Abstract
Abnormal c-Src expression and activation has been observed in a number of tumors. To determine the therapeutic potential of Src inhibitors for ovarian cancer patients, this study aimed to explore the expression patterns of c-Src and phospho-Src in epithelial ovarian cancer. A total of 82 patients with epithelial ovarian cancer treated at Sun Yat-sen University Cancer Center from January 1999 to December 2005 were enrolled along with 25 patients with benign ovarian lesions; 20 normal ovarian tissues served as controls. Expression of c-Src and phospho-Src (Tyr416) was examined using immunohistochemistry. Survival analyses were performed using Kaplan–Meier curves. As compared to the control group, a significantly greater proportion of ovarian cancer tissues were positive for c-Src and phospho-Src expression (P < 0.001). c-Src expression was associated with age, while phospho-Src expression was significantly associated with age, FIGO stage, histology grade, and residual tumor size after surgery (all P < 0.05). The mean survival time was associated with phospho-Src expression, but not with c-Src expression. The mean survival times of patients with phospho-Src-positive tumors were significantly greater than those with phospho-Src-negative tumors (87.4 months, 95 % CI = 74.3–100.5 months and 91.5 months, 95 % CI = 84.7–98.2 months, respectively; P = 0.013). The increased c-Src expression and activation in epithelial ovarian cancer suggests that ovarian cancer patients may benefit from tyrosine kinase inhibitors such as Dasatinib. Activation of c-Src through phosphorylation at Tyr416 may play a role in the early stages of ovarian cancer development, and evaluation of its expression may be a useful prognostic marker of epithelial ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mortality rate of ovarian cancer is among the highest of malignant gynecological tumors; standard treatment methods include resection, followed by platinum-based chemotherapy. Although the majority of tumors are initially responsive, most patients eventually relapse with a median progression-free survival of 18 months [1]. Therefore, identification of new treatment options along with early identification is crucial for improving the clinical outcomes for these patients. For example, bevacizumab, a neutralizing monoclonal antibody specific for vascular endothelial growth factor (VEGF), increased progression-free survival in ovarian cancer patients [2]. In addition, analysis of the efficacy of small molecule inhibitors of Bcr-Abl and Scr family kinases, including dasatinib, are presently underway [3–5].
Upregulation of c-Src, a non-receptor protein tyrosine kinase, in cancer has been widely reported [6–8]. Furthermore, in tumor cells harboring activated forms of Src, phosphorylation of numerous c-Src substrates, which have been linked to tumorigenicity and metastasis, has been reported [9]. Src activity is regulated by tyrosine phosphorylation at two sites with opposing effects. Phosphorylation of tyrosine 416 (Tyr416) in the activation loop of the kinase domain upregulates the c-Src activity, and phosphorylation of tyrosine 527 (Tyr527) in the carboxyl-terminal tail reduces enzyme activity [10]. Although Tyr527 is phosphorylated in normal cells, the phosphorylation status of c-Src has not been determined in ovarian cancer.
To evaluate the possible use of Src kinase inhibitors, such as Dasatinib, for ovarian cancer, the expression of c-Src and its phosphorylation status must be assessed. In this study, the expression of c-Src and phospho-Src (Tyr416) was assessed in ovarian tissue derived from patients with epithelial ovarian cancer, benign ovarian lesions, and normal tissue using immunohistochemistry. Correlation between c-Src and phospho-Src expression and clinicopathological parameters, as well as the prognostic significance of c-Src and phospho-Src expression was evaluated. Determination of phosphorylated c-Src expression in ovarian cancer may form the basis for future studies to evaluate its therapeutic and/or prognostic significance.
Patients and methods
Patients and controls
This study was approved by the Institutional Review Board of Sun Yat-sen University, and all enrolled patients signed written informed consents. A total of 82 patients (mean 47.5 years, range 18–67 years) with epithelial ovarian cancer treated with tumor resection at the Cancer Center of Sun Yat-sen University from January 1999 to December 2005 were enrolled in this study. The inclusion criteria for these patients included the following: (1) pathological diagnosis and initial treatment at the Cancer Center of Sun Yat-sen University; (2) no adjuvant chemotherapy or radiotherapy prior to the surgery; (3) availability of detailed clinical and follow-up data; and (4) the absence of other tumors. In addition, 25 patients with benign ovarian lesions and 20 patients with normal ovarian tissue were enrolled during the same period as controls (mean 36 years, range 17–80 years). Of the patients with benign lesions, pathological diagnosis revealed 15 cases of simple ovarian cyst, 2 cases of corpus luteum cyst, 2 cases of inclusion cyst, 4 cases of follicular cyst, and 2 cases of chronic inflammation. Normal ovarian tissue was obtained from women following surgery for reasons other than ovarian pathology; the presence of normal ovarian epidermis was confirmed upon pathological examination. FIGO staging was performed as described in Heintz et al. [11].
Follow-up was undertaken by contacting the patients by telephone and was completed by October 15, 2010. The frequencies and treatment regimens used in the ovarian cancer group stratified by c-Src and phosphor-Src expression groups were summarized in the supplementary Table.
Immunohistochemistry
Immunohistochemistry analysis was carried out using the PV-9000 kit that enhances sensitivity 3–4-fold (Zhongshan Goldenbridge Biotechnology, Beijing, China). Formalin-fixed, paraffin-embedded archived tissues were cut into 4 μm sections after which they were dewaxed and rehydrated, and endogenous peroxidase was blocked with hydrogen peroxide. Antigen retrieval was undertaken by boiling the sections in a microwave in 10 mM citrate buffer (pH 6.0). After nonspecific antibody binding was blocked with 5 % goat serum, sections were incubated with a rabbit anti-Src antibody (#2108, Cell Signaling Technology, Danvers, MA, USA; dilution of 1:60) or rabbit anti-phospho-Src (Tyr416) antibody (#2101, Cell Signaling Technology dilution of 1:40) overnight at 4 °C, followed by horseradish peroxidase-labeled secondary antibody (Cell Signaling Technology) for 30 min at room temperature. The sections were developed with diaminobenzidine tetrahydrochloride (DAB, Zhongshan Goldenbridge Biotechnology) and counterstained with hematoxylin. The primary antibody was replaced with PBS in the negative control.
A double-blind method was used to evaluate the staining intensity. Two pathologists independently selected ten fields stochastically under a high power objective, and the percentage of positive cells was determined. The results of each pathologist were averaged. The following nomenclature was used to denote the percentage of positive cells: <5 % was classified as (−), 5–30 % were (+), 31–55 % were (++), 56–80 % were (+++), and >80 % were (++++). For statistical analysis, (+) to (++++) were considered positive and (−) was considered negative.
Statistical analysis
Subjects’ age and the expression rates of c-Src and phospho-Src were summarized as n (%). Differences in subjects’ age and the expression rates of c-Src and Phospho-Src between groups were compared using Pearson’s Chi square test or Fisher’s exact test. Comparisons of the expression rates of c-Src and phospho-Src with clinical characteristics were performed using non-parametric methods, including Mann–Whitney U or Kruskall–Wallis tests. Furthermore, Log-rank tests were used to examine the association of survival time with clinical characteristics. Cumulative survival rates were analyzed using Kaplan–Meier analysis. All statistical assessments were considered significant when P < 0.05. Statistical analyses were performed using PASW 18.0 statistics software (SPSS Inc, Chicago, IL, USA).
Results
c-Src and phospho-Src expression in ovarian cancer
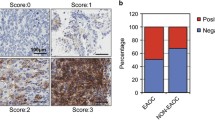
A total of 127 participants, including 45 in the control group and 82 in the ovarian cancer group, were enrolled. A significantly greater percentage of participants in the ovarian cancer group was >40 years as compared to the control group (P = 0.007; Table 1). As determined by immunohistochemistry analysis, c-Src and phospho-Src (Tyr416) expression was observed within the cell membrane and the cytoplasm (Fig. 1). As shown in Table 1, significant differences in c-Src and phospho-Src expression were observed between the groups; a significantly greater proportion of patients in the ovarian cancer group had positive c-Src and phospho-Src expression (P < 0.001).
c-Src and phospho-Src expression in ovarian cancer. c-Src and phospho-Src expression was assessed using immunohistochemistry. Representative results of anti-c-Src staining in ovarian cancer (A) and follicular cyst (B) were shown. Representative results of anti-phospho-Src (Tyr416) staining in ovarian cancer (C) and germinal ovarian epithelium (D) were shown. Scale bar 100 μm
Association of c-Src and phospho-Src expression with clinical characteristics
The association of c-Src and phospho-Src (Tyr416) expression with the clinical characteristics in 82 patients with ovarian cancer was next evaluated. Whereas c-Src expression was significantly associated with increased age (P = 0.027), phospho-Src expression was significantly associated with increased age, FIGO stage, histology grade, and residual tumor size after surgery (P = 0.002, 0.044, <0.001, and 0.032, respectively; Table 2).
Association of c-Src and phospho-Src expression with patient survival
During the follow-up period, 23 patients (28 %) died, and the mean survival time during the study period was 95.7 months (95 % CI = 84.9–106.5 months). The estimated 6-month, 1, 3, and 5-year survival rates were 97.6, 92.7, 81.3, and 67.4 %, respectively. The overall survival (OS) was significantly associated with FIGO stage II (HR = 6.92, 95 % CI = 2.03–23.66, P = 0.002) and residual tumor size (HR = 5.25, 95 % CI = 2.26–12.23, P < 0.001; data not shown).
As shown in Fig. 2, the mean survival time was associated with phospho-Src levels, but not c-Src levels. The mean survival times of patients with c-Src-negative and positive tumors were 61.5 months (95 % CI = 0–125.9 months) and 96.2 months (95 % CI = 85.3–107.1 months), respectively (P = 0.458). However, the mean survival times of patients with phospho-Src-negative tumors were 87.4 months (95 % CI = 74.3–100.5 months) while those with phospho-Src-positive tumors were 91.5 months (95 % CI = 84.7–98.2 months) (P = 0.013).
Discussion
Since c-Src is aberrantly expressed and/or activated in a number of tumors [4–6], this study sought to investigate the expression and activation (i.e., phosphorylation at Tyr416) of c-Src in epithelial ovarian cancer. As compared to tissues in the control group, a significantly greater proportion of ovarian cancer tissues expressed c-Src and phospho-Src. Phospho-Src expression was significantly associated with increased age, FIGO stage, histology grade, and residual tumor size after surgery and was associated with the mean survival time. Individuals with phospho-Src-positive tumors had significantly greater mean survival time as compared to those whose tumors were negative.
c-Src is an oncogene that plays a critical role in cell growth and differentiation [10]. Increased expression and activation of c-Src protein has been observed in lung, breast, pancreatic, esophageal, head and neck, and stomach cancers [6–8, 10, 12–15]. In colon cancer, c-Src expression was significantly increased as compared with the surrounding normal tissues, which led to the hypothesis that activation of c-Src was related to colonic carcinogenesis [16, 17]. This hypothesis was supported by work in an in vivo model in which tumor growth was inhibited through suppressing c-Src expression [18].In addition, silencing of Src increased docetaxel cytotoxicity and reduced tumor growth and microvessel density in both SKOV3ip1 and HeyA8 cells [18, 19]. Inhibition of Src restored paclitaxel sensitivity to resistant ovarian cancer cells [20], possibly through increasing apoptosis, autophagy, and microtubule stability [21]. In tumors derived from the mouse ovarian cancer cell line, ID8 cells, positive expression of c-Src and phospho-Src (Tyr418) was observed while remaining were absent in the normal ovarian tissues [22]. Furthermore, increased expression and activation (i.e., dephosphorylation at Tyr530) of c-Src in ovarian cancer tissues has been reported [23], which is consistent with the increased c-Src and phospho-Src (Tyr416) expression observed in ovarian cancer patients in the present study.
Although significantly more ovarian cancer tissues were positive for phospho-Src (Tyr416) as compared to control tissues, the positive rate was only 23.2 % in the present study. It is possible that phosphorylation at Tyr416 occurs only at a certain stage of ovarian cancer progression, which is supported by Wiener et al. [23]. Of the nine cases of advanced ovarian cancer, activated c-Src as detected by dephosphorylation at Tyr530 was observed in seven [23]. Thus, activation of c-Src by tyrosine phosphorylation at Tyr416 and dephosphorylation at Tyr527 may occur at different stages of ovarian cancer. Further larger studies will be undertaken to explore this possibility.
The present study was the first to assess the possible correlation of c-Src and phospho-Src with patient clinicopathological characteristics and evaluate their prognostic significance. The expression of c-Src was not significantly related to pathological type, histology grade, and clinical stage of ovarian cancer; however, its expression was significantly greater in patients >40 years of age. The relevance of c-Src and the clinicopathological characteristics may be underestimated due to the limited number of cases analyzed, requiring further larger studies. Although c-Src was not correlated with most patient characteristics analyzed, phospho-Src (Tyr416) was related to clinical stage, histology grade, and size of residual tumor following surgery. The percentage of early stage tumors positive for phospho-Src expression was greater than that observed for late-stage tumors, suggesting that c-Src phosphorylation at Tyr416 may occur early in ovarian cancer development. In addition, a greater percentage of moderate to well-differentiated tumors expressed phospho-Src (Tyr416). These results are consistent with Weber et al. [24] in which the highest levels of c-Src activity in human colon tumors were observed in moderately-to-well-differentiated tumors. In contrast, Src kinase activation was not observed in the endometrial cancer progression [25]. Although it remains possible that activation of c-Src by Tyr416 phosphorylation may promote cell differentiation as well as cell survival, proliferation, and metastasis [23], the influence of c-Src overexpression and/or activation on ovarian tumor cell growth and differentiation requires further analysis.
In the present study, the 5-year OS rate was higher in patients with phospho-Src-positive tumors as compared to those whose tumors were negative, suggesting that c-Src protein phosphorylation at Tyr416 may be related to better prognosis in ovarian cancer patients. These results are inconsistent with those reported for tongue cancer in which an inverse correlation between phospho-Src (Tyr416) expression and patient prognosis was observed [26]. Although the effects of phospho-Src (Tyr416) expression may be cancer type-specific, further larger studies are necessary to determine the prognostic value of examining phospho-Src (Tyr416) in ovarian cancer.
In summary, activation of c-Src protein through phosphorylation at Tyr416 may play a role in the early stages of ovarian cancer development. Since phospho-Src (Tyr416) expression was related to better survival in ovarian patients, it might represent a new prognostic marker for ovarian cancer. Furthermore, the results of the present study offer a theoretical basis for the use of c-Src inhibitors, including Dasatinib, for treating ovarian cancer.
Abbreviations
- VEGF:
-
Vascular endothelial growth factor
- DAB:
-
Diaminobenzidine tetrahydrochloride
- FIGO:
-
The International Federation of Gynecology and Obstetrics
References
Rubin SC, Randall TC, Armstrong KA et al (1999) Ten-year followup of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol 93:21–24
Kim A, Ueda Y, Naka T, Enomoto T (2012) Therapeutic strategies in epithelial ovarian cancer. J Exp Clin Cancer Res 31:14–22
Jinawath N, Vasoontara C, Jinawath A et al (2010) Oncoproteomic analysis reveals co-upregulation of RELA and STAT5 in carboplatin resistant ovarian carcinoma. PLoS ONE 5:e11198
Secord AA, Teoh DK, Barry WT et al (2012) A phase I trial of Dasatinib, an SRC-family kinase inhibitor, in combination with Paclitaxel and Carboplatin in patients with advanced or recurrent ovarian cancer. Clin Cancer Res 18:5489–5498
Schilder RJ, Brady WE, Lankes HA et al (2012) Phase II evaluation of Dasatinib in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 127:70–74
Egan C, Pang A, Durda D et al (1999) Activation of Src in human breast tumor cell lines: elevated levels of phosphotyrosine phosphatase activity that preferentially recognizes the Src carboxy terminal negative regulatory tyrosine 530. Oncogene 18:1227–1237
Mazurenko NN, Kogan EA, Zborovskaya IB et al (1992) Expression of pp 60c-src in human small cell and non-small cell lung carcinomas. Eur J Cancer 28:372–377
Lutz MP, Esser IB, Flossmann-Kast BB et al (1998) Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun 243:503–508
Brown MT, Cooper JA (1996) Regulation, substrates and functions of src. Biochim Biophys Acta 1287:121–149
Xu W, Allbritton N, Lawrence DS (2012) SRC kinase regulation in progressively invasive cancer. PLoS ONE 7:e48867
Heintz APM, Odicino F, Maisonneuve P et al (2006) Carcinoma of the ovary. Int J Gynecol Obstet 95:S161–S192
Verbeek BS, Vroom TM, Adriaansen-Slot SS et al (1996) c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J Pathol 180:383–388
Kumble S, Omary MB, Cartwright CA, Triadafilopoulos G (1997) Src activation in malignant and premalignant epithelia of Barrett’s esophagus. Gastroenterology 112:348–356
Irby RB, Yeatman TJ (2000) Role of Src expression and activation in human cancer. Oncogene 19:5636–5642
Frame MC, Fincham VJ, Carragher NO et al (2002) v-Src’s hold overactin and cell adhesions. Nature Rev Mol Cell Biol 3:233–245
Talamonti MS, Roh MS, Curley SA et al (1993) Increase in activity and level of pp 60c-src in progressive stages of human colorectal cancer. J Clin Investig 91:53–60
Peng Z, Raufman JP, Xie G (2012) Src-mediated cross-talk between farnesoid x and epidermal growth factor receptors inhibits human intestinal cell proliferation and tumorigenesis. PLoS ONE 7:e48461
Han LY, Landen CN, Travino JG et al (2006) Antiangiogenic and antitumor effects of SRC inhibition in ovarian carcinoma. Cancer Res 66:8633
Kim HS, Han HD, Armaiz-Pena GN et al (2011) Functional roles of Src and Fgr in ovarian carcinoma. Clin Cancer Res 17:1713–1721
Le XF, Bast RC Jr (2011) Src family kinases and paclitaxel sensitivity. Cancer Biol Ther 12:260–269
Kong L, Deng Z, Zhao Y et al (2011) Down-regulation of phospho-non-receptor Src tyrosine kinases contributes to growth inhibition of cervical cancer cells. Med Oncol 28:1495–1506
Pengetnze Y, Steed M, Roby KF et al (2003) Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem Biophys Res Commun 309:377–383
Wiener JR, Windham C, Veronica C et al (2003) Activated src protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol 88:73–79
Weber TK, Steele G, Summerhayes IC (1992) Differential pp 60c-src activity in well and poorly differentiated human colon carcinomas and cell lines. J Clin Investig 90:815–821
Desouki MM, Rowan BG (2004) Src kinase and mitogen-activated protein kinases in the progression from normal to malignant endometrium. Clin Cancer Res 10:546–555
Ben-Izhak O, Cohen-Kaplan V, Nagler RM (2010) The prognostic role of phospho-Src family kinase analysis in tongue cancer. J Cancer Res Clin Oncol 136:27–34
Acknowledgments
This study was funded by research grants from the Technology Project of Guangdong Province (No. 2009B030801021). The study sponsors had no role in the study.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Article note
Yong-Wen Huang and Chen Chen contributed equally to this work as co-first authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, YW., Chen, C., Xu, MM. et al. Expression of c-Src and phospho-Src in epithelial ovarian carcinoma. Mol Cell Biochem 376, 73–79 (2013). https://doi.org/10.1007/s11010-012-1550-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1550-1