Abstract
Neuroinflammation is an important pathological feature in neurodegenerative diseases. Accumulating evidence has suggested that neuroinflammation is mainly aggravated by activated microglia, which are macrophage like cells in the central nervous system. Therefore, the inhibition of microglial activation may be considered for treating neuroinflammatory diseases. p38 mitogen-activated protein kinase (MAPK) has been identified as a crucial enzyme with inflammatory roles in several immune cells, and its activation also relates to neuroinflammation. Considering the proinflammatory roles of p38 MAPK, its inhibitors can be potential therapeutic agents for neurodegenerative diseases relating to neuroinflammation initiated by microglia activation. This study was designed to evaluate whether NJK14047, a recently identified novel and selective p38 MAPK inhibitor, could modulate microglia-mediated neuroinflammation by utilizing lipopolysaccharide (LPS)-stimulated BV2 cells and an LPS-injected mice model. Our results showed that NJK14047 markedly reduced the production of nitric oxide and prostaglandin E2 by downregulating the expression of various proinflammatory mediators such as nitric oxide synthase, cyclooxygenase-2, tumor necrosis factor-α and interleukin-1β in LPS-induced BV2 microglia. Moreover, NJK14047 significantly reduced microglial activation in the brains of LPS-injected mice. Overall, these results suggest that NJK14047 significantly reduces neuroinflammation in cellular/vivo model and would be a therapeutic candidate for various neuroinflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia are the primary resident immune cells of the brain and play a central role in the innate immune responses and tissue repair in the central nervous system (CNS) [1]. Under normal conditions, microglia maintain resting phenotypes in which they perform diverse physical functions including the release of neurotrophic factors, support of neurogenesis, and regulation of brain development [2,3,4]. When the brain is exposed to injury, microglia are activated to produce various proinflammatory mediators, such as prostaglandin E2 (PGE2) and nitric oxide (NO), as well as proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [5, 6]. Although these microglia-derived inflammatory products have been released to resolve the injury conditions, the inflammation do not subside and continue in neuroinflammatory disease. And, in this condition, these products are considered responsible for exacerbation of various CNS disorders such as multiple sclerosis, Parkinson’s disease (PD), Alzheimer’s disease (AD), and Huntington’s disease [7,8,9,10]. Therefore, reduction in microglial activation might be a promising approach for treating inflammatory-mediated neurodegenerative diseases.
Lipopolysaccharide (LPS), which is a major pathogenic component of gram-negative bacteria, is one of the most potent activators of CNS microglia [11, 12]. Once primed by LPS, microglia transform from a resting state to an activated state and secrete several proinflammatory and neurotoxic molecules including TNF-α, IL-1β, IL-6, NO, eicosanoids, proteinases, and reactive oxygen species [13, 14]. Thus, LPS-stimulated microglia have been widely used as a research tool to study the characteristics of microglia-mediated inflammatory responses, both in vitro and in vivo [15, 16].
Mitogen-activated protein kinases (MAPKs) are serine/threonine protein kinases that regulate cellular properties in response to several extracellular stimuli such as growth factors, inflammatory cytokines, and G protein-coupled receptors [17]. Of interest is p38 MAPK, which is comprised of α, β, γ, and δ isoforms; it has been found to play an essential role in the immune response via the regulation of proinflammatory signaling networks and cytokine biosynthesis [18, 19]. The inhibition of p38 MAPK has been shown to effectively alleviate symptoms of inflammatory diseases such as rheumatoid arthritis, cardiovascular disease, and inflammatory pain [20, 21]. In particular, the suppression of p38 MAPK signaling has been reported to markedly alleviate symptoms of neuroinflammatory diseases such as AD [22]. Thus, p38 MAPK has been considered as an attractive therapeutic target, and a considerable amount of research has been aimed at attempting to develop novel p38 inhibitors. Recently, we developed a class of novel biphenyl amide p38 MAPK inhibitors and found that certain inhibitors have very potent inhibitory activity [23, 24]. Most notably, NJK14047 (N-cyclopropyl-4′-(4-(2,3-dihydroxypropoxy)benzoyl)-2-methyl-[1,1′-biphenyl]-4-carboxamide) exerted potent p38α MAPK inhibitory activity (IC50 = 27 nM) with high kinase selectivity [23, 24].
In the present study, we examined the anti-inflammatory effects of NJK14047 on LPS-induced inflammatory responses in microglia to address the hypothesis that specific p38 MAPK inhibition would be an effective strategy to ameliorate neuroinflammation and lead to the development of potential therapeutic agents for neuroinflammatory diseases.
Materials and Methods
Chemicals and Reagents
NJK14047 (Fig. 1a and Supplementary Fig. 1) was synthesized by a previously reported procedure [23, 24]. NJK14047 had more than 97% purity which was confirmed by 1H-NMR and Waters 1525 HPLC system (Supplementary Fig. 1). Dulbecco’s modified Eagle’s medium (DMEM), 10% Fetal Bovine Serum (FBS), 100 units/ml of penicillin and 100 µg/ml of streptomycin were purchased from GE Healthcare HyClone™. Cell culture plates were purchased from SPL (#20100, #30006, #30096) and were used without additional coating. LPS from Escherichia coli serotype O55:B5 (L6529, ≥ 500,000 EU/mg) and SB203580 (S8307) were purchased from Sigma-Aldrich. LPS was dissolved in PBS at 10 mg/ml and NJK14047 and SB203580 were dissolved in DMSO at 10 mg/ml. The aliquots of them were stored at − 20 °C. Anti-p38 MAPK rabbit polyclonal antibody (#9212s, RRID: AB_330713), anti-phospho-p38 MAPK (Thr180/Tyr182) (3D7) rabbit monoclonal antibody (#9215s, RRID: AB_331762), anti-iNOS (mouse specific) rabbit polyclonal antibody (#2982s, RRID: AB_1078202), and anti-COX-2 rabbit polyclonal antibody (#4842s, RRID: AB_10694771) were purchased from Cell Signaling Technology. Anti-actin (C-11) goat polyclonal antibody (sc-1615, RRID: AB_630835) was purchased from SantaCruz Biotechnology. Anti-ionized calcium-binding adapter molecule 1 (Iba-1) rabbit polyclonal antibody (#019-19741, RRID: AB_839504) was purchased from Wako. Goat anti-mouse IgG-HRP conjugate antibody (#1706516, RRID: AB_11125547) and goat anti-rabbit IgG-HRP conjugate antibody (#1706515, RRID: AB_11125142) were purchased from Bio-rad and goat anti-rabbit IgG-Alexa Fluor® 488 conjugate antibody (A-11008, RRID: AB_143165) was purchased from Invitrogen. TNF-α (558534) and IL-6 (555240) ELISA kits were purchased from BD biosciences. NO (KA1342), PGE2 (ADI-900-001) and mouse IL-1β (ab100704) assay kits were obtained from Abnova, Enzo Life Sciences and Abcam, respectively. WST-1 (#05-015-944-001) was obtained from Roche. LDH assay kit (DG-LDH500) was purchased from Dogen.
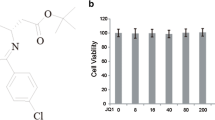
NJK14047 inhibits p38 MAPK activation in LPS-stimulated microglia. a Chemical structure of NJK14047. b Effect of NJK14047 on the cell viability in control and LPS-stimulated microglia. BV2 cells were pretreated with different concentrations (0–20 µM) of NJK14047 for 2 h, then cells were treated with LPS (500 ng/ml) for 22 h. Cell viability was measured by WST-1 assay (n = 10–12 per group) and LDH assay (n = 12 per group). c, d Inhibition of LPS-mediated p38 MAPK activation by NJK14047. BV2 cells were pretreated with different concentrations (0–20 µM) of NJK14047 and SB203580 for 2 h, then cells were treated with LPS (500 ng/ml) for 22 h. The levels of p38 MAPK activation were measured by immunoblotting. c Representative immunoblotting of phospho-p38 levels in whole cell lysates. d Quantification of phospho-p38 MAPK level (n = 6 per group). All experiments were repeated in three times independently. (###P < 0.001 vs. control; **P < 0.01 and ***P < 0.001 vs. LPS; †P < 0.05 and †††P < 0.001 vs. SB. All error bars indicate SEM)
Cell Culture and Treatment
The murine microglia cell line, BV2 (RRID: CVCL_0182) was kindly provided by Dr. Myung sook Oh [25]. We used BV2 cell line passage number #38~#43 confirmed with an absence of mycoplasma contamination using MycoAlert PLUS Mycoplasma detection kit (Lonza, LT07). Cells were cultured in DMEM with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37 °C in humidified atmosphere of 5% CO2. BV2 cells were treated with 0–20 µM of NJK14047 for 2 h and incubated with or without 500 ng/ml of lipopolysaccharides (LPS) for further 22 h.
Assessment of Cell Viability and Cytotoxicity of NJK14047
Cell viability was measured using 4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) assay. BV2 cells were seeded in 96-well plates at a density of 1 × 104 cells/well. After 12 h, the cells were treated with NJK14047 and LPS as described above. The cell culture media were replaced with fresh serum free medium with 10% WST-1 solution. After 4 h, the absorbance of the media was measured by using a microplate reader at 440 nm. Cytotoxicity was assessed by lactate dehydrogenase (LDH) assay kit. Cells were plated in 96-well plates and incubated with the same protocol of WST-1 assay above. After 24 h of NJK14047 treatment, the conditioned media were collected and the concentration of LDH in the conditioned media was measured according to the manufacturer’s instructions.
Measurement of NO and PGE2
Cells were seeded at a density of 5 × 105 cells/well in 6-well plates. After 12 h, cells were treated with 0–20 µM of NJK14047 for 2 h and 500 ng/ml of LPS was added for further 22 h. The concentration of NO and PGE2 in culture supernatants were assessed according to the manufacturer’s instructions.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
mRNA levels of cytokines were analyzed by qRT-PCR. Total RNA was extracted from BV2 cells using the RNeasy Plus Mini kit (Qiagen, Korea, Ltd) according to the manufacturer’s instructions. Concentrations and purities of the RNA samples were assessed by NanoDrop™-2000c (Thermo Scientific™) and cDNA synthesis was conducted using RNA to cDNA EcoDry™ Premix kit (Takara). cDNA samples were subjected to qRT-PCR using SYBR Green Mix (TOYOBO, Osaka, Japan) and the CFX Connect real-time PCR system (Bio-Rad Laboratories, USA). qRT-PCR protocol was as follows; first holding stage at 95 °C for 3 min, followed by cycling stage at 95 °C for 10 s, 55 °C for 10 s, 72 °C for 30 s, 30 cycles, after that last holding stage at 95 °C for 10 s. Primers used for the study are as follows; TNF-α: Forward, 5′-GATTATGGCTCAGGGTCCAA-3′, Reverse, 5′-GCTCCAGTGAATTCGGAAAG-3′; IL-1β: Forward, 5′-CCCAAGCAATACCCAAAGAA-3′, Reverse, 5′-GCTTGTGCTCTGCTTGTGAG-3′; IL-6: Forward, 5′-CCGGAGAGGAGACTTCACAG-3′, Reverse, 5′-TTGCCATTGCACAACTCTTT-3′; GAPDH: Forward, 5′-TGAATACGGCTACAGCAACA-3′, Reverse, 5′-AGGCCCCTCCTGTTATTATG-3′. The optimum melting temperature of the primers is 58 ± 2 °C.
Western Blotting
Western blot analysis was performed as previously described [26]. Cells were collected and washed in ice-cold PBS and then lysed in RIPA buffer (Cell Signaling Technology). Lysates were sonicated, centrifuged at 4 °C and supernatants were collected. The cortical tissues were weighed and homogenized in 10 × volume of RIPA buffer. Lysates were centrifuged at 13,000 rpm, 4 °C, 15 min and the supernatants were collected. Protein concentration was determined using Bradford technique (Bio-Rad, Hercules, CA, USA). Equal amounts of proteins were fractionated by SDS–PAGE, transferred to PVDF membranes. Immunoblotting was carried out with antibodies against phospho-p38 (1:1000), p38 (1:1000), iNOS (1:1000), COX-2 (1:1000), and β-actin (1:500). The membranes were incubated with corresponding secondary antibodies. Western blots were developed by enhanced chemiluminescence detection system (ECL; Amersham Biosciences).
Enzyme-Linked Immunosorbent Assay (ELISA)
BV2 cell conditioned media and mouse cortical protein samples were used for cytokine ELISA. Cell conditioned media were prepared with centrifugation at 3000 rpm, 3 min for removal of any cells. Mouse cortical protein samples were prepared with the same protocol in western blotting without Bradford assay. TNF-α, IL-1β and IL-6 ELSIAs were performed in duplicate according to the manufacturer’s instructions.
Animals and Treatments
Male C57BL/6 mice (8 weeks) were purchased from Daehan Biolink Co., Ltd (Eumseong, Korea). The mice lived in an individual ventilated cage, 12 h light/dark cycle and 22 °C condition. After a week for acclimation, they were divided into three groups, control, LPS, and LPS + NJK. Mice in LPS + NJK group were administered 5 mg/kg NJK14047 in 100 µl of 10% DMSO p.o. once daily for 4 consecutive days and other groups were administered the vehicle. 2 h after final administration of NJK14047, 5 mg/kg LPS in 100 µl saline was injected intraperitoneally to LPS and LPS + NJK groups and the vehicle was injected to control group. At 6 or 24 h after LPS injection, the mice were anesthetized by 0.1 ml/10 g body weight intraperitoneal injection of 2.5% Avertin (2,2,2-Tribromoethanol) and analysis was done. Mouse studies were approved by the Kyung Hee University Institutional Animal Care and Use Committee (IACUC).
Immunofluorescence
The mice were anesthetized with 2.5% Avertin and immediately cardiac perfused with PBS followed by 4% paraformaldehyde in PBS. After perfusion, brains were excised, post-fixed in 4% paraformaldehyde overnight at 4 °C, and incubated in 30% sucrose at 4 °C until equilibrated. For cryosection, the brains were set into O.C.T. compound block and incubated at − 70 °C. Sequential 30 µm coronal section was taken on a cryostat (CM30 50S; Leica) and every tenth section (300 µm apart) of the brain (Bregma − 1.30 ~ − 2.70 mm) were stored until used. Brain sections were rinsed in PBS and then incubated with rabbit anti-Iba-1 antibody (1:1000) for 24 h at 4 °C. For visualization, the primary antibody was developed by incubating with Alexa Fluor 488-conjugated secondary antibodies for 1 h at RT. Four cortex areas per section and four sections per mouse were used for quantification. The images were analyzed using Olympus BX51 microscope and the threshold distinguishing Iba-1 from background was determined by Image J software (NIH, Bethesda, MD, USA). Data analysis were proceeded in double-blinded and unaware of group allocation throughout the experiments.
Statistical Analysis
All data were expressed as the mean ± standard error of the mean (SEM) using Graph Pad Prism 5.0 software (Graph Pad software Inc., San Diego, CA, USA). The results were analyzed statistically by one-way analysis of variance followed by Tukey’s post-hoc test. p value less than 0.05 was considered statistically significant.
Results
NJK14047 Inhibits p38 MAPK Activation in LPS-Stimulated Microglia
We first investigated the cytotoxic effect of NJK14047 on microglia. To test this, various concentrations of NJK14047 were added with or without LPS (500 ng/ml) to BV2 microglia for 24 h. Cell viability was determined by the WST-1 assay and LDH assay. As shown Fig. 1b, either treatment with NJK14047 alone or NJK14047 with LPS had no cytotoxic effect on BV2 cells within the range of concentrations used in both different principle-based cytotoxicity assays. In our previous studies, NJK14047 showed a dose-dependent inhibitory effect on p38 MAPK in macrophage and hepatocyte cell cultures [23, 27]. To confirm p38 MAPK inhibition in microglia, BV2 cells were treated with LPS for 24 h in the presence or absence of NJK14047 and were analyzed by immunoblotting. As expected, LPS induced p38 MAPK activation, but treatment with NJK14017 significantly decreased p38 MAPK phosphorylation, without affecting total protein levels of p38 MAPK (Fig. 1c, d). The inhibitory effect of NJK14047 on p38 MAPK phosphorylation was significantly more potent than SB203580, one of well-known p38 MAPK inhibitors. These results suggest that NJK14047 can reduce p38 MAPK activation without causing cytotoxic effects on LPS-stimulated microglia.
NJK14047 Attenuates NO and PGE2 Production by Regulating iNOS and COX-2 Expression in LPS-Stimulated BV2 Microglia
Next, we examined whether NJK14047 inhibits the LPS-induced production of the inflammatory mediators such as NO and PGE2 in BV2 microglia. Cells were treated with LPS in the presence or absence of NJK14047 and SB203580 for 24 h. Compared with the control group, the production of NO was significantly increased in LPS-treated cells (Fig. 2a). However, NJK14047 dramatically suppressed this production in a concentration-dependent manner (Fig. 2a). These effects were stronger in NJK14047-treated group than SB203580 treated group. In the case of PGE2 inhibition assay, NJK14047 and SB203580 similarly decreased the production of PGE2 in LPS-treated BV2 cells (Fig. 2b).
NJK14047 attenuates NO and PGE2 production in LPS-stimulated BV2 microglia. a, b Effects of NJK14047 on the NO and PGE2 production in control and LPS-stimulated microglia. BV2 cells were pretreated with different concentrations (0–20 µM) of NJK14047 and SB203580 for 2 h, then cells were treated with LPS (500 ng/ml) for 22 h. Culture supernatants were collected and productions of NO (a) and PGE2 (b) were measured (n = 4–6 per group). c, d Inhibition of LPS-mediated iNOS and COX-2 expression by NJK14047. BV2 cells were pretreated with different concentrations (0–20 µM) of NJK14047 for 2 h, then cells were treated with LPS (500 ng/ml) for 22 h. The levels of iNOS and COX-2 were measured by immunoblotting. c Representative immunoblotting of iNOS and COX-2 in whole cell lysates. d Quantification of iNOS (n = 3 per group) and COX-2 levels (n = 3–6 per group). All experiments were repeated in three times independently. (##P < 0.01 and ###P < 0.001 vs. control; *P < 0.05, **P < 0.01 and ***P < 0.001 vs. LPS; †P < 0.05, ††P < 0.01 and †††P < 0.001 vs. SB. All error bars indicate SEM)
The production of NO and PGE2 in microglia are primarily regulated by the iNOS and COX-2 enzymes. To determine whether the suppression of NO and PGE2 by NJK14047 is associated with the modulation of iNOS and COX-2, we analyzed their expression levels by performing Western blotting of LPS-stimulated BV2 cells. Compared with control cells, the exposure of BV2 cells to LPS for 24 h led to the induction of iNOS and COX-2 expression (Fig. 2c, d). NJK14047 pretreatment significantly suppressed LPS-induced iNOS and COX-2 expression in BV2 cells (Fig. 2c, d). These results indicate that the NJK14047-mediated downregulation of NO and PGE2 in LPS-treated BV2 cells is due to the reduction in the expression levels of iNOS and COX-2 enzymes.
NJK14047 Reduces the Expression of Proinflammatory Cytokines in LPS-Stimulated Microglia
Activated microglia are known to be a major source of inflammatory cytokines, such as TNF-α, IL-1β and IL-6, which can be upregulated and detected in various acute and chronic inflammatory diseases [5]. Therefore, we examined the effect of NJK14047 on the expression levels of proinflammatory cytokines in LPS-stimulated microglia. The treatment of BV2 cells with LPS led to a remarkable increase in TNF-α, IL-1β and IL-6 expression (Fig. 3a–c) However, NJK14047 pretreatment reduced the expression levels of TNF-α, IL-1β and IL-6 in LPS-induced BV2 microglia in a dose-dependent manner (Fig. 3a–c). In a parallel experiment, we analyzed the effects of NJK14047 on TNF-α, IL-1β and IL-6 production following LPS treatment. Cells were pretreated with NJK14047 for 2 h and exposed to LPS for an additional 22 h. TNF-α, IL-1β and IL-6 proteins significantly increased in the culture medium of LPS-stimulated BV2 cells. However, pretreatment with NJK14047 resulted in a significant decrease in a concentration-dependent manner (Fig. 3d–f). Collectively, these results suggest that NJK14047 clearly suppresses LPS-triggered inflammatory cascades. Given that sequential inflammatory cascades are mainly involved with p38 MAPK activation, these results are likely due to direct p38 MAPK inhibition by the novel and selective p38 MAPK inhibitor [16].
NJK14047 reduces expression of pro-inflammatory cytokines in LPS-stimulated microglia. Inhibitory effects of NJK14047 on LPS-stimulated microglia. BV2 cells were pretreated with different concentrations (0–20 µM) of NJK14047 for 2 h, then cells were treated with LPS (500 ng/ml) for 22 h. Total mRNA was harvested and mRNA levels of TNF-α (a), IL-1β (b) and IL-6 (c) were measured by qRT-PCR (n = 4–6 per group). GAPDH was used as an internal control. Protein levels of TNF-α (d), IL-1β (e) and IL-6 (f) were determined in the medium (n = 5–6 per group). All experiments were repeated in three times independently. (##P < 0.01 and ###P < 0.001 vs. control; *P < 0.05, **P < 0.01 and ***P < 0.001 vs. LPS; All error bars indicate SEM)
NJK14047 Ameliorates LPS-Induced Neuroinflammation in the Brain of Mice
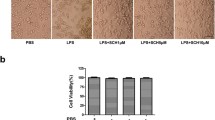
To confirm whether NJK14047 could ameliorate neuroinflammation in vivo, we used LPS-injected mice, a well-known neuroinflammation model showing microglial activation [28,29,30]. We orally administrated NJK14047 four days prior to the i.p. injection of LPS (Fig. 4a). To examine microglial activation induced by LPS, we first performed immunofluorescence analysis using Iba-1 antibody on sections taken 24 h after LPS injection. As expected, compared with control mice, % area of Iba-1 positive cells was markedly increased in LPS-treated mice. Compared with LPS injection, NJK14047 administration dramatically reduced % area of Iba-1 positive cells (Fig. 4b, c). In addition, immunoblotting analysis showed that LPS injection markedly elevated iNOS and COX-2 expression levels in the cortex of mice. However, NJK14047 effectively lowered these expression levels (Fig. 4d, e). In accordance with these results, ELISA analysis also revealed that NJK14047 could attenuate TNF-α, IL-β and IL-6 cytokines in the brain of LPS-injected mice model (Fig. 4f). Taken together, these results suggest that treatment with NJK14047 suppress microglial activation in the brains of mice, thereby reducing neuroinflammation.
NJK14047 reduces LPS-induced neuroinflammation in the brain of mice. a Experiment protocol. Mice were pretreated with NJK14047 at 5 mg/kg once daily for 4 consecutive days. LPS was injected at 5 mg/kg on the fourth day. Analysis was done at 24 h after LPS injection. Representative image (b) and quantification (c) of Iba-1 in the cortex of LPS-induced neuroinflammation mice model (four cortex areas per section and four sections per mouse; n = 3–4 per each group; Scale bar = 50 µm). d, e Inhibition of LPS-mediated iNOS and COX-2 expression by NJK14047 in the mice brains. d Representative immunoblotting of iNOS and COX-2 in samples derived from mice brains. e Quantification of iNOS and COX-2 levels (n = 3–4 per group). f Cytokine levels of TNF-α, IL-1β and IL-6 were assessed by ELISA. Brains were excised 6 h after LPS injection and proteins from the cortexes were used for ELISA. (n = 3–4 per group) (#P < 0.05 and ###P < 0.001 vs. control; *P < 0.05 and **P < 0.01 vs. LPS; All error bars indicate SEM)
Discussion
In the present study, we showed for the first time that NJK14047, a novel and selective p38 inhibitor, suppressed microglial cell activation and reduced the secretion of proinflammatory cytokines such as TNF-α, IL-1β and IL-6 through the inhibition of p38 MAPK activation in BV2 microglia. In addition, we confirmed the anti-inflammatory effect of NJK14047 in an LPS-induced neuroinflammation mouse model.
Although many researchers have attempted to develop p38 MAPK inhibitors, their development for clinical use remains challenging, partly due to kinase selectivity issues. In an effort to develop novel and selective p38 MAPK inhibitors, we recently reported several novel biphenyl amides and showed that some could significantly inhibit p38 MAPK activities in an enzyme assay [23]. Among them, NJK14047 displayed potent and selective p38 MAPK inhibitory effects on macrophages [16]. In the present study, we demonstrated that NJK14047 has anti-inflammatory effects on LPS-stimulated microglia and in an LPS-induced neuroinflammation mouse model, which were the result of the downregulation of p38 MAPK phosphorylation.
NO and PGE2 are key inflammatory and neurotoxic mediators released from activated microglia [31, 32]. The overproduction of these mediators may contribute to the pathogenesis of neurodegenerative diseases. In vivo and in vitro studies have shown that the enhanced production of NO and PGE2 due to increased iNOS and COX-2 expression, respectively, contribute to CNS diseases [31, 33]. We showed that NJK14047 could inhibit the production of NO and PGE2 in LPS-stimulated microglia. Moreover, NJK14047 had more potency in NO inhibition than SB203580, one of representative p38 MAPK inhibitors. We confirmed in vitro and in vivo that these inhibitory effects of NJK14047 were due to the suppression of iNOS and COX-2 expression. Thus, these results indicate the anti-inflammatory effects of NJK14047 and suggest that NJK14047 can be utilized for reducing neuroinflammation in various CNS diseases.
Neuroinflammation is further mediated by the secretion of proinflammatory cytokines such as TNF-α, IL-1β and IL-6 derived from microglia. These cytokines are associated with worse final outcomes from brain injury [34]. In the present study, we demonstrated the ability of NJK14047 to reduce the expression of TNF-α, IL-1β and IL-6 in LPS-treated microglia. The anti-neuroinflammatory effects of NJK14047 we confirmed in the mouse experiments might suggest that the compound can penetrate the blood–brain barrier (BBB). Because NJK14047 is a small molecule with a low molecular weight (MW = 445.515) and has appropriate physicochemical properties including lipophilicity (cLogP: 3.12), it might be expected to cross the BBB and penetrate the CNS even though it has a slightly polar diol moiety. Similarly, fingolimod (Gilenya®, Novartis), a sphingosine-1-phosphate receptor modulator possessing a propane diol moiety, has been shown to cross the BBB and attenuate multiple sclerosis, which suggests that other diol compounds might do so as well. Taken together, our results showing the NJK14047-mediated inhibition of proinflammatory cytokines further support the possible use of NJK14047 as an anti-inflammatory agent.
In conclusion, our observations demonstrate the anti-inflammatory effects of NJK14047 in LPS-induced neuroinflammation. NJK14047 mitigated LPS-induced inflammatory mediators in activated microglia, which was associated with a downregulation of the p38 MAPK pathway. Moreover, the increased proinflammatory cytokine productions from LPS-stimulated microglia were diminished by NJK14047. One of the limitations of this study is that technically we showed a protective effect of NJK14047 against LPS-induced neuroinflammation. Because NJK14047 treatment was followed by LPS-stimulus. Another limitation is that there were differences in inflammatory conditions between our experimental models and human patients. Therefore, the anti-inflammatory effect of NJK14047 against existing neuroinflammation and the question whether similar effects by NJK14047 occur in human remains to be explored. From a translational perspective, our results suggest that NJK14047 is an anti-inflammatory agent that can be used to ameliorate microglia-mediated neuroinflammatory disease.
References
Biber K, Moller T, Boddeke E, Prinz M (2016) Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat Rev Drug Discov 15:110–124
Cunningham CL, Martinez-Cerdeno V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33:4216–4233
Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T (2013) Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16:543–551
Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17:400–406
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Kaminska B (2005) MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754:253–262
Witcher KG, Eiferman DS, Godbout JP (2015) Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci 38:609–620
Norden DM, Godbout JP (2013) Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 39:19–34
Kim EK, Choi EJ (2015) Compromised MAPK signaling in human diseases: an update. Arch Toxicol 89:867–882
Perry VH, Holmes C (2014) Microglial priming in neurodegenerative disease. Nat Rev Neurol 10:217–224
Bodea LG, Wang Y, Linnartz-Gerlach B, Kopatz J, Sinkkonen L, Musgrove R, Kaoma T, Muller A, Vallar L, Di Monte DA, Balling R, Neumann H (2014) Neurodegeneration by activation of the microglial complement-phagosome pathway. J Neurosci 34:8546–8556
Fan K, Li D, Zhang Y, Han C, Liang J, Hou C, Xiao H, Ikenaka K, Ma J (2015) The induction of neuronal death by up-regulated microglial cathepsin H in LPS-induced neuroinflammation. J Neuroinflammation 12:54
Henry CJ, Huang Y, Wynne AM, Godbout JP (2009) Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun 23:309–317
Dutta G, Zhang P, Liu B (2008) The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundam Clin Pharmacol 22:453–464
Kaneko YS, Mori K, Nakashima A, Sawada M, Nagatsu I, Ota A (2005) Peripheral injection of lipopolysaccharide enhances expression of inflammatory cytokines in murine locus coeruleus: possible role of increased norepinephrine turnover. J Neurochem 94:393–404
Lund S, Christensen KV, Hedtjarn M, Mortensen AL, Hagberg H, Falsig J, Hasseldam H, Schrattenholz A, Porzgen P, Leist M (2006) The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol 180:71–87
Pearson G, Robinson F, Gibson BT, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183
Escos A, Risco A, Alsina-Beauchamp D, Cuenda A (2016) p38gamma and p38delta mitogen activated protein kinases (MAPKs), new stars in the MAPK galaxy. Front Cell Dev Biol 4:31
Yokota T, Wang Y (2016) p38 MAP kinases in the heart. Gene 575:369–376
Arthur JS, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13:679–692
Denise Martin E, De Nicola GF, Marber MS (2012) New therapeutic targets in cardiology: p38 alpha mitogen-activated protein kinase for ischemic heart disease. Circulation 126:357–368
Lee JK, Kim NJ (2017) Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules 22(8):E1287
Heo J, Shin H, Lee J, Kim T, Inn KS, Kim NJ (2015) Synthesis and biological evaluation of N-cyclopropylbenzamide-benzophenone hybrids as novel and selective p38 mitogen activated protein kinase (MAPK) inhibitors. Bioorganic Med Chem Lett 25:3694–3698
Choi MS, Heo J, Yi CM, Ban J, Lee NJ, Lee NR, Kim SW, Kim NJ, Inn KS (2016) A novel p38 mitogen activated protein kinase (MAPK) specific inhibitor suppresses respiratory syncytial virus and influenza A virus replication by inhibiting virus-induced p38 MAPK activation. Biochem Biophys Res Commun 477:311–316
Hwang DS, Gu PS, Kim N, Jang YP, Oh MS (2018) Effects of Rhei Undulati Rhizoma on lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Environ Toxicol 33:23–31
Lee JK, Jin HK, Bae JS (2010) Bone marrow-derived mesenchymal stem cells attenuate amyloid beta-induced memory impairment and apoptosis by inhibiting neuronal cell death. Curr Alzheimer Res 7:540–548
Kim SY, Kim H, Kim SW, Lee NR, Yi CM, Heo J, Kim BJ, Kim NJ, Inn KS (2017) An effective antiviral approach targeting hepatitis B virus with NJK14047, a novel and selective biphenyl amide p38 mitogen-activated protein kinase inhibitor. Antimicrob Agents Chemother 61(8):e00214–e00217
Wang J, Chen L, Liang Z, Li Y, Yuan F, Liu J, Tian Y, Hao Z, Zhou F, Liu X, Cao Y, Zheng Y, Li Q (2017) Genipin inhibits LPS-induced inflammatory response in BV2 microglial cells. Neurochem Res 42:2769–2776
Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM (2011) Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacol 36:857–870
Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP (2008) Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation 5:15
Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. J Clin Investig 122:1164–1171
Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149:2736–2741
Chinta SJ, Ganesan A, Reis-Rodrigues P, Lithgow GJ, Andersen JK (2013) Anti-inflammatory role of the isoflavone diadzein in lipopolysaccharide-stimulated microglia: implications for Parkinson’s disease. Neurotox Res 23:145–153
Kim SH, Smith CJ, Van Eldik LJ (2004) Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1 beta production. Neurobiol Aging 25:431–439
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), which was funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1A5A2014768 and NRF-2016R1A2B4015169).
Author information
Authors and Affiliations
Contributions
MSG and SYK performed the experiments, analyzed the data, and prepared the manuscript. NK and SJL performed animal and HPLC experiment, respectively. MSO, HKJ, JSB, KSI, NJK and JKL interpreted the data and reviewed the paper. NJK and JKL designed the study and wrote the manuscript. All authors discussed results and commented on the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gee, M.S., Kim, SW., Kim, N. et al. A Novel and Selective p38 Mitogen-Activated Protein Kinase Inhibitor Attenuates LPS-Induced Neuroinflammation in BV2 Microglia and a Mouse Model. Neurochem Res 43, 2362–2371 (2018). https://doi.org/10.1007/s11064-018-2661-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2661-1