Abstract
Memantine is a N-methyl-D-aspartate (NMDA) receptor antagonist and several studies have pointed to the NMDA receptor antagonists as a potential therapeutic target for the treatment of depression. The present study was aimed to evaluate the behavioral and physiological effects of administration of memantine in rats exposed to the chronic mild stress (CMS) model. To this aim, after 40 days of exposure to CMS procedure, rats were treated with memantine (20 mg/kg) for 7 days. In this study, sweet food consumption, adrenal gland weight, corticosterone levels, and brain-derived-neurotrophic factor (BDNF) protein levels in the prefrontal cortex, hippocampus and amygdala were assessed. Our results demonstrated that chronic stressful situations induced anhedonia, hypertrophy of adrenal gland weight, and an increase of corticosterone levels in rats, but did not alter BDNF protein levels in the rat brain. Memantine treatment reversed anhedonia and the increase of adrenal gland weight, normalized corticosterone levels and increased BDNF protein levels in the prefrontal cortex in stressed rats. Finally, these findings further support the hypothesis that NMDA receptor antagonists such as memantine could be helpful in the pharmacological treatment of depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a mood disorder, which affects up to 20% of the world population (Nestler et al. 2002; Manji et al. 2001). For more than 50 years, drugs that increase the synaptic availability of biogenic amines have been used to treat depression, but these antidepressant drugs require 2–4 weeks (or more) to produce a clinical improvement in depressive symptomatology (Skolnick et al. 2009). In addition, patients have a low (about 30%) remission rate (Krishnan and Nestler 2008). For these reasons new research has been conducted for more effective agents (Feier et al. 2011; Magalhães et al. 2011).
There is growing evidence that an increase of glutamatergic neurotransmission is involved with the pathophysiology of depression. In addition, studies have been suggested that NMDA receptor antagonists play an important role in the treatment of depression (Hashimoto 2009, 2011; Skolnick et al. 2009). In fact, patients with depression presented a significant increase of serum levels of glutamate, compared with healthy controls (Kim et al. 1982; Mitani et al. 2006), and several preclinical and clinical studies have demonstrated that NMDA antagonists, such as ketamine, memantine, amantadine and others, display antidepressant effects (Berman et al. 2000; Ferguson and Shingleton 2007; Garcia et al. 2008a, b, 2009; Moryl et al. 1993; Parsons et al. 1999; Quan et al. 2011; Réus et al. 2010; Roman et al. 2009; Zarate et al. 2006). The memantine is clinically used for neurological disorders, including Alzheimer’s disease. In addition to the NMDA receptor, other systems are involved in the action of memantine, for example, serotonin and dopamine uptake, nicotinic acetylcholine, serotonin and sigma-1 receptors (for a review see Johnson and Kotermanski 2006).
Given the fact of the latency time being 3–4 weeks for antidepressants to exert their initial pharmacological effects, some studies have suggested that downstream neural adaptation (for example: the BDNF-TrkB receptor signaling pathway) rather than an elevation in synaptic monoamine levels itself may be responsible for their therapeutic effects (Hashimoto et al. 2004; Hashimoto 2011; Nestler et al. 2002). BDNF is a member of the neurotrophin family (Lewin and Barde 1997). A series of in depth studies have pointed to an involvement of BDNF in the pathophysiology of depression. In fact, postmortem studies have demonstrated that depressed subjects have decreased BDNF levels (Altar 1999). In contrast, antidepressants drugs induce an increase in BDNF levels in humans and animals (Pittenger and Duman 2008). Moreover, studies from many laboratories have showed that NMDA receptor antagonists induce increases in BDNF levels (Garcia et al. 2008a; Marvanová et al. 2001; Réus et al. 2010; Rogóz et al. 2008).
Animal models of depression are very important for our understanding of depression and antidepressant drug action, as well as for the development of new antidepressant treatments (Bylund and Reed 2009). The CMS is a widely used model of depression and it induces a lower consumption of sucrose (sweet food), changes in hypothalamic-pituitary-adrenal (HPA) axis and adrenal hypertrophy, which leads to corticosterone hypersecretion (Fortunato et al. 2010; Katz et al. 1981; Lucca et al. 2008, 2009a, b; Vollmayr and Henn 2003).
Studies evaluating the effects of memantine in rats submitted to a stress protocol, and linking the effects of memantine on behaviour and parameters linked to depression, such as glucocorticoids and BDNF have not been fully characterized. So, the present study aimed to investigate behavioral and physiological effects of repeated administration of memantine in rats submitted to the chronic mild stress procedure.
Experimental procedures
Animals
Male Adult Wistar rats (60 days old) were obtained from the UNESC (Universidade do Extremo Sul Catarinense, Criciúma, SC, Brazil) breeding colony. They were housed five per cage with food and water available ad libitum (except for the stressed group during the period when the stressor applied required no food or water) and were maintained on a 12-h light/dark cycle (lights on at 7:00 a.m.). All experimental procedures involving animals were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care and with approval by local Ethics Committee under protocol number 059/2008.
Drugs and treatment
Memantine was obtained from APSEN Pharmaceutical Industry (São Paulo) Brazil, and a dose of 20 mg/kg was injected intraperitoneally once a day over 7 days (chronic treatment) after the CMS procedure. The protocol was performed in accordance with previous studies (Fortunato et al. 2010; Garcia et al. 2009) and the dose of 20 mg/kg of memantine was chosen because in a previous study from our group it showed a better effect on the forced swimming test (Réus et al. 2010). The treatments were administered in a volume of 1 ml/kg. To develop this study we employed 60 animals (n = 15 per group) separated into four groups, as follows: 1) non stressed—saline; 2) non stressed—memantine; 3) stressed—saline; 4) stressed—memantine. The treatment with memantine was performed during the anhedonia test, once a day over 7 days, 60 min prior to the behavioral assessment.
Experimental procedure
The chronic mild stress (CMS) protocol was adapted from the procedure described by Gamaro et al. (2003). The animals were divided in two groups: control and stressed. The Control groups were kept undisturbed in their home cages during the 40 days of treatment receiving only ordinary daily care with daily supports of food and water. The 40-days chronic mild stress paradigm was used for the animals in the stressed group. Individual stressors and length of time applied each day as follows. The following stressors were used: (i) 24 h food deprivation was applied on days 2, 7, 15, 21 and 30; (ii) 24 h water deprivation on days 1, 10, 20 and 33; (iii) 1–3 h restraint on days 9, 23 and 31, (iv) 1.5–2 h restraint at 4°C on days 13, 26 and 34; (v) forced swimming for a duration of 10 or 15 min on days 8, 16, 27, 35 and 40; (vi) flashing light over a duration of 120–210 min on days 6, 14, 22, 28, 32 and 39; (vii) isolation on days 3, 4, 5, 17, 18, 19, 24, 25, 36 and 37. Stressor stimuli were applied at different times every day, in order to minimize its predictability. The Restraint test was carried out by placing the animal in a 25 × 7 cm plastic tube and adjusting it with plaster tape on the outside, so that the animal was unable to move. There was a 1 cm hole at the far end for breathing. The Forced swimming was carried out by placing the animal in a glass tank measuring 50 × 47 cm with 30 cm of water at 23 ± 2°C. The exposure to flashing light test was made by placing the animal in a 60 × 60 × 25 cm plywood made box divided in 16 cells of 15 × 15 × 25 cm with a frontal glass wall. A 40 w lamp, flashing at frequency of 60 flashes/min, was used.
Open-field test
The rats were treated with memantine (20 mg/kg), and saline 60 min before the exposure to the open-field apparatus, in order to assess possible effects of drug treatment on spontaneous locomotor activity. Analysis of the rats spontaneous activity was carried out in an open field apparatus, which is an arena 45 × 60 cm surrounded by 50 cm high walls made of brown plywood with a frontal glass wall. The floor of the open field was divided into 9 rectangles (15 × 20 cm each) by black lines (Frey et al. 2006). Animals were gently placed on the left rear quadrant, and left to explore the arena for 5 min. The number of horizontal (crossings) and vertical (rearings) activities performed by each rat during the 3 min observation period were counted by an expert observer.
Sweet food consumption (anhedonia test)
After 40 days of treatment, the consumption of sweet food was measured to verify anhedonia. The animals were placed in a lit rectangular box (40 × 15 × 20 cm) with a glass ceiling, floor and side walls made of wood and divided into 9 equal rectangles by black lines. Ten Froot Loops (Kellogg’s® pellets of wheat and sucrose) were placed in one extremity of the box. Animals were submitted to 5 sessions of 3 min each, once a day, in order to become familiarized with this food. After being habituated, the animals were exposed to two test sessions, 3 min each, where the number of ingested pellets were counted (Garcia et al. 2009; Lucca et al. 2009a, b). This task was undertaken during the light cycle and the evaluation was realized by an observer with no knowledge of the groups. Results were noted when the animal ate 1, 1/2, or 1/4 part of the Froot Loops, in accordance with previous studies (Gamaro et al. 2003; Katz et al. 1981). These evaluations were made since food deprivation, which is used in many behavior tasks as a motivating stimulus, may also be an acute stressor (Gamaro et al. 2003; Katz et al. 1981). At the seventh day after consumption of the sweet food, the rats were placed under anesthesia and blood was collected by cardiac puncture for subsequent analyses of corticosterone serum levels. After the decapitation of rats, the skulls were immediately removed and the prefrontal cortex, hippocampus and amygdala were quickly isolated by hand dissection using a magnifying glass and a thin brush (dissection was based on histological distinctions) described by Paxinos and Watson (1986) and it was stored at −70°C for analyses of BDNF protein levels. The adrenal gland was removed through laparotomy, and weighed in an analytical balance. Adrenal gland weight was used in this study as an indirect parameter of hypothalamic-pituitary-adrenal axis activation (Katz et al. 1981).
Corticosterone circulating levels
The rats were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) administrated i.p. and after blood was collected to evaluate corticosterone serum levels. Corticosterone levels were determined using enzyme immunoassay kits. The analyses were performed in a commercial laboratory (Biolabor Laboratório de Análises Clínicas, Criciúma, SC, Brazil), blinded to the experimental conditions.
BDNF analysis
BDNF levels in prefrontal cortex, hippocampus and amygdala were measured by anti-BDNF sandwich-ELISA, according to the manufacturers instructions (Chemicon, USA). Briefly, the rat prefrontal cortex, hippocampus and amygdala were homogenized in phosphate buffer solution (PBS) with 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM (EGTA). Microtiter plates (96-well flat-bottom) were coated for 24 h with the samples diluted 1:2 in sample diluent and standard curve ranged from 7.8 to 500 pg/ml of BDNF. The plates were then washed four times with sample diluent and a monoclonal anti-BDNF rabbit antibody diluted 1:1000 in sample diluent was added to each well and incubated for 3 h at room temperature. After washing, a peroxidase conjugated anti-rabbit antibody (diluted 1:1000) was added to each well and incubated at room temperature for 1 h. After addition of streptavidin-enzyme, substrate and stop solution, the amount of BDNF was determined by absorbance in 450 nm. The standard curve demonstrates a direct relationship between Optical Density (OD) and BDNF concentration. Total protein was measured by Lowry’s method using bovine serum albumin as a standard, as previously described by Lowry et al. (1951).
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) 16.0 was utilized for statistical analyses. All data is presented as mean ± S.E.M. Differences among experimental groups were determined by one-way ANOVA followed by Tukey’s post hoc test for sweet food intake, locomotory activity, adrenal gland weight, ACTH circulating levels and BDNF protein levels. P < 0.05 were considered statistically significant.
Results
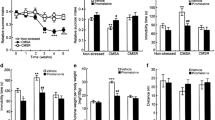
As depicted in Fig. 1a, stressed rats treated with saline decreased sweet food intake, compared with control rats treated with saline (p = 0.03). Statistical analyses revealed that treatment with memantine increased sweet food intake, when compared with stressed rats treated with saline. The Fig. 1b shows that the behavior of the rats subjected to the open-field test, after 40 days of chronic stressful stimuli, and that this did not alter the number of crossings (p = 0.21) and rearings (p = 0.13) displayed by all groups.
The effects of the CMS protocol in the adrenal gland this illustrated in Fig. 2. CMS procedure induced an increase of adrenal gland weight in stressed rats treated with saline, compared with non-stressed rats injected with saline. Interestingly, the treatment with memantine re-established to a normal range the adrenal glands weight in stressed rats (p = 0.02).
Corticosterone levels this illustrated in Fig. 3. Rats subjected to the CMS paradigm, and treated with saline, displayed increased corticosterone levels compared with non-stressed rats injected with saline. However, the CMS-induced increases in circulating corticosterone levels were reversed by memantine (p = 0.02).
Figure 4 shows the BDNF protein levels in the prefrontal cortex, hippocampus and amygdala. Stressed rats injected with saline did not alter BDNF protein levels in the prefrontal cortex, hippocampus and amygdala, but interestingly, the treatment with memantine in stressed animals increased BDNF protein levels in the prefrontal cortex (p = 0.01).
Discussion
In the present study we demonstrated that: (1) CMS rats displayed reduced sweet food intake, without significant changes in locomotor activity; (2) CMS rats displayed hypertrophy of adrenal gland, compared to non-stressed rats; (3) CMS rats displayed increase of corticosterone levels, compared to non-stressed rats; (4) administration of memantine reversed the anhedonic behavior induced by chronic stressful stimuli, and reversed the increase of adrenal gland weight and corticosterone levels; (5) memantine increased BDNF protein levels in the prefrontal cortex in CMS rats.
Memantine is used for treatment of moderate to severe Alzheimer’s disease (Lipton 2004; Réus et al. 2008), it is a low-to-moderate-affinity non-competitive NMDA receptor (Rammes et al. 2008). Several recent studies have shown the role of memantine in depression. Our group recently demonstrated that acute and chronic treatments with memantine (5, 10 and 20 mg/kg) reduced immobility time in the rat forced swimming test, without affecting spontaneous locomotor activity (Réus et al. 2010). Other studies also showed that memantine enhances antidepressive-like effects in animals (Almeida et al. 2006; Rogóz et al. 2008). One open label study investigating the effect of memantine on depression demonstrated that the depressives symptoms improved within 1 week and reached and maintained maximal improvement from weeks 8 to 12 (Ferguson and Shingleton 2007). However, Zarate et al. (2006) in a randomized controlled trial it was concluded that memantine did not have antidepressant effects in patients with major depression.
Our results also showed that administration of memantine antagonized anhedonic behavior assessed in CMS, without affecting the consumption of sweet food in non-stressed rats. The present data is in agreement with literature studies, which show that rats exposed to the CMS procedure and treated with saline consumed less sweet food, compared to non-stressed rats treated with saline (Allaman et al. 2008; Garcia et al. 2009; Lucca et al. 2008, 2009a, b). In our data we have also shown an increase in adrenal gland weight in CMS rats, compared with non-stressed rats. Other groups also have shown an increase of rat adrenal weight after CMS paradigm (Garcia et al. 2009; Harro et al. 2001; Konarska et al. 1990; Lucca et al. 2008, 2009a, b). The hypertrophy of adrenal gland could increase corticosterone levels (O’Connor et al. 2000). In fact, we showed in this study an increase in the corticosterone levels in stressed rats. Moreover, an adaptive response to physical or psychological stress in the activation of the HPA axis, and consequently, a series of hormones are released, such as corticosterone. In addition, the high levels of glucocorticoids in the brain acting in turn to increase brain glutamate levels (Jacobs et al. 2000). In fact, glucocorticoids appear to exert their effects via a downstream action on NMDA receptors (Moghaddam et al. 1994). Additionally, in the present study memantine regulated hypertrophy of the adrenal gland and hormonal alteration, thereby; the effects of memantine in the present study may be related, at least in part, by its regulation of glucocorticoids via NMDA receptor. Indeed, glutamate antagonists can attenuate or block some of the effects of chronic glucocorticoids excess on morphology in the hippocampus (Magariños and McEwen 1995).
Stress has profound effects on brain areas associated with mood, such as the prefrontal cortex, hippocampus and amygdala (Pittenger and Duman 2008). In the prefrontal cortex and hippocampus, both are reduced in size and activity in major depression; in contrast, in the amygdala’s size and activity are increased (Drevets 2003). BDNF exerts its effects promoting the growth and development of immature neurons and enhancing the survival and function of adult neurons (Lindsay et al. 1994). The role of BDNF in depression has been extensively studied in recent years. Several forms of stress in preclinical studies, as well as postmortem human with depression reduce BDNF-mediated signaling in the hippocampus; on the other hand, chronic antidepressant treatment increases BDNF-mediated signaling (Karege et al. 2005; Nestler et al. 2002). However, the opposite effects have been shown. In fact, chronic stress increases BDNF levels in the nucleus accumbens (Berton et al. 2006). In the present study we demonstrated that the BDNF protein levels were not altered in the prefrontal cortex, hippocampus and amygdala in CMS rats, though our results have shown a tendency to decrease BDNF protein levels in the prefrontal cortex and hippocampus and to increase in the amygdala in CMS rats, suggesting that the effects of stress on BDNF levels may be related to the brain area. Allaman et al. (2008) demonstrated that exposure of the rats to the CMS paradigm does not alter BDNF mRNA expression in the hippocampus and in the basolateral amygdala, suggesting that BDNF mRNA levels were not modulated in these limbic brain structures during experimental conditions that cause anhedonia and chronic administration of the tricyclic antidepressant imipramine, which reverses CMS-induced anhedonia, does not regulate BDNF mRNA expression in the hippocampus and in the basolateral amygdala. Additionally, 7 days of treatment with antidepressant escitalopram activated intracellular pathways linked to BDNF and increased the levels of pro-BDNF in the rat prefrontal cortex (Alboni et al. 2010). In the present data we showed that 7 days of treatment with NMDA receptor antagonist memantine increased the BDNF protein levels in the prefrontal cortex from CMS rats. Fascinatingly, NMDA receptors and BDNF have important interactive actions as modulators of plasticity and survival. Furthermore, NMDA receptors play an important role in transcriptional regulation of BDNF formation (Yamada and Nabeshima 2004).
Some recent studies have demonstrated that NMDA receptor antagonists induced an increase in BDNF levels. Fluoxetine (10 mg/kg) in the hippocampus, and fluoxetine (5 and 10 mg/kg) and NMDA receptor antagonist, amantadine (10 mg/kg) in the cerebral cortex, significantly elevated the BDNF mRNA levels; in addition, joint administration of fluoxetine (5 or 10 mg/kg) and amantadine (10 mg/kg) induced a larger increase in BDNF gene expression in the cerebral cortex, but not in the hippocampus (Rogóz et al. 2009). Moreover studies from our group have shown that acute, but not chronic treatment with NMDA receptor antagonists, ketamine or memantine increase BDNF protein levels in the hippocampus (Garcia et al. 2009; Réus et al. 2010), suggesting adaptive mechanisms or the development of tolerance to the drugs effects on hippocampal levels. Additionally, Marvanová et al. (2001) showed that memantine increased BDNF mRNA levels in the limbic cortex in rat brain by in situ hybridization, suggesting new possibilities of pharmacologically regulating the expression of neurotrophic factors in the brain.
Other protective effects have been attributed to memantine. In vitro study showed that memantine in low concentrations (0.05–2 lM) could attenuate neuronal apoptosis induced by staurosporine, a protein kinases inhibitor and classical inducer of caspase 3-dependent apoptosis, in 7 day in vitro cortical neurons (Jantas-Skotniczna et al. 2006). In addition, Jantas et al. (2009) demonstrated that memantine can protect mouse primary cortical cultured neurons against staurosporine-evoked apoptosis in NMDAR-independent way by increasing phosphorylation of Akt and increasing BDNF protein level, providing a new insight into the anti-apoptotic action of memantine and its beneficial effects in chronic neurodegenerative disorders. Furthermore, memantine-injection promoted cellular proliferation in mice (Namba et al. 2010). It is important to note that memantine acts in other systems, involved with depression, such as 5-HT3 receptor and this effect might contribute to its therapeutic efficacy (Rammes et al. 2001). However, has been shown that antidepressants, such as desipramine and fluoxetine were found to be low-affinity NMDA antagonists (Szasz et al. 2007), suggesting that classic antidepressant also acts in NMDA receptors, may contribute to the clinical effects of antidepressants.
In conclusion, our data suggest that the anhedonic behavior observed in rats subjected to chronic unpredictable stressful situations were reversed by treatment with memantine. In addition, administration of memantine reversed hypertrophy of adrenal glands, and corticosterone levels, which may have been regulated by controlling levels of glutamate by memantine. In fact, an increase in brain glucocorticoids activity can increase brain glutamate levels (Jacobs et al. 2000). Moreover memantine treatment increased BDNF protein levels in the prefrontal cortex, an effect seen by traditional antidepressant. Finally, these findings further support the hypothesis that NMDA receptor antagonists such as memantine could be helpful in the pharmacological treatment of depression. In addition, future double-blind, placebo-controlled studies would be necessary to confirm these observations in patients with major depression and to evaluate whether memantine could be a new option for this impairment disorder.
References
Alboni SC, Benatti G, Capone D, Corsini F, Caggia F, Tascedda J, Mendlewicz N, Brunello (2010) Time-dependent effects of escitalopram on brain derived Neurotrophic factor (BDNF) and neuroplasticity related targets in the central nervous system of rats. Eur J Pharmacol 643:180–187
Allaman I, Papp M, Kraftsik R, Fiumelli H, Magistretti PJ, Martin JL (2008) Expression of brain-derived Neurotrophic factor is not modulated by chronic mild stress in the rat hippocampus and amygdale. Pharmacol Report 60:1001–1007
Almeida RC, Felisbino CS, López MG, Rodrigues ALS, Gabilan NH (2006) Evidence for the involvement of l-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effect of memantine in mice. Behav Brain Res 168:318–322
Altar CA (1999) Neurotrophins and depression. Trends Pharmaco Sci 20:59–61
Berman RB, Cappielo A, Anand A, Oren DA, George RH, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868
Bylund DB, Reed AL (2009) Childhood and adolescent depression: why do children and adults respond differently to antidepressant drugs? J Psychopharmacol 23:295–304
Drevets WC (2003) Neuroimaging abnormalities in the amygdala in mood disorders. Ann NY Acad Sci 985:420–444
Feier G, Valvassori SS, Rezin GT, Búrigo M, Streck EL, Kapczinski F, Quevedo J (2011) Creatine kinase levels in patients with bipolar disorder: depressive, manic, and euthymic phases. Rev Bras Psiquiatr 33:171–175
Ferguson JM, Shingleton RN (2007) An open-label, flexible-dose study of memantine in major depressive disorder. Clin Neuropharmacol 30:136–144
Fortunato JJ, Réus GZ, Kirsch TR, Stringari RB, Fries GR, Kapczinski F, Hallak JE, Zuardi AW, Crippa JA, Quevedo J (2010) Effects of beta-carboline harmine on behavioral and physiological parameters observed in the chronic mild stress model: further evidence of antidepressant properties. Brain Res Bull 81:491–496
Frey BN, Andreazza AC, Ceresér KMM, Martins MR, Valvassori SS, Réus GZ, Quevedo J, Kapczinski F (2006) Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci 79:281–286
Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C (2003) Effects stress on feeding behavior and on monoamine levels in structures. Neurochem Int 42:107–114
Garcia LB, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008a) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatr 32:140–144
Garcia LB, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008b) Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting Hippocampal brain-derived Neurotrophic factor protein levels. Basic Clin Pharmacol Toxicol 103:502–506
Garcia LSB, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J (2009) Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatr 30:450–455
Harro J, Tonissaar M, Eller M, Kask A, Oreland L (2001) Chronic variable stress and partial 5-HT denervation by parachloroamphetamine treatment in the rat: effects on behavior and monoamine neurochemistry. Brain Res 899:227–239
Hashimoto K (2009) Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev 61:105–123
Hashimoto K (2011) The role of glutamate on the action of antidepressants. Prog Psychopharmacol Biol Psychiatr 35:1558–1568
Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Shimizu E, Iyo M (2004) Protective effects of N-acetyl-L-cysteine on the reduction of dopamine transporters in the striatum of monkeys treated with methamphetamine. Neuropsychopharmacol 29:2018–2023
Jacobs BL, Praag H, Gage FH (2000) Molecular brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry 5:262–269
Jantas D, Szymanska EM, Budziszewska EB, Lason EW (2009) An involvement of BDNF and PI3-K/Akt in the anti-apoptotic effect of memantine on staurosporine-evoked cell death in primary cortical neurons. Apoptosis 14:900–912
Jantas-Skotniczna D, Kajta M, Lason W (2006) Memantine attenuates staurosporine-induced activation of caspase-3 and LDH release in mouse primary neuronal cultures. Brain Res 1069:145–153
Johnson JW, Kotermanski SE (2006) Mechanism of action of memantine. Curr Opin Pharmacol 6:61–67
Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R (2005) Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 136:29–37
Katz RJ, Roth KA, Carroll BJ (1981) Animal models and human depressive disorders. Neurosci Biobehav Rev 5:231–246
Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH (1982) Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr 23:299–304
Konarska M, Stewart RE, McCarty R (1990) Predictability of chronic intermittent stress: effects on sympathetic-adrenal medullary responses of laboratory rat. Behav Neural Biol 53:231–243
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902
Lewin GR, Barde YA (1997) Physiology of the neurotrophins. Annu Rev Neurosci 19:215–221
Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS (1994) Neurotrophic factors: from molecule to man. Trends Neurosci 17:182–190
Lipton SA (2004) Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer’s disease and other neurological disorders. J Alzheimer’s Dis 6:61–74
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Lucca G, Comim CM, Valvassori SS, Pereira JG, Stertz L, Gavioli EC, Kapczinski F, Quevedo J (2008) Chronic mild stress paradigm reduces sweet food intake in rats without affecting brain derived Neurotrophic factor protein levels. Curr Neurovasc Res 5:207–213
Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, Gavioli EC, Dal-Pizzol F, Quevedo J (2009a) Increased oxidative stress in submitocondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatr Res 43:864–869
Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, Dal-Pizzol F, Gavioli EC, Quevedo J (2009b) Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 54:358–362
Magalhães PV, Dean OM, Bush AI, Copolov DL, Malhi GS, Kohlmann K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Berk M (2011) N-acetylcysteine for major depressive episodes in bipolar disorder. Rev Bras Psiquiatr 33:374–188
Magariños AM, McEwen BS (1995) Stress-induced atrophy of apical dendrites of Hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69:89–98
Manji HK, Drevets WC, Charney DS (2001) The cellular neurobiology of depression. Nature Med 7:541–547
Marvanová M, Lakso M, Pirhonen J, Nawa N, Hiroyoki N, Wong G, Castrén E (2001) The neuroprotective agent memantine induces brain-derived Neurotrophic factor and trkb receptor expression in rat brain. Mol Cell Neurosci 18:247–258
Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR Jr, Kawahara R (2006) Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatr 30:1155–1158
Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R (1994) Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res 655:251–254
Moryl E, Danysz W, Quack G (1993) Potential antidepressive properties of Amantadine, memantine and bifemelane. Pharmacol Toxicol 72:394–397
Namba T, Yabe T, Gonda Y, Ichikawa N, Sanagi T, Arikawa-Hirasawa E, Mochizuki H, Kohsaka S, Uchino S (2010) Pigment epithelium-derived factor up-regulation induced by memantine, an N-methyl-D-aspartate receptor antagonist, is involved in increased proliferation of Hippocampal progenitor cells. Neuroscience 167:372–383
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia M (2002) Neurobiology of depression. Neuron 34:13–25
O’Connor TM, O’Halloran DJ, Shanahan F (2000) The stress response and the hypothalamic–pituitary–adrenal axis: from molecule to melancholia. Q J Med 93:323–333
Parsons CG, Danysz W, Quack G (1999) Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist–a review of preclinical data. Neuropharmacology 38:735–767
Paxinos G, Watson C (1986) The rat brain: stereotaxic coordinates, 2nd edn. Academic, Australia
Pittenger C, Duman R (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109
Quan MN, Zhang N, Wang YY, Zhang T, Yang Z (2011) Possible antidepressant effects and mechanisms of memantine in behaviors and synaptic plasticity of a depression rat model. Neuroscience 182:88–97
Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsons CG (2001) The N-methyl-D aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non competitive manner. Neurosci Lett 306:81–84
Rammes G, Danysz W, Parsons CJ (2008) Pharmacodynamics of memantine: an update. Curr Neuropharmacol 6:55–78
Réus GZ, Valvassori SS, Machado RA, Martins MR, Gavioli EC, Quevedo J (2008) Acute treatment with low doses of memantine does not impair aversive, nonassociative and recognition memory in rats. Naunyn Schmiedeberg’s Arch Pharmacol 376:295–300
Réus GZ, Stringari RB, Kirshi TR, Fries GR, Kapczinski F, Roesler R, Quevedo J (2010) Neurochemical and behavioural effects of acute and chronic memantine administration in rats: further support for NMDA as a new pharmacological target for the treatment of depression? Brain Res Bull 81:585–589
Rogóz Z, Skuza G, Legutko B (2008) Repeated co-treatment with fluoxetine and Amantadine induces brain-derived Neurotrophic factor gene expression in rats. Pharmacol Rep 60:817–826
Rogóz Z, Kubera M, Rogóz K, Basta-Kaim A, Budziszewska B (2009) Effect of co-administration of fluoxetine and amantadine on immunoendocrine parameters in rats subjected to a forced swimming test. Pharmacol Rep 61:1050–1060
Roman A, Rogóz Z, Kubera M, Nawrat D, Nalepa I (2009) Concomitant administration of fluoxetine and Amantadine modulates the activity of peritoneal macrophages of rats subjected to a forced swimming test. Pharmacol Rep 61:1069–1077
Skolnick P, Popik P, Trullas R (2009) Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci 30:563–569
Szasz BK, Mike A, Karoly R, Gerevich Z, Illes P, Vizi ES, Kiss JP (2007) Direct inhibitory effect of fluoxetine on N-methyl-D-aspartate receptors in the central nervous system. Biol Psychiatry 62:1303–1309
Vollmayr B, Henn FA (2003) Stress models of depression. Clin Neurosci Res 3:245–251
Yamada K, Nabeshima T (2004) Interaction of BDNF/TrkB signaling with NMDA receptor in learning and memory. Drug News Perspect 17:435–438
Zarate CA, Singh JB, Carson PJ, Brutshe NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-d-aspartate antagonist in treatment resistant major depression. Arch Gen Psychiatry 63:856–864
Acknowledgements
This study was supported in part by grants from ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico’ (CNPq-Brazil—JQ and FK), from the Instituto Cérebro e Mente (JQ) and UNESC (JQ). JQ and FK are recipients of CNPq (Brazil) Productivity Fellowships. GZR is holder of a Capes studentship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Réus, G.Z., Abelaira, H.M., Stringari, R.B. et al. Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats. Metab Brain Dis 27, 175–182 (2012). https://doi.org/10.1007/s11011-012-9281-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-012-9281-2