Abstract
Neuroinflammation resulting from microglial activation is involved in the pathogenesis of neurodegenerative diseases, including Parkinson’s diseases. Microglial activation plays an important role in neuroinflammation and contributes to several neurological disorders. Hence, inhibition of both microglial activation and the generation of pro-inflammatory cytokines may lead to an effective treatment for neurodegenerative diseases. In the present study, the anti-neuroinflammatory effects of galangin were investigated in lipopolysaccharide (LPS)-stimulated BV-2 microglial cells. Galangin significantly decreased the generation of nitric oxide, interleukin-1β, and inducible nitric oxide synthase in LPS-stimulated BV-2 microglial cells. In addition, galangin inhibited the phosphorylation of p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase 1/2. Furthermore, it was observed that activation of both IκB-α and nuclear factor kappa B (NF-κB) was significantly increased following LPS stimulation, and this effect was suppressed by galangin treatment. In conclusion, galangin displayed an anti-neuroinflammatory activity in LPS-stimulated BV-2 microglial cells. Galangin inhibited LPS-induced neuroinflammation via the MAPK and NF-κB signaling pathways and might act as a natural therapeutic agent for the treatment of various neuroinflammatory conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroinflammation plays an important role in Parkinson’s and Alzheimer’s disease and other neurodegenerative conditions [1, 2]. Neuroinflammatory responses are characterized by reactive oxygen species, inflammatory cytokines, and reactive nitrogen species, which have pivotal functions in the development and maintenance of the response [3,4,5,6]. Parkinson’s and Alzheimer’s disease are a very common progressive neurodegenerative disorder and have increased in recent years with the advent of improved imaging of cerebral dopamine distribution allowing presymptomatic diagnosis [7,8,9].
Microglia is the resident macrophage of the central nervous system (CNS) and the primary effector cells mediating neuroinflammation. Moreover, they are known to act as phagocytes in the brain and account for 10–15% of total brain cells [10]. Microglia plays important roles in the neuroinflammatory response, which is the major defense mechanism against exogenous pathogens and injury to the CNS. Because damage to the CNS could be catastrophic, this process is very sensitive to small pathological changes in the surrounding environment [11]. The CNS is capable with an elaborated response repertoire termed “neuroinflammation,” which enables it to cope with toxin and pathogens [9].
For example, microglia can be activated by lipopolysaccharide (LPS), an endotoxin released from the outer membrane of gram-negative bacteria, and quickly initiate a neuroinflammatory response by accelerating the production of pro-inflammatory mediators, such as nitric oxide (NO), and pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [12, 13]. Conversely, neuroinflammation resulting from microglial activation is considered a major event in the pathogenesis of several neurodegenerative disorders [14]. Therefore, modulation of both microglial activation and the production of pro-inflammatory mediators and cytokines is considered a promising strategy in mediating the onset and progression of neurodegenerative diseases.
Galangin (3,5,7-trihydroxyflavone) has three hydroxyl groups and carbon rings and belongs to the flavonols, which are a class of flavonoids. It is abundant in Zingiber officinale (ginger), Alpinia officinarum, and Helichrysum aureonitens. In previous studies, it has been reported that galangin has useful biological activities such as anti-fibrosis, anti-cancer, and as an antioxidant [15,16,17,18]. However, the effect of galangin on microglia-mediated neuroinflammation has not yet been established. Therefore, our present study investigated the effect of galangin on LPS-stimulated BV-2 microglial cells in vitro and explored the underlying molecular mechanisms.
Materials and methods
Chemicals and reagents
Galangin (HPLC ≥ 95.0%) was purchased from Sigma Chemical Co. (MO, USA). The molecular structure of galangin is shown in Fig. 1a. Griess reagent, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), was purchased from Sigma. TNF-α, IL-1β, and IL-6 ELISA detection kit were purchased from BioLegend (CA, USA). All antibodies were purchased from Santa Cruz biotechnology (CA, USA) and cell signaling (MA, USA).
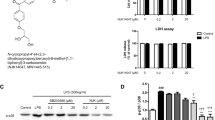
Galangin has no effect on viability in BV-2 microglial cells. Microglia were seeded in 96-well cell culture plates and treated with various concentrations of galangin (0–30 µM) (a), with the exception of controls (0.03% DMSO). The number of viable cells was assessed by an MTT assay after 24 h, as described in the “Materials and methods.” Data are reported as the percentage of viable cells present in each condition versus the control cells (b). The data represent the average (± SD) of four replicate wells and are representative of three separate experiments
Cell culture
BV-2 microglial cells were obtained from American Type Culture Collection (VA, USA). Briefly, cells were cultured at 37 °C in the presence of 5% CO2 in DMEM supplemented with 10% FBS, 200 IU/ml penicillin, 200 µg/ml streptomycin, 4 mM l-glutamine and 1 mM sodium pyruvate. Galangin was reconstituted in dimethyl sulfoxide (DMSO) and then diluted to the desired concentration in DMEM (final DMEM concentration 0.05% v/v). In the control (untreated) samples, equal amount DMSO was added.
MTT cell viability assay
Cells viability was assessed by using MTT reagent. The cells (1 × 104 cells/well) were seeded in 96-well culture plates. Seeded BV-2 microglial cells were pretreated with galangin (5, 10, 20, and 30 µM) or with LPS for 24 h. MTT solution (0.5 mg/mL) was added for 4 h at 37 °C in the presence of 5% CO2. After incubation in 37 °C for 4 h, MTT solution was removed and formazan product was dissolved in solubilization solution (1:1 = dimethyl sulfoxide:ethanol) into a colored solution. Absorbance of the formazan solution was quantified by an ELISA microplate reader at 570 nm.
NO assay
BV-2 microglial cells (5 × 104 cells/well) were seeded in 96-well culture plates in DMEM. Cultured cells were pretreated with various concentrations of galangin (0–30 µM) for 2 h and then cells were incubated for 22 h in the absence or presence of LPS. After incubation, the cultured medium was mixed with an equivalent volume of 1× Griess Reagent and incubated for 15 min at room temperature. After incubation, absorbance was measured by ELISA microplate reader at 540 nm of absorbance.
Reverse transcription (RT)-PCR
BV-2 microglial cells (1 × 106 cells/well) were seeded in 6-well culture plates in DMEM. Cultured cells were pretreated with various concentrations of galangin (0–30 µM) for 2 h and then cells were incubated for 6 h in the absence or presence of LPS. After incubation, the cells were collected by centrifugation and total RNA was isolated from galangin-treated cells using TRI reagent according to manufacturer’s protocol. To synthesize cDNA, 0.5 µg of total RNA was primed with oligo dT and reacted with mixture of M-MLV RTase, dNTP, and reaction buffer (Promega, WI, USA). To measure the mRNA level of inflammatory cytokines, cDNA was amplified using Gene Atlas G02 gradient thermal cycler system (Astec, Japan) e-Taq DNA polymerase kit (solgent, Daejeon, Korea) and indicated primers. And then, PCR products were visualized by fluorescent dye and UV transilluminator.
Enzyme-linked immunosorbent assay (ELISA)
BV-2 microglial cells (5 × 104 cells/well) were seeded in 96-well culture plates. Cells were pretreated with various concentrations of galangin for 2 h, thereafter incubated in the absence or presence of LPS for 22 h. Supernatant was used for samples and the quantification of IL-1β release was measured by Mouse IL-1β, IL-6, or TNF-α ELISA MAX™ Deluxe Sets (BioLegend, CA, USA), according to manufacturer’s protocol. Briefly, standards and samples were incubated on capture antibody coated plate at 4 °C, overnight. Detection antibody was incubated for 1 h and Avidin-HRP binds to detection antibody. For visualization, substrate solution was added to each well, and then the reaction was stopped by stop solution (2N H2SO4). Absorbance was measured by ELISA microplate reader at 405 nm wavelength.
Western blot analysis
The cells were exposed to LPS (200 ng/ml) in the absence or presence of 30 µM of galangin pretreatment. Following 15, 30, 45 min, or 24 h of incubation at 37 °C, cells were washed twice with cold PBS and lysed with modified RIPA buffer containing 150 mM sodium chloride, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris (pH 8.0), 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 µg/mL leupeptin, 1 µg/mL pepstatin, 1 mM sodium orthovanadate, and 100 mM sodium fluoride for 30 min at 4 °C. Lysates were cleared by centrifuging at 14,000×g for 15 min at 4 °C. The protein content of cell lysates was determined using the Micro BCA assay kit (Pierce, Rockford, IL, USA). Equivalent amounts of proteins were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to Polyvinylidene Difluoride (PVDF) transfer membrane. The membrane was placed into a blocking solution (5% non-fat milk) at room temperature for 1 h. After blocking, anti-ERK1/2, anti-phospho-ERK1/2 (p-ERK), anti-JNK, anti-phospho-JNK, anti-p38, phospho-p38 (p-p38), anti-iNOS, anti-COX-2, or anti-β-actin antibodies (Santa Cruz Biotechnology, CA, USA) was used as the primary antibodies. Horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies (Santa Cruze Biotechnology) were used as the secondary antibodies. Band detection was performed using the enhanced chemiluminescence (ECL) detection system and exposed to radiographic film. Pre-stained protein marker was used for molecular weight determination.
Immunofluorescence analysis
BV-2 microglial cells grown on coverslips were fixed with 4% paraformaldehyde in PBS for 15 min, permeabilized with 0.1% Triton X-100 for 15 min, and then blocked with 5% bovine serum albumin in PBS for 30 min. Coverslips were then incubated with primary antibodies against mouse p-p65 (eBioscience, San Diego, CA, USA) at a dilution of 1:100, and then with secondary antibodies at a dilution of 1:200, both at room temperature for 1 h. Cells were washed with PBS, and nuclei were counterstainedwith4′,6-diamidino-2-phenylindole (DAPI). Cover slips were mounted in 70% glycerol, and micrographs were obtained with an Olympus BX50 fluorescence microscope (Tokyo, Japan).
Statistical analysis
The results are presented as the mean ± standard deviation. The data were analyzed by one-way analysis of variance (ANOVA) followed by Scheffe’s post hoc test using SPSS. The differences were considered statistically significant at p < 0.01.
Results
Galangin inhibits the production of NO in LPS-stimulated BV-2 microglial cells
This study investigated the effect of galangin on cell viability and NO regulation. To determine cell viability, BV-2 microglial cells were cultured for 24 h in the presence of various concentrations of galangin (0, 5, 10, 20, or 30 µM). We found that galangin had no detectable cytotoxic effects on BV-2 microglial cells in doses up to 30 µM (Fig. 1b). Subsequently, four non-cytotoxic doses of galangin were chosen to test for anti-inflammatory effects. To this end, we measured the levels of nitrite released in the culture medium using an NO detection assay. BV-2 microglial cells were pretreated with galangin for 2 h and then stimulated with LPS (200 ng/ml). The Griess assay was used to investigate whether galangin could effectively regulate NO production at these concentrations. We found that LPS-induced NO production was significantly decreased by galangin treatment in a dose-dependent manner (Fig. 2). These results indicate that galangin effectively inhibits NO production in LPS-stimulated BV-2 microglial cells.
Galangin reduces LPS-induced NO production in BV-2 microglial cells. BV-2 microglial cells were seeded in 96-well cell culture plates. Microglia were pretreated with various concentrations of galangin for 2 h and stimulated with LPS (200 ng/ml). After 22 h, NO levels were determined using an NO assay, as described in the “Materials and methods.” Briefly, supernatants were mixed with a Griess reagent and the absorbance was measured by ELISA and a microplate reader. The data represent the average (± SD) of four replicate wells and are representative of three separate experiments (**P < 0.01 vs. LPS only groups)
Galangin inhibits the expression of pro-inflammatory genes and inducible enzymes in LPS-stimulated BV-2 microglial cells
Given the strong inhibitory effects of galangin on NO production in LPS-stimulated microglia cells, we decided to further investigate galangin for anti-inflammatory effects by measuring the expression of inflammatory mediators in LPS-stimulated BV-2 microglial cells. Specifically, we investigated the expression of pro-inflammatory genes, including IL-1β, IL-6, and TNF-α, as well as the levels of pro-inflammatory enzymes, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2. An RT-PCR analysis was used to determine if galangin inhibits the expression of pro-inflammatory genes and inducible enzymes. Microglia were pretreated for 2 h with galangin and then stimulated with LPS (200 ng/ml) for 6 h. As shown in Fig. 3a, mRNA expression levels of iNOS, COX-2, IL-6, TNF-α, and IL-1β were increased in LPS-stimulated BV-2 microglial cells. Interestingly, the LPS-induced expression of iNOS and IL-1β were significantly blunted by galangin treatment, in a dose-dependent manner. We next investigated whether galangin also blunted the LPS-induced expression of iNOS at the level of protein expression. As shown in Fig. 3b, the levels of iNOS protein were decreased by galangin treatment in LPS-stimulated BV-2 microglial cells. However, neither COX-2 mRNA nor protein levels were regulated by galangin treatment. These results indicate that galangin inhibits the expression of iNOS and IL-1β mRNA, as well as the levels of iNOS protein, in LPS-stimulated microglia.
Galangin suppresses the expression of pro-inflammatory factors at the level of mRNA and protein. a Microglia were pretreated with galangin (0, 5, 10, 20, or 30 µM) for 2 h and stimulated with LPS for 6 h. The levels of inflammatory genes were determined by RT-PCR using GAPDH as an internal control. b Microglia were stimulated with LPS for 24 h, following pretreatment with galangin. Western blots were used to determine protein levels using β-actin as an internal control. Data are expressed as the average (± SD) of triplicate cultures (**P < 0.01 vs. LPS only groups)
Galangin inhibits the production of the IL-1β cytokine
IL-1β is a pro-inflammatory cytokine that is released by microglia following exposure to LPS and other inflammatory stimuli. As shown in Fig. 3a, the expression of IL-1β dramatically increased in response to LPS; however, production was significantly inhibited by galangin. Therefore, to further examine the effects of galangin on inflammatory cytokine production in LPS-stimulated BV-2 microglial cells, the levels of IL-1β, TNF-α, and IL-6 protein were determined by an enzyme-linked immunosorbent assay (ELISA). As shown Fig. 4a, d, stimulation of microglia with LPS increased the secretion of IL-1β, which was significantly blunted by galangin in a dose-dependent manner. Conversely, production of the TNF-α and IL-6 cytokines was not regulated by galangin. These results suggest that galangin selectively reduced the production of IL-1β protein in LPS-stimulated BV-2 microglial cells.
Galangin attenuates production of IL-1β in LPS-stimulated BV-2 microglial cells. BV-2 microglial cells were pretreated with various concentrations (0, 5, 10, 20, or 30 µM) of galangin, and stimulated with LPS for 24 h. Supernatants were measured for the release of IL-1β, TNF-α, and IL-6 by ELISA (a). The pro-IL-1β proteins were analyzed by Western blot analysis using anti-IL-1β antibody (b). Data are expressed as the average (± SD) of triplicate cultures (*P < 0.05, **P < 0.01 vs. LPS only groups)
Galangin inhibits the phosphorylation of p38 MAPK and JNK1/2 in LPS-stimulated BV-2 microglial cells
To investigate the mechanism by which galangin exerts its anti-inflammatory effects, we examined the mitogen-activated protein kinase (MAPK) signaling pathways, which are known to play an important role in pro-inflammatory gene expression by modulating transcription factors such as nuclear factor kappa B (NF-κB). Additionally, the MAPK pathways include extracellular signal-regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK)1/2, and p38 MAPK. Therefore, we measured LPS-induced phosphorylation of members of the MAPK pathway by western blot analysis. As shown in Fig. 5a, phosphorylation of ERK1/2, JNK1/2, and p38 MAPK was increased by LPS stimulation at 15, 30, and 45 min. However, treatment with galangin significantly blunted the LPS-induced phosphorylation of p38 MAPK and JNK1/2. These results indicate that galangin alters LPS-stimulated microglial activation by inhibiting p38 MAPK and JNK1/2 phosphorylation.
Galangin inhibits phosphorylation of the MAPK pathway and NF-κB activation. Cells were starved in serum-free DMEM for 4 h and pre-treated with 30 µM galangin for 2 h. Cells were then stimulated with 200 ng/ml LPS for 15, 30, or 45 min. Cells were lysed and equal amount of whole cell proteins were separated by SDS-PAGE and transferred to PVDF membranes. The MAPK proteins were then analyzed by Western blot analysis using anti-ERK, anti-p-ERK, anti-JNK, anti-p-JNK, anti-p-38, and anti-p-p-38 antibodies (a). Anti-IκB-α, anti-p-65, and anti-p-p-65 antibodies were used (b, c). We used β-actin as an internal control. This result is from one representative experiment and is one of three that represents a similar pattern. Data are expressed as the average (± SD) of triplicate cultures (**P < 0.01 vs. LPS only groups)
Galangin attenuates the activation of NF-κB in LPS-stimulated BV-2 microglial cells
Phosphorylation of MAPK can activate IκB kinase (IKK), which phosphorylates Iκ-B, resulting in the ubiquitination of Iκ-B and its degradation by the proteasome. Degradation of IκB-α, which is an inhibitor of NF-κB, allows for translocation of NF-κB from the cytosol to the nucleus. As shown Fig. 5b, IκB-αlevels were decreased in BV-2 microglial cultures after 15, 30, and 45 min of LPS-stimulation. However, treatment with galangin slightly reduced LPS-induced IκB-α degradation. NF-κB activation plays an important role in inducing the transcription of inflammatory genes, such as iNOS, COX-2, and IL-1β, in LPS-stimulated BV-2 microglial cells. Therefore, we next investigated the effect of galangin on the activation of the NF-κB subunit NF-κB-p65 in LPS-stimulated BV-2 microglial cells. We found that galangin inhibited the LPS-induced phosphorylation of NF-κB-p65 in BV-2 microglial cells (Fig. 5c). These results suggest that the MAPK pathway, along with the NF-κB pathway, may contribute to the anti-inflammatory effects of galangin, specifically, the down-regulation of pro-inflammatory mediators such as iNOS and IL-1β.
Discussion
Neuroinflammation, caused by activated glial cells, is very important in the development of neurodegenerative diseases [19]. Activated microglia can produce various pro-inflammatory cytokines and neurotoxic mediators that contribute to neuronal degeneration [20]. Microglia is an important immune cell in the brain and act as the first major safeguard of the CNS. Additionally, microglia can be phagocytic and are able to act as antigen-presenting cells. In the current study, we demonstrated that galangin can inhibit the neuroinflammatory response following LPS-induced microglial activation and identified a potential molecular mechanism [21, 22]. Our results show that galangin inhibits the production of the pro-inflammatory mediators NO, iNOS, and IL-1β in LPS-stimulated microglia. Moreover, galangin regulated LPS-induced phosphorylation of the MAPK pathway and NF-κB activation.
NO is a biological marker of inflammatory responses and can be regulated by iNOS expression, along with other NO metabolites. NO is produced by activated microglia and inflammatory stimuli and plays an important role in both acute and chronic inflammation. Therefore, suppression of NO production maybe a principal therapeutic approach in the development of anti-neuroinflammatory agents [23,24,25]. As shown in Figs. 2 and 3, we found that galangin reduced NO production and iNOS expression in LPS-stimulated microglia. However, the expression of COX-2 was not altered by galangin treatment. It is known that NO and prostaglandin E2 (PGE2) production are primarily mediated by iNOS and COX-2, respectively. Additionally, TNF-α and IL-1β are also important pro-inflammatory mediators and are actively involved in the progression of neuroinflammatory diseases. To this end, we found that galangin inhibited IL-1β gene expression and cytokine production in LPS-stimulated BV-2 microglia cells (Figs. 3, 4). Taken together, these results suggested that galangin may be a potential candidate for the treatment of neuroinflammatory diseases.
The MAPK family includes serine/threonine kinases that have crucial roles in iNOS modulation in diverse biological systems. MAPKs are known to modulate the expression of cell survival genes as well as pro-inflammatory enzymes and cytokines in response to various stimuli. LPS initiates signaling cascades for pro-inflammatory gene expression by activating toll-like receptor 4. After recognition of LPS by the receptor, several intracellular signaling molecules, including the IκB complex and MAPKs, are activated, and these cytoplasmic molecules activate downstream transcription factors, including NF-κB. It is well known that NF-κB activation is regulated by several processes such as IKK and IκB phosphorylation, IκB degradation, and the nuclear translocation of NF-κB [26,27,28]. Additionally, expression of iNOS is dependent on MAPK activation in LPS-stimulated BV-2 microglial cells [29, 30]. For this reason, we determined the effect of galangin on phosphorylation of the MAPK pathway in LPS-stimulated microglia. As shown in Fig. 5, phosphorylation of JNK1/2, p38 MPAK, and NF-κB-p65 were significantly suppressed by galangin pretreatment in LPS-activated microglia.
In summary, our study showed that galangin can exhibit anti-neuroinflammatory activity in vitro by suppressing the expression of pro-inflammatory mediators in LPS-stimulated BV-2 microglial cells. These beneficial effects could be a result of inhibition of the MAPK and NF-κB inflammatory signaling pathways. To our knowledge, this is the first comprehensive study to investigate the in vitro anti-neuroinflammatory effects of galangin. Collectively, the present study demonstrated that galangin possesses anti-neuroinflammatory properties that inhibit microglial activation and represents a potential therapeutic agent for neuroinflammatory diseases.
References
Guo C, Yang L, Wan CX, Xia YZ, Zhang C, Chen MH, Wang ZD, Li ZR, Li XM, Geng YD, Kong LY (2016) Anti-neuroinflammatory effect of Sophoraflavanone G from Sophora alopecuroides in LPS-activated BV2 microglia by MAPK, JAK/STAT and Nrf2/HO-1 signaling pathways. Phytomedicine 23:1629–1637. https://doi.org/10.1016/j.phymed.2016.10.007
Tiwari PC, Pal R (2017) The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialogues Clin Neurosci 19:71–80
Parsa R, Lund H, Tosevski I, Zhang XM, Malipiero U, Beckervordersandforth J, Merkler D, Prinz M, Gyllenberg A, James T, Warnecke A, Hillert J, Alfredsson L, Kockum I, Olsson T, Fontana A, Suter T, Harris RA (2016) TGFbeta regulates persistent neuroinflammation by controlling Th1 polarization and ROS production via monocyte-derived dendritic cells. Glia 64:1925–1937. https://doi.org/10.1002/glia.23033
Fan H, Wu PF, Zhang L, Hu ZL, Wang W, Guan XL, Luo H, Ni M, Yang JW, Li MX, Chen JG, Wang F (2015) Methionine sulfoxide reductase A negatively controls microglia-mediated neuroinflammation via inhibiting ROS/MAPKs/NF-kappaB signaling pathways through a catalytic antioxidant function. Antioxid Redox Signal 22:832–847. https://doi.org/10.1089/ars.2014.6022
Schain M, Kreisl WC (2017) Neuroinflammation in neurodegenerative disorders—a review. Curr Neurol Neurosci Rep 17:25. https://doi.org/10.1007/s11910-017-0733-2
Chen WW, Zhang X, Huang WJ (2016) Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep 13:3391–3396. https://doi.org/10.3892/mmr.2016.4948
Hawkes C (2003) Olfaction in neurodegenerative disorder. Mov Disord 18:364–372. https://doi.org/10.1002/mds.10379
Zablocka A (2006) Alzheimer’s disease as neurodegenerative disorder. Postepy Hig Med Dosw 60:209–216
Barlow BK, Cory-Slechta DA, Richfield EK, Thiruchelvam M (2007) The gestational environment and Parkinson’s disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol 23:457–470. https://doi.org/10.1016/j.reprotox.2007.01.007
Park SY, Jin ML, Wang Z, Park G, Choi YW (2016) 2,3,4′,5-tetrahydroxystilbene-2-O-beta-d-glucoside exerts anti-inflammatory effects on lipopolysaccharide-stimulated microglia by inhibiting NF-kappaB and activating AMPK/Nrf2 pathways. Food Chem Toxicol 97:159–167. https://doi.org/10.1016/j.fct.2016.09.010
Lee YY, Lee EJ, Park JS, Jang SE, Kim DH, Kim HS (2016) Anti-inflammatory and antioxidant mechanism of tangeretin in activated microglia. J Neuroimmune Pharmacol 11:294–305. https://doi.org/10.1007/s11481-016-9657-x
Lopes PC (2016) LPS and neuroinflammation: a matter of timing. Inflammopharmacology 24:291–293. https://doi.org/10.1007/s10787-016-0283-2
Schmidt AF, Kannan PS, Chougnet CA, Danzer SC, Miller LA, Jobe AH, Kallapur SG (2016) Intra-amniotic LPS causes acute neuroinflammation in preterm rhesus macaques. J Neuroinflammation 13:238. https://doi.org/10.1186/s12974-016-0706-4
Khan MS, Ali T, Kim MW, Jo MH, Jo MG, Badshah H, Kim MO (2016) Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem Int 100:1–10. https://doi.org/10.1016/j.neuint.2016.08.005
Wang X, Gong G, Yang W, Li Y, Jiang M, Li L (2013) Antifibrotic activity of galangin, a novel function evaluated in animal liver fibrosis model. Environ Toxicol Pharmacol 36:288–295. https://doi.org/10.1016/j.etap.2013.04.004
Ren K, Zhang W, Wu G, Ren J, Lu H, Li Z, Han X (2016) Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed Pharmacother 84:1748–1759. https://doi.org/10.1016/j.biopha.2016.10.111
Chien ST, Shi MD, Lee YC, Te CC, Shih YW (2015) Galangin, a novel dietary flavonoid, attenuates metastatic feature via PKC/ERK signaling pathway in TPA-treated liver cancer HepG2 cells. Cancer Cell Int 15:15. https://doi.org/10.1186/s12935-015-0168-2
Aloud AA, Veeramani C, Govindasamy C, Alsaif MA, El Newehy AS, Al-Numair KS (2016) Galangin, a dietary flavonoid, improves antioxidant status and reduces hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. Redox Rep 22:290–300. https://doi.org/10.1080/13510002.2016.1273437
Hong H, Kim BS, Im HI (2016) Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. Int Neurourol J 20:S2–S7. https://doi.org/10.5213/inj.1632604.302
Walter L, Neumann H (2009) Role of microglia in neuronal degeneration and regeneration. Semin Immunopathol 31:513–525. https://doi.org/10.1007/s00281-009-0180-5
Kim BW, Koppula S, Hong SS, Jeon SB, Kwon JH, Hwang BY, Park EJ, Choi DK (2013) Regulation of microglia activity by glaucocalyxin-A: attenuation of lipopolysaccharide-stimulated neuroinflammation through NF-kappaB and p38 MAPK signaling pathways. PLoS ONE 8:e55792. https://doi.org/10.1371/journal.pone.0055792
Culbert AA, Skaper SD, Howlett DR, Evans NA, Facci L, Soden PE, Seymour ZM, Guillot F, Gaestel M, Richardson JC (2006) MAPK-activated protein kinase 2 deficiency in microglia inhibits pro-inflammatory mediator release and resultant neurotoxicity. Relevance to neuroinflammation in a transgenic mouse model of Alzheimer disease. J Biol Chem 281:23658–23667. https://doi.org/10.1074/jbc.M513646200
Chen YF, Wang YW, Huang WS, Lee MM, Wood WG, Leung YM, Tsai HY (2016) Trans-cinnamaldehyde, an essential oil in cinnamon powder, ameliorates cerebral ischemia-induced brain injury via inhibition of neuroinflammation through attenuation of iNOS, COX-2 expression and NFkappa-B signaling pathway. Neuromolecular Med 18:322–333. https://doi.org/10.1007/s12017-016-8395-9
Raposo C, Nunes AK, Luna RL, Araujo SM, da Cruz-Hofling MA, Peixoto CA (2013) Sildenafil (Viagra) protective effects on neuroinflammation: the role of iNOS/NO system in an inflammatory demyelination model. Mediators Inflamm. https://doi.org/10.1155/2013/321460
Zhou M, Wang CM, Yang WL, Wang P (2013) Microglial CD14 activated by iNOS contributes to neuroinflammation in cerebral ischemia. Brain Res 1506:105–114. https://doi.org/10.1016/j.brainres.2013.02.010
Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu XF, Zhou QG, Chen YY, Guo AZ, Hu CM (2017) Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation 40:1–12. https://doi.org/10.1007/s10753-016-0447-7
Wang W, Liu P, Hao C, Wu L, Wan W, Mao X (2017) Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-kappaB pathways. Sci Rep 7:44252. https://doi.org/10.1038/srep44252
Park EJ, Kim YM, Park SW, Kim HJ, Lee JH, Lee DU, Chang KC (2013) Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem Toxicol 55:386–395. https://doi.org/10.1016/j.fct.2012.12.027
Park J, Min JS, Kim B, Chae UB, Yun JW, Choi MS, Kong IK, Chang KT, Lee DS (2015) Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci Lett 584:191–196. https://doi.org/10.1016/j.neulet.2014.10.016
Dai JN, Zong Y, Zhong LM, Li YM, Zhang W, Bian LG, Ai QL, Liu YD, Sun J, Lu D (2011) Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS ONE 6:e21891. https://doi.org/10.1371/journal.pone.0021891
Acknowledgements
This study was supported by research fund from Chosun University 2015 (K206212009).
Author information
Authors and Affiliations
Contributions
MEK, PRP, and JSL conceived and designed the experiments; MEK, and PRP, JYN, IJ, and JHC performed experiments and analyzed the data. MEK and JSL wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, M.E., Park, P.R., Na, J.Y. et al. Anti-neuroinflammatory effects of galangin in LPS-stimulated BV-2 microglia through regulation of IL-1β production and the NF-κB signaling pathways. Mol Cell Biochem 451, 145–153 (2019). https://doi.org/10.1007/s11010-018-3401-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3401-1