation of peripheral endothelial cells (ECs) under normal conditions (Chinchilla et al. 2010; Renault et al. 2010).
Further studies have found that Shh could be secreted by astrocytes under oxidative stress and protected astrocytes and neurons against oxidative stress, suggesting that endogenous Shh derived from astrocytes may acts as an angiogenic factor and also participates in ischemic insults (Dai et al. 2011; Xia et al. 2012). Furthermore, RhoA/ROCK pathway also plays an essential role on the angiogenesis of endothelial cells, and is found to be involved in VEGF-induced angiogenesis (Bryan et al. 2010; van der Meel et al. 2011). ROCK inhibitor, Fasudil has been found to inhibit the angiogenesis (Washida et al. 2011). Besides, RhoA/ROCK is likewise involved in angiogenesis of HCT116 and LS174T colon carcinoma cells (Croft et al. 2004). In addition, ROCK inhibitor HA1077 is reported to block the glioma-induced angiogenesis (Nakabayashi and Shimizu 2011).
By using a co-culture of astrocytes with brain microvascular endothelial cells (BMECs) under oxygen–glucose deprivation (OGD) condition, Quanwei He et al. found that the secretion of Shh by astrocytes significantly up-regulated after OGD, and migration, proliferation, and tube formation of BMECs co-cultured with astrocytes significantly enhanced. But the migration, proliferation, and tube formation of BMECs after OGD could be blocked by Shh antagonist cyclopamine, silencing RhoA gene of BMECs or ROCK antagonist Y27632 (He et al. 2013). These findings suggested that RhoA/ROCK pathway may be involved in the angiogenesis mediated by activated astrocytes-produced shh after cerebral ischemia.
In addition, RhoA/ROCK pathway has been found to be involved in Shh-mediated tube formation of peripheral vascular ECs (Chinchilla et al. 2010; Polizio et al. 2011), cellular proliferation, promotion of fibroblast migration, and patterning during vertebrate development (Kasai et al. 2004; Polizio et al. 2011). In view of the fact that upregulation of angiogenesis induced by activated astrocytes-derived Shh after oxygen–glucose deprivation (OGD) are remarkably reversed by downregulating RhoA or by treatment with special ROCK antagonist (Chinchilla et al. 2010; He et al. 2013; Renault et al. 2010). These findings further confirmed that RhoA/ROCK pathway not surprisingly participates in endogenous Shh-induced angiogenesis after ischemia. In conclusion, RhoA/ROCK pathway is involved in the astrocyte-mediated angiogenesis via facilitating the effect of astrocytes-derived Shh.
RhoA/ROCK pathway in astrocyte‐mediated neurogenesis
Neurogenesis in the cerebral cortex is significantly influenced by Shh during the embryonic stage, and possesses a multiple effect to the development of central nervous system (CNS). Shh induces neural cells specialization, proliferation, as well as growth of dendrites and axons in various CNS regions, including hindbrain, forebrain, and spinal cord. Shh also acts as a mitogen to regulate survival and proliferation of neural progenitor cells (NPCs) and neural stem cells (NSCs) (Alvarez-Buylla and Ihrie 2014). Besides, the notable importance at the beginning of life, Shh also plays a key role in regulating proliferation of NPCs in adult hippocampus (Lai et al. 2003; Yao et al. 2016). Down-regulation of Shh holds close correlation with senescence, which makes the body more susceptible to aging associated disorders (Dashti et al. 2012). Accordingly, the activation of shh signaling plays particularly role on maintaining the activity of neurons in adults (Han et al. 2008). Several lines of studies have reported the crucial role of Shh in neurological diseases due to the capability of neurogenesis (Alvarez-Buylla and Ihrie 2014; Yao et al. 2016). Salvianolic acid, a free radical scavenger and an antioxidant, has been found to promote neurogenesis and functional recovery via activation of Shh after stroke in mice (Zhang et al. 2017).
Astrocytes are ubiquitous glial cells throughout the CNS, and play vital and diverse roles in the normal function and development of the CNS (Ben Haim and Rowitch 2017). Increasing studies have reported that astrocytes could regain multipotency and even revert back to its initiative undifferentiated state after stroke, focal ischemia, kainate-induced neural excitotoxicity, etc. (Feng et al. 2014; Shin et al. 2013). More importantly, increasing research groups have further revealed that astrocytes could revert to lineage-restricted neural progenitors, pluripotent neural stem cells (NSCs), and even certain subtypes of neurons by application of specific interventions or by forced expression of defined factors (Gao et al. 2017; Niu et al. 2015; Wang et al. 2015). Recent research revealed that mature astrocytes could directly convert into NSCs induced by Oct4, a single transcription factor. These induced-NSCs displayed authentic NSC gene expression, typical neurosphere morphology, multipotency and self-renewal capacity(Yang et al. 2019). Moreover, the neurons derived from induced NSC possessed functionality as neurons. Surprisingly, continuous Shh stimulation could potentiate the Oct4-mediated reprogramming of astrocytes into induced NSCs, which was as demonstrated by increased conversion efficiency and a sped-up reprogramming (Yang et al. 2019).
The RhoA/ROCK pathway is best known for its effect on cytoskeletal rearrangement, resulting in retraction and growth cone collapse. Inhibition of Rho kinase in neurons by using ROCK inhibitors rescues embryonic stem cell-derived neural precursor cells from apoptosis after transplantation (Koyanagi et al. 2008), blocks the effect of neurite outgrowth inhibitors, promotes neurite outgrowth and axon regeneration and sprouting following injury (Murakoshi et al. 2011). Inhibition of RhoA/ROCK pathway could induce the changes of neurogenesis in adult hippocampal and increase the survival of new born neurons, which could aid in the recovery of brain function following injury to the brain (Christie et al. 2013). In addition, Jing Ding et al. have found that ROCK inhibitor Fasudil could up-regulate astrocytes to produce granulocyte colony-stimulating factor (G-CSF), which could trigger neurogenesis and protect neurons in astrocyte-conditioned medium containing G-CSF (Ding et al. 2009).
In a word, activation of RhoA/ROCK pathway blocks the neurogenesis, which is harmful to the neural recovery after ischemic stroke. However, the relationship between the RhoA/ROCK pathway and astrocyte-mediated neurogenesis is not fully understood.
Astrocyte‐mediated neuroprotection
Astrocytes, most abundant cell types in the brain, are critical functional and structural part of the neurovascular unit (NVU) and the tripartite synapses, which communicate with oligodendrocytes, neurons and endothelial cells. The branches of astrocyte finely contact all parts of neurons, containing soma, axons, dendrites and synaptic terminals, and envelop all cellular components throughout the CNS (Scemes et al. 2000). As integral part of the NVU, astrocytes are involved in many housekeeping functions, including formation of blood brain barrier (BBB), structural support, maintenance of the extracellular environment, neuronal metabolism, regulation of cerebral blood flow, mediation of cell-cell communications, defense against oxidative stress, and modulation of neurotransmitter synthesis. Furthermore, as aforementioned, astrocytes play key roles in adult angiogenesis and neurogenesis after stroke (Becerra-Calixto and Cardona-Gomez 2017).
The astrocytic processes form a physical barrier to limit diffusion of the neurotransmitter away from the synapse via enveloping the pre- and post-synaptic terminals, termed as tripartite synapse. To maintain the concentration of extracellular neurotransmitters, astrocytes rapidly remove the K+ accumulation induced by neuronal activity, and convert the glutamate to glutamine and uptake it back into presynaptic terminals released during neurotransmission. More than 90 % of glutamate uptake dues to astrocyte glutamate transporters, GLT-1 and GLAST in most brain regions. Besides, astrocytes play the major role with neurons in synaptic transmission via regulating release of synaptically active molecules including glutamate, D-serine, GABA etc. Glutamate released by astrocytes activates NMDA receptors in pre-synaptic terminals and promotes communication between neurons. Release and reuptake of neurotransmitters is essential for neuronal plasticity and brain function, and loss of proper communication between astrocytes and neurons aggravates excitotoxicity after brain injury (Xing et al. 2012).
Astrocytes not only play key role in passive homeostatic control of adequate conditions for synaptic function, but also actively regulate synaptic transmission and neuronal excitability in synaptic function, and promote the brain functional output via the coordinated activity of complex neuronal networks, containing neurons and glia (Farhy-Tselnicker and Allen 2018). Hence, astrocytes are emerging as key regulatory elements involving in the structural plasticity of synaptic connections within the nervous system during both initial establishment and ongoing remodeling, and have a great ability to mediate synaptic strength in response to changes in neuronal activity (Stellwagen and Malenka 2006).
Dendritic spines are quite dynamic, and have a powerful ability to respond to many events with rapid alteration as long-term potentiation (LTP) and long-term depression (LTD), which are synaptic plasticity and associated with increases and decreases in spine size, respectively. It has been reported that astrocytes could extend and retract rapidly to engage and disengage from motile postsynaptic dendritic spines consistent with changes in spines (Haber et al. 2006), suggesting the active role of astrocytes in regulating synaptic plasticity.
Furthermore, neurotrophic factors such as BDNF play vital roles in LTP as well as in learning and memory via enhancing the synaptic plasticity (Akaneya et al. 1997). BDNF is secreted in its precursor form (pro-BDNF) and cleaved to form its mature form by proteolytic cleavage, then cleared from the extracellular space via rapid uptake by nearby astrocytes (Bergami et al. 2008), indicating that astrocytes play the important role in the neuronal clearance of neurotrophic factors and subsequent recycling of the endocytic neurotrophin, and modulating both its spatial and temporal availability. Hereby, Recycling of neurotrophin by astrocytes may thus contribute to the mediation of synaptic plasticity by glia.
The ischemic stroke is known to result in generation of glial scar, which is a general tissue response after central nervous system (CNS) injury. Glial scar is composed of highly migrating and proliferating glial cells, many of them are reactive astrocytes (Burda et al. 2016). The formation of glial scar decreases the spread of inflammatory responses from the damaged area to other parts. Along with the formation of glial scar, expression of extracellular matrix (ECM) molecules such as chondroitin sulfate proteoglycans (CSPGs), glycosaminoglycan proteoglycans (GAG), dermatan sulfate proteoglycans (DSPGs), heparin sulfate proteoglycans (HSPGs), and keratin sulfate proteoglycans (KSPGs) also upregulate in injured brain (Soleman et al. 2013). Glial scar consisted of these proteoglycans forms a thick and protective sheet around the damaged site so as to rapidly seal the disrupted blood-brain barrier.
Furthermore, the proteoglycan derived from astrocytes regulates the growth and direction of newly generated neurons and plays a vital role throughout the development of the brain. CSPGs have been found to be upregulated in brain of embryonic rat and given repulsive guidance to the growth of axons. The CSPGs expression reduces gradually throughout the brain development phase and increases rapidly only after major brain injury. Altogether, these findings indicate that proper balance of CSPGs and other proteoglycans is vital to neuronal development (Didangelos et al. 2014).
In a few words, astrocytes provide neuroprotective potential by releasing neurotrophins and limiting lesion extension via anti-excitotoxicity effects after ischemic stroke.
Astrocytes‐mediated the exacerbation of cerebral ischemic injury
In the cerebral ischemic tissues, cytokines produced by glial cells in the core and by injured neurons in the penumbra and core of the lesion increase within minutes after injury, and then, cytokines trigger astrocyte activation, which is known as reactive astrogliosis (Sofroniew 2009). As all we know, the exhibition of reactive astrogliosis mainly contains cellular hypertrophy and hyperplasia, enhanced expression of the intermediate filament proteins such as CSPGs, vimentin, glial fibrillary acidic protein (GFAP) and nestin. In addition, reactive astrogliosis is considered to alter the expression of many other molecules, their functions are involved in cell structure, energy metabolism, gene transcription, membrane transporters and intracellular signaling, etc. (Osborn et al. 2016).
Activated astrocytes in the area around the ischemic infarction exhibit elongated processes (Kajihara et al. 2001). Within a few days after the ischemia, glial scar mainly generated by reactive astrocytes is forming around the necrotic brain tissues of the infarct (Bidmon et al. 1998; Silver and Miller 2004). Depending on the severity of injury, several forms of reactive astrogliosis exist permanent, while in minor cases, reactive astrogliosis can resolve over time. Reactive astrocytes in the scar express a large number of inhibitory molecules against axonal regeneration, such as CSPGs, which is major barrier to axon extension and thus lead regeneration failure in the CNS of mammals. Accumulating studies have found that CSPGs could inhibit neurite outgrowth of various neuronal cell types (Bradbury et al. 2002), these findings have been reinforced by the study of Bradbury and colleagues that chondroitinase treatment supports neuron regeneration (Mukherjee et al. 2020).
CSPGs, the key inhibitory to block axonal regeneration following injury, is secreted by reactive astrocytes instantaneously after injury in the adult mammalian CNS and lasts for a long time (Tang et al. 2003). A transmembrane protein tyrosine phosphatase (PTPσ), Nogo receptor (NgR) and Leukocyte common antigen-related phosphatase (LAR) mediate CSPGs-induced inhibition of axon growth. CSPGs interact with the PTPσ, NgR or PTPσ, and then activate the RhoA/ROCK pathway and block the neurite growth (Mukherjee et al. 2020). Inactivation of the RhoA/ROCK pathway by using specific inhibitor C3 transferase or ROCK inhibitor blocks the inhibitory effects of CSPGs on neurite outgrowth (Monnier et al. 2003). These findings confirmed that RhoA/ROCK pathway mediates the inhibition of CSPGs secreted by reactive astrocytes on neurite outgrowth.
In conclusion, astrocytic inflammatory response to stroke may exacerbate the ischemic injury by up-regulation of CSPGs that activates the RhoA/ROCK pathway.
Conclusions
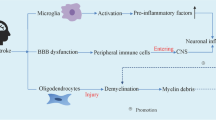
The increased CSPGs is expressed rapidly by reactive astrocytes after injury, and is highest at the center of the necrotic area and reduces gradually into the penumbra. Although the glial scar can undoubtedly limit axon regeneration by inhibiting axonal sprouting in the adult mammal, the glial scar also plays an important role in isolating the injury site and inhibiting its extension, then protecting cells against harmful factors released from the necrotic area. Thereby, the functional role of glial scar in stroke remain controversial, treatment of cerebral injury becomes complicated for these two contrasting factors (Fig. 1).
RhoA/ROCK pathway affects numerous cellular processes such as cell motility and contraction in brain, many of them in endothelial cells, neurons, glia, vascular smooth muscle etc., which makes RhoA/ROCK pathway a unique multifaceted objective of ischemic research. In addition, ROCK signaling is important in astrocyte secreted shh in neurogenesis and angiogenesis after cerebral ischemia. However, the mechanism of astrocytes changes mediated by RhoA/ROCK signaling pathway need further study.
Data availability
Not applicable.
References
Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H (1997) Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci 17:6707–6716
Alvarez-Buylla A, Ihrie RA (2014) Sonic hedgehog signaling in the postnatal brain. Semin Cell Dev Biol 33:105–111. https://doi.org/10.1016/j.semcdb.2014.05.008
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970. https://doi.org/10.1038/nm747
Becerra-Calixto A, Cardona-Gomez GP (2017) The Role of Astrocytes in Neuroprotection after Brain Stroke: Potential in Cell Therapy. Front Mol Neurosci 10:88. https://doi.org/10.3389/fnmol.2017.00088
Ben Haim L, Rowitch DH (2017) Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18:31–41. https://doi.org/10.1038/nrn.2016.159
Bergami M, Santi S, Formaggio E, Cagnoli C, Verderio C, Blum R, Berninger B, Matteoli M, Canossa M (2008) Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol 183:213–221. https://doi.org/10.1083/jcb.200806137
Bidmon HJ, Jancsik V, Schleicher A, Hagemann G, Witte OW, Woodhams P, Zilles K (1998) Structural alterations and changes in cytoskeletal proteins and proteoglycans after focal cortical ischemia. Neuroscience 82:397–420. https://doi.org/10.1016/s0306-4522(97)00289-3
Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640. https://doi.10.1038/416636a
Brea D, Sobrino T, Ramos-Cabrer P, Castillo J (2009) Reorganisation of the cerebral vasculature following ischaemia. Rev Neurol 49:645–654
Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK, D’Amore PA (2010) RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J 24:3186–3195. https://doi.org/10.1096/fj.09-145102
Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275(Pt 3):305–315. https://doi.org/10.1016/j.expneurol.2015.03.020
Chen X, Hou XM, Fan YF, Jin YT, Wang YL (2016) Sonic hedgehog protein regulates fibroblast growth factor 8 expression in metanephric explant culture from BALB/c mice: Possible mechanisms associated with renal morphogenesis. Mol Med Rep 14:2929–2936. https://doi.org/10.3892/mmr.2016.5614
Chinchilla P, Xiao L, Kazanietz MG, Riobo NA (2010) Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle 9:570–579. https://doi.org/10.4161/cc.9.3.10591
Christie KJ, Turbic A, Turnley AM (2013) Adult hippocampal neurogenesis, Rho kinase inhibition and enhancement of neuronal survival. Neuroscience 247:75–83. https://doi.org/10.1016/j.neuroscience.2013.05.019
Codega P, Silva-Vargas V, Paul A, Maldonado-Soto AR, Deleo AM, Pastrana E, Doetsch F (2014) Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 82:545–559. https://doi.org/10.1016/j.neuron.2014.02.039
Croft DR, Sahai E, Mavria G, Li S, Tsai J, Lee WM, Marshall CJ, Olson MF (2004) Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res 64:8994–9001. https://doi.org/10.1158/0008-5472.CAN-04-2052
Dai RL, Zhu SY, Xia YP, Mao L, Mei YW, Yao YF, Xue YM, Hu B (2011) Sonic hedgehog protects cortical neurons against oxidative stress. Neurochem Res 36:67–75. https://doi.org/10.1007/s11064-010-0264-6
Dashti M, Peppelenbosch MP, Rezaee F (2012) Hedgehog signalling as an antagonist of ageing and its associated diseases. BioEssays 34:849–856. https://doi.org/10.1002/bies.201200049
Didangelos A, Iberl M, Vinsland E, Bartus K, Bradbury EJ (2014) Regulation of IL-10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. J Neurosci 34:16424–16432. https://doi.org/10.1523/JNEUROSCI.2927-14.2014
Ding J, Yu JZ, Li QY, Wang X, Lu CZ, Xiao BG (2009) Rho kinase inhibitor Fasudil induces neuroprotection and neurogenesis partially through astrocyte-derived G-CSF. Brain Behav Immun 23:1083–1088. https://doi.org/10.1016/j.bbi.2009.05.002
Du K, Zhao C, Wang L, Wang Y, Zhang KZ, Shen XY, Sun HX, Gao W, Lu X (2019) MiR-191 inhibit angiogenesis after acute ischemic stroke targeting VEZF1. Aging 11:2762–2786. https://doi.10.18632/aging.101948
Farhy-Tselnicker I, Allen NJ (2018) Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev 13:7. https://doi.org/10.1186/s13064-018-0104-y
Feng GD, He BR, Lu F, Liu LH, Zhang L, Chen B, He ZP, Hao DJ, Yang H (2014) Fibroblast growth factor 4 is required but not sufficient for the astrocyte dedifferentiation. Mol Neurobiol 50:997–1012. https://doi.org/10.1007/s12035-014-8649-1
Gao L, Guan W, Wang M, Wang H, Yu J, Liu Q, Qiu B, Yu Y, Ping Y, Bian X, Shen L, Pei G (2017) Direct Generation of Human Neuronal Cells from Adult Astrocytes by Small Molecules. Stem Cell Rep 8:538–547. https://doi.org/10.1016/j.stemcr.2017.01.014
Haber M, Zhou L, Murai KK (2006) Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci 26:8881–8891. https://doi.org/10.1523/JNEUROSCI.1302-06.2006
Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A (2008) Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci 11:277–284. https://doi.org/10.1038/nn2059
He QW, Xia YP, Chen SC, Wang Y, Huang M, Huang Y, Li JY, Li YN, Gao Y, Mao L, Mei YW, Hu B (2013) Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Mol Neurobiol 47:976–987. https://doi.org/10.1007/s12035-013-8396-8
He H, Huang M, Sun S, Wu Y, Lin X (2017) Epithelial heparan sulfate regulates Sonic Hedgehog signaling in lung development. PLoS Genet 13:e1006992. https://doi.org/10.1371/journal.pgen.1006992
Hirota S, Liu Q, Lee HS, Hossain MG, Lacy-Hulbert A, McCarty JH (2011) The astrocyte-expressed integrin alphavbeta8 governs blood vessel sprouting in the developing retina. Development 138:5157–5166. https://doi.org/10.1242/dev.069153
Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA (2003) Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci 24:171–189. https://doi.org/10.1016/s1044-7431(03)00159-3
Kajihara H, Tsutsumi E, Kinoshita A, Nakano J, Takagi K, Takeo S (2001) Activated astrocytes with glycogen accumulation in ischemic penumbra during the early stage of brain infarction: immunohistochemical and electron microscopic studies. Brain Res 909:92–101. https://doi.org/10.1016/s0006-8993(01)02640-3
Kasai K, Takahashi M, Osumi N, Sinnarajah S, Takeo T, Ikeda H, Kehrl JH, Itoh G, Arnheiter H (2004) The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells 9:49–58. https://doi.org/10.1111/j.1356-9597.2004.00701.x
Kawagishi H, Xiong J, Rovira II, Pan H, Yan Y, Fleischmann BK, Yamada M, Finkel T (2018) Sonic hedgehog signaling regulates the mammalian cardiac regenerative response. J Mol Cell Cardiol 123:180–184. https://doi.org/10.1016/j.yjmcc.2018.09.005
Koyanagi M, Takahashi J, Arakawa Y, Doi D, Fukuda H, Hayashi H, Narumiya S, Hashimoto N (2008) Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res 86:270–280. https://doi.org/10.1002/jnr.21502
Lai K, Kaspar BK, Gage FH, Schaffer DV (2003) Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci 6:21–27. https://doi.org/10.1038/nn983
Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C (2000) Treatment-induced cortical reorganization after stroke in humans. Stroke 31:1210–1216. https://doi.org/10.1161/01.str.31.6.1210
Liu Z, Chopp M (2016) Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol 144:103–120. https://doi.org/10.1016/j.pneurobio.2015.09.008
Liu J, Wada Y, Katsura M, Tozawa H, Erwin N, Kapron CM, Bao G, Liu J (2018) Rho-associated coiled-coil kinase (ROCK) in molecular regulation of angiogenesis. Theranostics 8:6053–6069. https://doi.org/10.7150/thno.30305
Maldonado H, Calderon C, Burgos-Bravo F, Kobler O, Zuschratter W, Ramirez O, Hartel S, Schneider P, Quest AF, Herrera-Molina R, Leyton L (2017) Astrocyte-to-neuron communication through integrin-engaged Thy-1/CBP/Csk/Src complex triggers neurite retraction via the RhoA/ROCK pathway. Biochim Biophys Acta Mol Cell Res 1864:243–254. https://doi.org/10.1016/j.bbamcr.2016.11.006
Malone K, Amu S, Moore AC, Waeber C (2019) The immune system and stroke: from current targets to future therapy. Immunol Cell Biol 97:5–16. https://doi.org/10.1111/imcb.12191
Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK (2003) The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 22:319–330. https://doi.org/10.1016/s1044-7431(02)00035-0
Mukherjee N, Nandi S, Garg S, Ghosh S, Ghosh S, Samat R, Ghosh S (2020) Targeting chondroitin sulfate proteoglycans: an emerging therapeutic strategy to treat CNS injury. ACS Chem Neurosci 11:231–232. https://doi.org/10.1021/acschemneuro.0c00004
Murakoshi H, Wang H, Yasuda R (2011) Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472:100–104. https://doi.org/10.1038/nature09823
Nakabayashi H, Shimizu K (2011) HA1077, a Rho kinase inhibitor, suppresses glioma-induced angiogenesis by targeting the Rho-ROCK and the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signal pathways. Cancer Sci 102:393–399. https://doi.org/10.1111/j.1349-7006.2010.01794.x
Niu W, Zang T, Smith DK, Vue TY, Zou Y, Bachoo R, Johnson JE, Zhang CL (2015) SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep 4:780–794. https://doi.org/10.1016/j.stemcr.2015.03.006
Nusslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801. https://doi.org/10.1038/287795a0
Osborn LM, Kamphuis W, Wadman WJ, Hol EM (2016) Astrogliosis: An integral player in the pathogenesis of Alzheimer’s disease. Prog Neurobiol 144:121–141. https://doi.org/10.1016/j.pneurobio.2016.01.001
Peng T, Frank DB, Kadzik RS, Morley MP, Rathi KS, Wang T, Zhou S, Cheng L, Lu MM, Morrisey EE (2015) Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526:578–582. https://doi.org/10.1038/nature14984
Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM (2001) The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 7:706–711. https://doi.org/10.1038/89083
Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA (2011) Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem 286:19589–19596. https://doi.org/10.1074/jbc.M110.197111
Rattner A, Williams J, Nathans J (2019) Roles of HIFs and VEGF in angiogenesis in the retina and brain. J Clin Invest 129:3807–3820. https://doi.org/10.1172/JCI126655
Renault MA, Roncalli J, Tongers J, Thorne T, Klyachko E, Misener S, Volpert OV, Mehta S, Burg A, Luedemann C, Qin G, Kishore R, Losordo DW (2010) Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol 49:490–498. https://doi.org/10.1016/j.yjmcc.2010.05.003
Rossini PM, Calautti C, Pauri F, Baron JC (2003) Post-stroke plastic reorganisation in the adult brain. Lancet Neurol 2:493–502. https://doi.org/10.1016/s1474-4422(03)00485-x
Sa-Pereira I, Brites D, Brito MA (2012) Neurovascular unit: a focus on pericytes. Mol Neurobiol 45:327–347. https://doi.org/10.1007/s12035-012-8244-2
Scemes E, Suadicani SO, Spray DC (2000) Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. The Journal of neuroscience: the official journal of the Society for Neuroscience 20:1435–1445
Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, Johnson RS, Ferrara N, Fruttiger M (2010) Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PloS One 5:e11863. https://doi.org/10.1371/journal.pone.0011863
Shibuya M (2009) Brain angiogenesis in developmental and pathological processes: therapeutic aspects of vascular endothelial growth factor. FEBS J 276:4636–4643. https://doi.org/10.1111/j.1742-4658.2009.07175.x
Shin YJ, Kim HL, Park JM, Cho JM, Kim SY, Lee MY (2013) Characterization of nestin expression and vessel association in the ischemic core following focal cerebral ischemia in rats. Cell Tissue Res 351:383–395. https://doi.org/10.1007/s00441-012-1538-x
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146–156. https://doi.org/10.1038/nrn1326
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647. https://doi.org/10.1016/j.tins.2009.08.002
Soleman S, Filippov MA, Dityatev A, Fawcett JW (2013) Targeting the neural extracellular matrix in neurological disorders. Neuroscience 253:194–213. https://doi.org/10.1016/j.neuroscience.2013.08.050
Spadafora R, Gonzalez FF, Derugin N, Wendland M, Ferriero D, McQuillen P (2010) Altered fate of subventricular zone progenitor cells and reduced neurogenesis following neonatal stroke. Dev Neurosci 32:101–113. https://doi.org/10.1159/000279654
Stellwagen D, Malenka RC (2006) Synaptic scaling mediated by glial TNF-alpha. Nature 440:1054–1059. https://doi.org/10.1038/nature04671
Stenzel D, Lundkvist A, Sauvaget D, Busse M, Graupera M, van der Flier A, Wijelath ES, Murray J, Sobel M, Costell M, Takahashi S, Fassler R, Yamaguchi Y, Gutmann DH, Hynes RO, Gerhardt H (2011) Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development 138:4451–4463. https://doi.org/10.1242/dev.071381
Tang X, Davies JE, Davies SJ (2003) Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res 71:427–444. https://doi.org/10.1002/jnr.10523
Thier MC, Hommerding O, Panten J, Pinna R, Garcia-Gonzalez D, Berger T, Worsdorfer P, Assenov Y, Scognamiglio R, Przybylla A, Kaschutnig P, Becker L, Milsom MD, Jauch A, Utikal J, Herrmann C, Monyer H, Edenhofer F, Trumpp A (2019) Identification of Embryonic Neural Plate Border Stem Cells and Their Generation by Direct Reprogramming from Adult Human Blood Cells. Cell Stem Cell 24:166–182 e113. https://doi.org/10.1016/j.stem.2018.11.015
Tonges L, Koch JC, Bahr M, Lingor P (2011) ROCKing regeneration: rho kinase inhibition as molecular target for neurorestoration. Front Mol Neurosci 4:39. https://doi.org/10.3389/fnmol.2011.00039
van der Meel R, Symons MH, Kudernatsch R, Kok RJ, Schiffelers RM, Storm G, Gallagher WM, Byrne AT (2011) The VEGF/Rho GTPase signalling pathway: a promising target for anti-angiogenic/anti-invasion therapy. Drug Discov Today 16:219–228. https://doi.org/10.1016/j.drudis.2011.01.005
Wang C, Fong H, Huang Y (2015) Direct reprogramming of RESTing astrocytes. Cell Stem Cell 17:1–3. https://doi.org/10.1016/j.stem.2015.06.011
Washida N, Wakino S, Tonozuka Y, Homma K, Tokuyama H, Hara Y, Hasegawa K, Minakuchi H, Fujimura K, Hosoya K, Hayashi K, Itoh H (2011) Rho-kinase inhibition ameliorates peritoneal fibrosis and angiogenesis in a rat model of peritoneal sclerosis. Nephrol Dial Transplant 26:2770–2779. https://doi.org/10.1093/ndt/gfr012
Wen JY, Gao SS, Chen FL, Chen S, Wang M, Chen ZW (2019) Role of CSE-Produced H2S on cerebrovascular relaxation via RhoA-ROCK inhibition and cerebral ischemia-reperfusion injury in mice. ACS Chem Neurosci 10:1565–1574. https://doi.org/10.1021/acschemneuro.8b00533
Xia YP, Dai RL, Li YN, Mao L, Xue YM, He QW, Huang M, Huang Y, Mei YW, Hu B (2012) The protective effect of sonic hedgehog is mediated by the phosphoinositide [corrected] 3-kinase/AKT/Bcl-2 pathway in cultured rat astrocytes under oxidative stress. Neuroscience 209:1–11. https://doi.org/10.1016/j.neuroscience.2012.02.019
Xing C, Hayakawa K, Lok J, Arai K, Lo EH (2012) Injury and repair in the neurovascular unit. Neurol Res 34:325–330. https://doi.org/10.1179/1743132812Y.0000000019
Yang H, Liu C, Fan H, Chen B, Huang D, Zhang L, Zhang Q, An J, Zhao J, Wang Y, Hao D (2019) Sonic hedgehog effectively improves oct4-mediated reprogramming of astrocytes into neural stem cells. Mol Ther 27:1467–1482. https://doi.org/10.1016/j.ymthe.2019.05.006
Yao PJ, Petralia RS, Mattson MP (2016) Sonic hedgehog signaling and hippocampal neuroplasticity. Trends Neurosci 39:840–850. https://doi.org/10.1016/j.tins.2016.10.001
Zhang Y, Zhang X, Cui L, Chen R, Zhang C, Li Y, He T, Zhu X, Shen Z, Dong L, Zhao J, Wen Y, Zheng X, Li P (2017) Salvianolic Acids for Injection (SAFI) promotes functional recovery and neurogenesis via sonic hedgehog pathway after stroke in mice. Neurochem Int 110:38–48. https://doi.org/10.1016/j.neuint.2017.09.001
Funding
This study was funded by grants for teaching and research project of Hefei Technology College (No. 2019JYXM022).
Author information
Authors and Affiliations
Contributions
Lu WZ and Wen JY: Participated in writing of the manuscript; Chen ZW: Participated in review design.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, W., Chen, Z. & Wen, J. RhoA/ROCK signaling pathway and astrocytes in ischemic stroke. Metab Brain Dis 36, 1101–1108 (2021). https://doi.org/10.1007/s11011-021-00709-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00709-4