Abstract
Background
The up regulation of Phospho-Src family kinase oncogene has been correlated with reduced post-operative survival in various cancers but never in tongue cancer.
Methods
We analyzed phospho-Src family kinase in 39 tongue (mobile) cancer patients by immunohistochemistry, compared these results with similar analysis for TUNEL and c-erbB-2 and with both clinical tumor characteristics and patient survival probability rates.
Results
Phospho-Src family kinase overexpression was found in most tongue cancer biopsies (62%), significantly correlating with tumors larger in size (P = 0.05), progression—lymph node metastasis (0.004) and stage (P = 0.05), and correlating with TUNEL (P = 0.01) and c-erbB-2 (P = 0.05) expression rates. At 60 months, survival probability for negative phospho-Src family kinase level (=0) patients was 67%, but 30% for positive phospho-Src family kinase level (>0) patients (P = 0.05).
Conclusions
Inverse correlation between phospho-Src family kinase and patient survival demonstrates the prognostic role of phospho-Src family kinase in tongue cancer. These findings suggest a novel link between phospho-Src family kinase and TUNEL and c-erbB-2 pathways, tilting the balance toward cell proliferation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 300,000 new cases of oral squamous cell carcinoma (SCC) are diagnosed annually worldwide. This aggressive epithelial malignancy is associated with high mortality and severe morbidity among the long-term survivors. The 5-year mortality rate is approximately 50% (Alho et al. 1999; Kantola et al. 2000; Myers et al. 2000) and the poor survival index has not changed significantly in the past half-century (Muir and Weiland 1995; Yeole et al. 2000; Ribeiro et al. 2000). Of those patients who survive, therapy frequently results in significant physical mutilation and compromised speech, taste, mastication and swallowing (Jackson et al. 1999). Improvement of patient survival and reduction of the therapeutic-related morbidity require a better understanding of the biological nature of the disease. Currently available diagnostic tools based on stage and grade rates are sometimes crude and often miss evaluating the true carcinogenic nature and aggressiveness of the tumor. Improved understanding is also expected to facilitate the development of novel and more efficient treatment modalities for this lethal disease (Nagler et al. 1999, 2006; Bahar et al. 2007). Thus, we have previously studied various biological aspects of salivary gland and tongue tumors (Ben-Izhak et al. 2005, 2006, 2007), examining an array of tumor markers that are part of pathways related to tumorogenicity, proliferation and apoptosis though until now we have not investigated the well-known oncogene phospho-Src family kinase. Up-regulation of phospho-Src family kinase, a nonreceptor tyrosine kinase, has been documented in numerous tumors including those derived from the colon, liver, lung and breast (Warmuth et al. 2003; Martin 2004; Ishizawar and Parsons 2004; Dehm and Bonham 2004; McCarty and Block 2005; Alper and Bowden 2005; Chong et al. 2005; Alvarez et al. 2006). The relationship between phospho-Src family kinase activation and cancer progression is significant, since phospho-Src family kinase has been found to be a critical component of multiple signaling pathways that regulate proliferation, survival, metastasis and angiogenesis. Furthermore, aberrant phospho-Src family kinase activation has been identified as one of the molecular alterations involved in human pancreatic carcinogenesis. It has been postulated that phospho-Src family kinase may induce transformation by causing the overexpression of the insulin-like growth factor-1 receptor (IGF-1R) in pancreatic tumor cell lines and indeed, significantly higher coexpression of both these molecules was found in human pancreatic ductal adenocarcinomas, suggesting an important role in transformation of pancreatic ductal cells (Hakam et al. 2003). In this respect it is worth mentioning that we have recently reported that the concentrations of salivary IGF was significantly higher by 117% (P = 0.03) in oral cancer patients when compared with healthy controls (Shpitzer et al. 2007).
Surprisingly phospho-Src family kinase examination in human oral SCC lesions has never been documented in the professional literature, though it was investigated once in an animal model and once in a cellular model of oral SCC (Tamura et al. 2003; Conway et al. 2006).
The purpose of the current study was to correlate phospho-Src family kinase level of expression with the expression of other cancer-related markers: TUNEL and c-erbB-2, which are related to tumorogenicity, proliferation and apoptosis, as well as with the “traditional” clinical characteristics of tongue cancer and with the survival probability rates. Such an examination performed concurrently in the same cohort of tongue tumors has never been documented, and is expected to shed further light on the biological pathways related with tongue SCC, hopefully contributing to the uncovering of new prognostic tools for this lethal cancer.
Patients and methods
Experimental design
This study included 39 patients (20 males and 19 females ages 64 ± 15 years) with tongue cancer (located at the lateral border of the oral—“mobile” tongue) whose archival paraffin-embedded pathological material was available for immunohistochemical analysis at the Department of Pathology of Rambam Health Care Campus, Haifa, Israel. Three of the patients were diagnosed with stage 1 disease, 16 patients with stage 2 disease and 8 and 12 patients were diagnosed with stage 3 and stage 4 disease, respectively. All patients had their tumors resected within 1–2 weeks after diagnosis, followed by radiation therapy to the head and neck region for patients at stages 2–4, with a mean dose of 60 Gy. The study protocol was approved by the Institutional Review Board. Clinical data included clinical characteristics, tumor-node-metastasis staging, histological grading status at the end of the study (alive or deceased), failure (local, regional or distant), time to failure, follow-up and survival and an immunohistochemical analysis of phospho-Src family kinase and various other molecular markers.
Pathological study
All specimens were formalin-fixed and paraffin-embedded following surgical harvesting. Shortly prior to the immunological evaluation, serial sections (4–5 μm in thickness) were prepared for hematoxylin and eosin staining, phospho-Src family kinase, c-erbB-2 (Her-2) and for the terminal deoxynucleotidyl transferase (TdT)-mediated biotinylated deoxyuridine-triphosphate (dUTP) nick end-labeling (TUNEL) staining.
TUNEL staining
Sections were stained by the in situ Death Detection POD kit (Roche Diagnostic GmbH, Mannheim, Germany), according to the manufacturer’s instructions. Briefly, after deparaffinization and rehydration, sections were incubated with proteinase K (20 μM/ml in 10 mm Tris/Hcl pH 7.4) for 30 min at 37°C. Slides were rinsed with phosphate-buffered saline (PBS) and incubated with 3% H2O2 in methanol for 10 min at room temperature to block endogenous peroxidase activity, followed by PBS washing and incubation in 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice (4°C). Sections were incubated with a mixture of TdT solution and fluorescein isothiocyanate dUTP solution in a humidified chamber at 37°C for 60 min. This was followed by washings with PBS and incubation with anti-fluorescein antibody Fab fragments conjugated with horse-radish peroxidase in a humidified chamber at 37°C for 30 min. After washing with PBS, amino ethyl carbazole solution was applied, followed by light counterstain with hematoxylin. Paraffin-embedded sections of normal tonsils were used as positive controls. Negative control was obtained by replacing the TdT solution with distilled water. The presence of clear nuclear staining was indicative of apoptotic cells. At least 1,000 tumor cell nuclei were examined in the most evenly and distinctly labeled areas. The number of TUNEL-positive tumor cell nuclei was counted, and the apoptotic index was the percentage of apoptotic cells in the tumor. The positive apoptotic indices were classified into three groups: 0–1% (+1), 1–3% (+2) and above 3% (+3).
c-erbB-2 staining
Four micrometers paraffin-embedded sections were dewaxed and rehydrated. Endogenous peroxidase was blocked by incubation with 3% H2O2 in methanol for 10 min. Nonspecific binding was blocked by incubation in 10% normal serum for 20 min. Sections were heated in a microwave oven for 15 min in 10 mm citrate buffer pH 6. The antibodies used were anti-c-erbB-2/Her-2/neu (polyclonal, 1:10,000, Dako).
Slides were incubated with the antibodies and antiserum for 60 min at room temperature, followed by the application of the streptavidin–biotin complex method (Histostain Plus, Zymed Laboratories). Color development was performed with amino ethyl carbazole followed by light hematoxylin counterstaining. Positive controls were run in parallel: breast tumor, known to strongly express the c-erbB-2 protein. Negative controls were obtained by substituting nonimmune mouse or rabbit serum for the primary antibody. Staining for c-erbB-2 was considered positive when more than 10% of tumor cells showed moderate (+1) to strong (+2) complete membranous staining.
c-Src staining
To identify phospho-Src family kinase protein expression, a phospho-Src family (Tyr416) antibody (Cell Signaling Technology, dilution 1:100, with antigen retrieval) was used. Antigen retrieval was performed prior to reactivity with phospho-Src family kinase antibody by microwaving (1,100 W) slides for four periods of 5 min each in 500 ml 0.01 M citrate buffer at pH 6.0. Staining was performed manually using the avidin–biotin–peroxidase complex method (Vectastain ABC Kit; Vector, Burlingame, CA, USA) at room temperature. Endogenous peroxidase and nonspecific background staining were blocked by incubating slides with 3% aqueous hydrogen peroxide for 10 min. After washing with PBS for 5 min, slides were blocked with normal serum for 20 min, followed by incubation with the primary antibodies for 60 min. After rinsing with PBS for 5 min, sections were incubated with a biotinylated secondary antibody for 20 min. Following washing with PBS for 5 min, slides were incubated with avidin–biotin complex for 30 min and washed again. Chromogen was developed with 10 mg of 3,3′-diaminobenzidine tetrahydrochloride (Sigma) diluted in 12 ml of Tris buffer at pH 7.6 for 2 min. All slides were lightly counterstained with Mayer’s hematoxylin for 30 s before dehydration and mounting. Positive controls and nonimmune protein-negative controls were used for each antibody. The stain was semiquantitatively examined by two independent pathologists using the Allred 8-unit system with a combination of a proportion score from 0 to 5, and an intensity score on a scale from 0 to 3 (none, weak, moderate, strong). A total score of 2–3 was considered low, 4–5 was considered intermediate and 6–8 was considered high.
Statistical analysis
For categorical variables, frequencies and percentages were calculated. Distributions for categorical variables were compared and analyzed by the Fisher–Irwin exact test (small sample). For continuous variables, ranges, mean values and standard deviation and standard errors were calculated. The results of continuous variables between subgroups of patients were compared and analyzed by one-way analysis of variance. The “Kaplan–Meier estimate” was used to calculate the probability of survival rates as a function of time. The “Log Rank test” was used to compare between survival curves.
Results
Phospho-Src family kinase staining
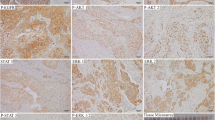
Of the 39 patients analyzed, 15 (38%) stained negatively for phospho-Src family kinase (0) while the other 24 (62%) stained positively. The phospho-Src family kinase positive group was further categorized according to the intensity of staining. Thus, weak staining (+1) was found in 31% (12/39) of specimens, while 18% (7/39) were stained moderately (+2) and 13% (5/39) were strongly stained (+3) for c-Src (Fig. 1). Adjacently, normal-looking tissue was not stained by the anti-c-Src antibody, thus serving as internal controls (Fig. 1). c-Src immunostaining was neihter significantly different between males and females (P = 0.90) nor significantly altered by age (P = 0.58).
Phospho-Src family kinase staining of predominantly membranous staining of tumor cells (a), and of focal granular cytoplasmic staining, in addition to areas of membranous staining of tumor cells (b), (×300). The antibody currently used was against phosphorylated Src family kinases and in this respect it is noteworthy that phosphorylation of Src results in its activation and recruitment to the plasma membrane which may explain this reported observation
Phospho-Src family kinase expression correlates with tongue tumor progression
Phospho-Src family kinase expression significantly correlated with the T, N and stage values of the disease but not with its pathological grade as presented in Tables 1, 2 and 3. Phospho-Src family kinase expression significantly correlated with tumor size (P = 0.05; Tables 1, 2). Here, 33% of the cases negative for phospho-Src family kinase originated from small tumors (T 1), while 83% of the phospho-Src family kinase positive cases were obtained from large tumors (T 2–4, >2 cm in diameter), differences that were statistically significant (P = 0.05). All five patients expressing the most intense phospho-Src family kinase staining (+3) were diagnosed with large tumors (T 2–4) (Tables 1, 2). Similar to the results obtained for the tumor T values were the results demonstrated for the N values, i.e., the correlation between phospho-Src family kinase staining level and the rate of neck lymph node metastatic involvement was significant (P = 0.004). Hence, while 80% of the patients with phospho-Src family kinase negatively stained tumors had no lymph node involvement (N = 0), only 58% of the patients with phospho-Src family kinase positively stained tumors had no neck metastasis diagnosed (Tables 1, 2). Also the correlation between the phospho-Src family kinase staining level and the stage rates was significant (P = 0.05). Hence, while all stage 1 patients exhibited phospho-Src family kinase negative staining, not more than 80% of the advanced-stage patients (stages 3–4) exhibited phospho-Src family kinase positive staining (Table 3).
Prognostic value of phospho-Src family kinase for tongue carcinoma
The frequency of patients with a positive phospho-Src family kinase level (>0) who died due to the disease (67%) was significantly higher (P = 0.05) than those patients with negative phospho-Src family kinase staining (=0), (33%). The probability of survival of patients with negative phospho-Src family kinase level (=0) at 60 months was 67% (with 95% confidence interval of 37–84%) while the probability of survival of patients with positive phospho-Src family kinase level (>0) at 60 months was 30% (with 95% confidence interval of 13–50%; P = 0.05; Fig. 2).
Cumulative survival by phospho-Src family kinase level (0 vs. >0). The probability of survival of patients with phospho-Src family kinase level (=0) at 60 months was 67% (with 95% confidence interval of 37–84%) while the probability of survival of patients with phospho-Src family kinase level (>0) at 30 months was 30% (with 95% confidence interval of 13–50%; P = 0.05)
Correlation between phospho-Src family kinase and other tumor markers expression
Of the 39 patients analyzed, 13 (33%) stained negatively (0) for TUNEL while the other 26 (66%) stained positively. The c-erbB2 staining analysis revealed that 34 patients (87%) stained negatively while 5 (13%) stained positively for c-erbB-2.
The phospho-Src family kinase level of staining significantly correlated with the levels of staining of TUNEL (P = 0.01) and c-erbB-2 (P = 0.05). In the subgroup of patients with negative phospho-Src family kinase level (=0) there were none with TUNEL level >1. In the subgroups of patients with phospho-Src family kinase level (+1) only 17% of patients had TUNEL level >1, while in the subgroups of patients with phospho-Src family kinase level (+2) only 14% of patients had TUNEL level >1, but in the subgroups of patients with phospho-Src family kinase level (+3) 60% of patients had TUNEL level >1 (Tables 4, 5).
Discussion
Most interesting is the finding that phospho-Src family kinase induction was noted in most examined biopsies of tongue cancer, correlating with tumors larger in size (Tables 1, 2) and in progression (lymph node metastasis and stage) and also correlating with TUNEL and c-erbB-2 expression rates (Tables 1, 2, 3, 4, 5) but not with grade. It is worth mentioning that we used an antibody against phosphorylated Src family kinases, whose drawback is that it is not specific to phospho-Src family kinase but cross-reacts with other members of the Src-kinase family. As such the levels of activated Src family kinases in tongue cancer are demonstrated rather than specifically phospho-Src family kinase levels. In the absence of a reliably specific antibody to phospho-c-Src, it is important to demonstrate the role of c-Src and phospho-c-Src via immunoprecipitation followed by immunoblotting experiments on protein extracts from the patient tumors. Furthermore, it is important to examine the expression of total phospho-Src family kinase in the tissues and correlate the levels to that of activated or phosphorylated Src. These experiments were beyond the scope of the current study but should certainly be performed in a further study. In any case, the current study’s most important result, however, is the novel demonstration of the prognostic role of phospho-Src family kinase in tongue cancer, given the inverse correlation found between phospho-Src family kinase expression and patient survival (Fig. 1). Reduced post-operative survival of cancer patients over-expressing phospho-Src family kinase is most likely due to increased tumor metastasis and indeed we found that the correlation between the level of phospho-Src family kinase expression and lymph node metastasis (N) was highly significant (P = 0.004; Tables 1, 2). This pro-metastatic function of phospho-Src family kinase is probably explained by the accumulating evidence which strongly suggest that phospho-Src family kinase functions as a pro-angiogenic mediator via the induction of the vascular epithelial growth factor (VEGF) and thus, it is likely that phospho-Src family kinase facilitates tumor vascularity and cell survival. This notion is further supported by the recently reported capacity of phospho-Src family kinase inhibitors to completely abrogate VEGF up-regulation (Zetser et al. 2006).
The currently demonstrated mutual expression of TUNEL and phospho-Src family kinase markers in the tongue lesion is especially intriguing as we have recently reported that higher expression of TUNEL in salivary gland malignancies is a significant predictor for poor prognosis (Ben-Izhak et al. 2007). Hence, this study adds credence to the current demonstration of phospho-Src family kinase’s prognostic role for poorer outcome. Further credence for the association between cancer and the mutual expression of phospho-Src family kinase and TUNEL may be found in the recently published studies of Ducker et al. (2005) and Fukuda et al. (2004). The former reported that inhibition of phospho-Src family kinase resulted in a reduction of the VEGF-mediated vascular permeability while the latter reported that a loss of activation of phospho-Src family kinase was associated with inhibition of cell replication. In any case, TUNEL staining of tongue SCC tumors for evaluation of their malignancy profile was performed in this study since TUNEL examination has been performed by researchers in other cancer types. Moreover, an unbalanced admixture of proliferative cells and apoptotic cells in neoplastic parenchyma is related to tumor growth. When the growth features of tumors are discussed, not only cell proliferation but also cell death, especially apoptosis, should be taken into consideration. However, TUNEL in situ technique for the detection of apoptosis is not completely specific, as overlap between apoptotic and necrotic cell death has been reported, possibly resulting in the fact that some of the apoptotic cells do not stain (Finlay et al. 1988; Wyllie et al. 1999). The report of Grasl-Kraupp et al. (1995), which found that TUNEL failed to distinguish between apoptotic and necrotic cells, strengthens this point. Thus, the exact biological pathway expressed by the mutually positive TUNEL phospho-Src family kinase staining is yet to be fully elucidated, and its exploration is highly warranted, as it may lead to finding novel anti-cancer cellular targets.
The currently demonstrated up-regulation of the c-erbB-2 oncogene is not surprising, as c-erbB-2 shares considerable homology with the epidermal growth factor receptor (EGFR), a tyrosine kinase involved in cell growth and differentiation and, consequently, with carcinogenesis, cell growth and differentiation (Akiyama et al. 1986). EGFR was shown to be involved in the pathogenesis of oral cancer (Nestor et al. 2007), yet it was never studied in relation to either TUNEL or to phospho-Src family kinase which might be important with respect to further elucidating the pathogenesis of the disease. It has been demonstrated that this proto-oncogene amplification and/or protein overexpression is associated with poor prognosis in breast carcinoma and other human neoplasms, including stomach, renal, pancreatic, ovarian and salivary gland carcinomas (Quenel et al. 1995; Hanna et al. 1999; Nagler et al. 2003). Further credence for these results is found in the reported mutual pro-carcinogenic role for c-erbB-2 and phospho-Src family kinase, previously shown in other cancers (though never in oral SCC). For example, Wilson et al. recently reported that in ductal carcinoma, high levels of activated phospho-Src family kinase correlated with c-erbB-2 positivity, high tumor grade, comedo necrosis and elevated epithelial proliferation, and that high activated phospho-Src family kinase level associated with lower recurrence-free survival at 5 years (Wilson et al. 2006). Similar results were reported by others as well (Vadlamudi et al. 2003; Kim et al. 2005).
Taken together, our results indicate that phospho-Src family kinase expression is enhanced in tongue tumors, and that the outcome in these patients inversely correlates with phospho-Src family kinase levels. The results further reveal a correlation between phospho-Src family kinase levels and tongue tumor progression, metastasis and size, and suggest a novel link between phospho-Src family kinase and the TUNEL and c-erbB-2 pathways, likely tilting the balance toward cell proliferation. This link seems to be part of a wide carcinogenetic network important in oral cancer progression and metastasis which contains various molecules such as heparanase, VEGF, PDGF and others (Nagler et al. 2007; Wood et al. 2000), some of whom are independent of phospho-Src family kinase while others are related. In any case the currently presented data further encourage the development of phospho-Src family kinase inhibitors for the treatment of this lethal cancer.
References
Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T (1986) The product of the human C-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 232:1644–1646. doi:10.1126/science.3012781
Alho OP, Kantola S, Pirkola U, Laara E, Jokinen K, Pukkala E (1999) Cancer of the mobile tongue in Finland—increasing incidence, but improved survival. Acta Oncol 38:1021–1024. doi:10.1080/028418699432293
Alper O, Bowden ET (2005) Novel insights into c-Src. Curr Pharm Des 11:1119–1130. doi:10.2174/1381612053507576
Alvarez RH, Kantarjian HM, Cortes JE (2006) The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer 107:1918–1929. doi:10.1002/cncr.22215
Bahar G, Feinmesser R, Shpitzer T, Popovtzer A, Nagler RM (2007) Salivary analysis in oral cancer patients: DNA and protein oxidation, reactive nitrogen species and antioxidant profile. Cancer 101:54–59. doi:10.1002/cncr.22386
Ben-Izhak O, Kablan F, Laster Z, Nagler RM (2005) Oropharyngeal cancer pathogenesis: ubiquitin proteolytic, apoptotic and epidermal growth factor related pathways act in concert—first report. Oral Oncol 41:851–860. doi:10.1016/j.oraloncology.2005.04.012
Ben-Izhak O, Kaplan-Cohen V, Ilan N, Gan S, Vlodavsky I, Nagler R (2006) Heparanase expression in malignant salivary gland tumors inversely correlates with long-term survival. Neoplasia 8:879–884. doi:10.1593/neo.06382
Ben-Izhak O, Laster Z, Araidy S, Nagler RM (2007) TUNEL—an efficient prognosis predictor of salivary malignancies. Br J Cancer 96:1101–1106. doi:10.1038/sj.bjc.6603655
Chong YP, Ia KK, Mulhern TD, Cheng HC (2005) Endogenous and synthetic inhibitors of the Src-family protein tyrosine kinases. Biochim Biophys Acta 1754:210–220
Conway WC, Van der Voort van Zyp J, Thamilselvan V, Walsh MF, Crowe DL, Basson MD (2006) Paxillin modulates squamous cancer cell adhesion and is important in pressure-augmented adhesion. J Cell Biochem 98:1507–1516. doi:10.1002/jcb.20819
Dehm SM, Bonham K (2004) SRC gene expression in human cancer: the role of transcriptional activation. Biochem Cell Biol 82:263–274. doi:10.1139/o03-077
Ducker CE, Upson JJ, French KJ, Smith CD (2005) Two N-myristoyltransferase isozymes play unique roles in protein myristoylation, proliferation and apoptosis. Mol Cancer Res 3:463–476. doi:10.1158/1541-7786.MCR-05-0037
Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, Levine AJ (1988) Activating mutations for transformation by p53 produce a gene product that forms an HSC70–p53 complex with an altered half-life. Mol Cell Biol 8:531–539
Fukuda S, Kaga S, Sasaki H, Zhan L, Zhu L, Otani H, Kalfin R, Das DK, Maulik N (2004) Angiogenic signal triggered by ischemic stress induces myocardial repair in rat during chronic infarction. J Mol Cell Cardiol 36:547–549. doi:10.1016/j.yjmcc.2004.02.002
Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R (1995) In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 21:1465–1469
Hakam A, Fang Q, Karl R, Coppola D (2003) Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci 48:1972–1978. doi:10.1023/A:1026122421369
Hanna W, Kahn HJ, Trudeau M (1999) Evaluation of HER-2/neu (erbB-2) status in breast cancer: from bench to bedside. Mod Pathol 12:827–834
Ishizawar R, Parsons SJ (2004) C-Src and cooperating partners in human cancer. Cancer Cell 6:209–214. doi:10.1016/j.ccr.2004.09.001
Jackson MS, Wrench AA, Soutar DS, Robertson AG (1999) Carcinoma of the tongue: the speech therapist’s perspective. Br J Oral Maxillofac Surg 37:200–204. doi:10.1054/bjom.1999.0032
Kantola S, Parikka M, Jokinen K, Hyrynkangs K, Soini Y, Alho OP, Salo T (2000) Prognostic factors in tongue cancer—relative importance of demographic, clinical and histopathological factors. Br J Cancer 83:614–619. doi:10.1054/bjoc.2000.1323
Kim H, Chan R, Dankort DL, Zuo D, Najoukas M, Park M, Muller WJ (2005) The c-Src tyrosine kinase associates with the catalytic domain of ErbB-2: implications for ErbB-2 mediated signaling and transformation. Oncogene 24:7599–7607. doi:10.1038/sj.onc.1208898
Martin GS (2004) The road to Src. Oncogene 23:7910–7917. doi:10.1038/sj.onc.1208077
McCarty MF, Block KI (2005) Multifocal angiostatic therapy: an update. Integr Cancer Ther 4:301–314. doi:10.1177/1534735405282475
Muir C, Weiland L (1995) Upper aerodigestive tract cancers. Cancer 75:147–153. doi:10.1002/1097-0142(19950101)75:1+<147::AID-CNCR2820751304>3.0.CO;2-U
Myers JN, Elkins T, Roberts D, Byers RM (2000) Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg 122:44–51. doi:10.1016/S0194-5998(00)70142-2
Nagler RM, Barak M, Ben-Aryeh H, Peled M, Filatov M, Laufer D (1999) Early diagnostic and treatment monitoring role of Cyfra 21–1 and TPS in oral squamous cell carcinoma. Cancer 35:1018–1025. doi:10.1002/(SICI)1097-0142(19990301)85:5<1018::AID-CNCR2>3.0.CO;2-R
Nagler RM, Kerner H, Ben-Eliezer S, Minkov I, Ben-Itzhak O (2003) Prognostic role of apoptotic, Bcl-2, c-erbB-2 and p53 tumor markers in salivary gland malignancies. Oncology 64:389–398. doi:10.1159/000070298
Nagler R, Bahar G, Shpitzer T, Feinmesser R (2006) Concomitant analysis of salivary tumor markers—a new diagnostic tool for oral cancer. Clin Cancer Res 12:3979–3984. doi:10.1158/1078-0432.CCR-05-2412
Nagler R, Ben-Izhak O, Cohen-Kaplan V, Shafat I, Vlodavsky I, Akrish S, Ilan N (2007) Heparanase up-regulation in tongue cancer: tissue and saliva analysis. Cancer 110:2732–2739. doi:10.1002/cncr.23095
Nestor M, Ekberg T, Dring J, van Dongen GA, Wester K, Tolmachev V, Anniko M (2007) Quantification of CD44v6 and EGFR expression in head and neck squamous cell carcinomas using a single-dose radioimmunoassay. Tumour Biol 28:253–263. doi:10.1159/000110898
Quenel N, Wafflart J, Bonichon F, de Mascarel I, Trojani M, Durand M, Avril A, Coindre JM (1995) The prognostic value of C-erbB-2 in primary breast carcinoma: a study of 942 cases. Breast Cancer Res Treat 35:283–291. doi:10.1007/BF00665980
Ribeiro KC, Kowalski LP, Latorre MR (2000) Impact of comorbidity, symptoms, and patients’ characteristics on the prognosis of oral carcinomas. Arch Otolaryngol Head Neck Surg 126:1079–1085
Shpitzer T, Bahar G, Feinmesser R, Nagler RM (2007) A comprehensive salivary analysis for oral cancer diagnosis. J Cancer Res Clin Oncol 133:613–617. doi:10.1007/s00432-007-0207-z
Tamura I, Sakaki T, Chaqour B, Howard PS, Ikeo T, Macarak EJ (2003) Correlation of P-cadherin and beta-catenin expression and phosphorylation with carcinogenesis in rat tongue cancer induced with 4-nitroquinoline 1-oxide. Oral Oncol 39:506–514. doi:10.1016/S1368-8375(03)00013-7
Vadlamudi RK, Sahin AA, Adam L, Wang RA, Kumar R (2003) Heregulin and HER2 signaling selectively activates c-Src phosphorylation at tyrosine 215. FEBS Lett 543(1–3):76–80. doi:10.1016/S0014-5793(03)00404-6
Warmuth M, Damoiseaux R, Liu Y, Fabbro D, Gray N (2003) SRC family kinases: potential targets for the treatment of human cancer and leukemia. Curr Pharm Des 9:2043–2059. doi:10.2174/1381612033454126
Wilson GR, Cramer A, Welman A, Knox F, Swindell R, Kawakatsu H, Clarke RB, Dive C, Bundred NJ (2006) Activated c-SRC in ductal carcinoma in situ correlates with high tumour grade, high proliferation and HER2 positivity. Br J Cancer 95:1410–1414. doi:10.1038/sj.bjc.6603444
Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F (2000) PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 60:2178–2189
Wyllie AH, Bellamy CO, Bubb VJ, Clarke AR, Corbet S, Curtis L, Harrison DJ, Hooper ML, Toft N, Webb S, Bird CC (1999) Apoptosis and carcinogenesis. Br J Cancer 80(Suppl 1):34–37
Yeole BB, Sankaranarayanan R, Sunny M, Sc L, Swaminathan R, Parkin DM (2000) Survival from head and neck cancer in Mumbai (Bombay), India. Cancer 89:437–444. doi:10.1002/1097-0142(20000715)89:2<437::AID-CNCR32>3.0.CO;2-R
Zetser A, Bashenko Y, Edovitsky E, Levy-Adam F, Vlodavsky I, Ilan N (2006) Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and Src activation. Cancer Res 66:1455–1463. doi:10.1158/0008-5472.CAN-05-1811
Acknowledgment
The authors thank Mrs S. Gan for her assistance in statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben-Izhak, O., Cohen-Kaplan, V. & Nagler, R.M. The prognostic role of phospho-Src family kinase analysis in tongue cancer. J Cancer Res Clin Oncol 136, 27–34 (2010). https://doi.org/10.1007/s00432-009-0633-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0633-1