Abstract
Antimicrobial peptides (AMPs) are short molecules produced by almost all organisms. Fish AMPs contain innate immune components as their primary immune molecules. The fish AMPs include piscidins, hepcidins, defensins, cathelicidins and histone-derived peptides. Piscidin is potent and broad-spectrum; this peptide was conserved among Acanthopterygii superorder and is therapeutically important among other AMPs. It was present mainly in the tissues of gills, muscle, head-kidney, skin and intestine of teleost. Piscidin AMP family includes piscidin, moronecidin, pleurocidin, epinecidin, gaduscidin, misgurin, dicentracin, chrysophsin and myxinidin. This review reports the structural properties of various piscidin and their mode of action as it is important to know their mechanism how the peptide involved in antimicrobial activity. In addition, the gene expression of piscidin which influenced the immune responses, their pharmaceutical importance and biological applications were described. Overall, the review explains a broad spectrum of knowledge on piscidin, its classes and types, structure, cytotoxicity, membrane permeabilization, properties and therapeutical implications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimicrobial peptides are ribosomally synthesized peptides less than 10 KDa molecular weight and having a positive net charge. These are evolutionarily preserved components (Arockiaraj et al. 2012; Shabir et al. 2018) which have a key role in the first line of defense mechanism (Hancock 2000; Arockiaraj et al. 2013) and play a major role in innate immunity (Campagna et al. 2007; Moon et al. 2007; Kumaresan et al. 2015). AMPs are derived from protein sequence by hydrolytic degradation which includes a signal sequence. AMPs are classified into five, based on their secondary structure which includes α-helical (Zasloff 2002; Arockiaraj et al. 2014a), β-sheet, (Andreu and Rivas 1998; Chaurasia et al. 2014) loop, extended coil and cyclic peptides (Hu et al. 2006; Arasu et al. 2017a; Shabir et al. 2018). AMPs have been derived from various vertebrates and invertebrates and even from plants (Lee et al. 2007; Katzenback 2015; Arockiaraj et al. 2015). They are very sensitive against different pathogenic microbes such as bacteria, fungi, viruses and parasites (Jenssen et al. 2006; Sung et al. 2008; Niu et al. 2013; Arasu et al. 2017b). It has a potential value as it is active against multi-drug resistant and biofilm-forming microorganisms (Hiemstra et al. 2016; Ravichandran et al. 2016), thus can replace antibiotics (Lee et al. 2007; Arockiaraj et al. 2014b).
The AMPs are short sequences present in mucosal, skin surfaces and mast cells of different aquatic organisms (Silphaduang et al. 2006; Arasu et al. 2014). As the surrounding environment of fish contains a wide range of pathogenic organisms, the innate immune system of fish has its importance (Bulet et al. 2004; Sathyamoorthi et al. 2017). The major route for entry of pathogenic microorganisms is through the epithelial cells of skin, gills and gastrointestinal tract, which provide the first line of defense by producing host defense peptides. The healthy fish can limit these infections by the presence of AMPs as well as other short defense proteins (Salger et al. 2016; Ravichandran et al. 2018). The AMPs present in the fish mucus prevents the colonization of bacteria, fungi, parasites and other pathogenic organisms (Pálffy et al. 2009; Shabir et al. 2018; Sannasimuthu et al. 2018). The presence of MHC I loci and the unique organization of the Toll-like receptor (TLR) in Atlantic cod (Gadus morhua L.) helps the innate immune mechanism, thus preventing pathogenic infection (Star et al. 2011; Ravichandran et al. 2017). The peptides with antimicrobial properties play a major role in such a preventive mechanism (Ruangsri et al. 2012a; Marimuthu et al. 2015). Along with antimicrobial property, these peptides also have anti-inflammation, wound healing, immune activation (Gordon et al. 2005; Sannasimuthu et al. 2019), antitumor, immune-modulatory and anti-diabetic effects (Diamond et al. 2009; Conlon et al. 2014; Timalata et al. 2015), hence these fish-derived peptides can be used as a potent product for improving immunity as well as health-related matters for various organisms including human (Salger et al. 2016; Kumaresan et al. 2019). In 1990s, identification and analysis of fish AMPs were initiated; and based on their structure, they were classified into five different families such as hepcidins, β-defensins, histone-derived peptides, cathelicidins and fish-specific piscidins (Katzenback 2015; Rajesh et al. 2018).
Apart from hepcidin present in human, it was also screened in fish species. It is a major component of innate immunity with antimicrobial activity. The gene expression showed that it is highly expressed in liver tissues (Wang et al. 2009; Sathyamoorthi et al. 2019). It has a small cysteine rich region with antimicrobial property and also has a role in iron homeostasis (Rodrigues et al. 2006; Akila et al. 2018). Hepcidin were formerly called as LEAP (Liver Expressed Antimicrobial Peptide) that contains a disulphide (cysteine) bond with β sheets (Cuesta et al. 2008; Sannasimuthu and Arockiaraj 2019). The importance of hepcidin in pharmaceuticals are for its anticancer and antibacterial properties (Chen et al. 2009a, b; Thirumalai et al. 2014). Wang et al. (2010) reported that hepcidin can be used as antiviral agent against nervous necrosis virus.
Defensins are components involved in innate molecules which include antimicrobial peptides and proteins (AMPPs). It is a cationic peptide with β-sheet structure which has biological properties and are conserved among plant and animal kingdoms. Fish defensins were first found in zebrafish (Danio rerio), green-spotted pufferfish (Tetraodon nigroviridis) and tiger pufferfish (Takifugu rubripes) through gene mining and later it was found in other fish species too (Ruangsri et al. 2013; Purabi et al. 2020). Β-defensins were reported to be present in brain, pituitary and testis and possessed antibacterial and antiviral properties (Jin et al. 2010; Raju et al. 2020). Studies on antiparasitic property were also analyzed against Trypanosoma cruzi and Toxoplasma gondii by pore formation and mitochondrial DNA fragmentation (Masso-Silva and Diamond 2014). The antiviral activity of defensin was isolated from Epinephelus coioides and was used against two viral pathogens, Singapore grouper iridovirus (SGIV), an enveloped DNA virus and viral nervous necrosis virus (VNNV), a non-enveloped RNA virus (Guo et al. 2012).
The histone derived peptides are a part of histone molecule with biological activities. Buforin I was isolated from Asian toad (Bufo bufo) and was the first histone H2A derived peptide. These histone derived peptide was found in many fish species with wide range of pathogenic activities (Masso-Silva and Diamond 2014; Prabha et al. 2019). The histone-derived AMPs were identified from fish species including catfish (Parasilurus asotus), Atlantic salmon (Salmon salar), rainbow trout (Oncorhynchus mykiss), and Atlantic halibut (Hippoglossus hippoglossus) and invertebrates pacific white shrimp (Litopenaeus vannamei) and Chinese scallop (Chlamys farreri) (Zoysa et al. 2009). The two major histones are core histones and linker histones such as H2A, H2B, H3, H4 and H1. Parasin, hipposin, buforin I and II, abhisin, himanturin, sunettin, and molluskin are represent histone H2A-derived peptides; and onchorhyncin II and SAM (Salmon antimicrobial protein) are represent histone H1 (Chaithanya et al. 2013).
Cathelicidin peptide has four cysteine residues forming two disulphide bridges, which is cationic and amphipathic in nature. Maier et al. (2008) suggested that it has major role in both innate and adaptive immunity. In mammals, both immune and non-immune activities have been exhibited. The gene expression was induced by different bacterial species and the expression were high in gill, liver, spleen and intestine (Masso-Silva and Diamond 2014). The major property of this peptide is its antimicrobial activity with no cytotoxicity (Lu et al. 2011).
Piscidin are a linear cationic α-helical peptide with broad-spectrum activity (Fernandes et al. 2010). These peptides were characterized in various species of teleost fish which comes under superorder Acanthopterygii (Ruangsri et al. 2012b; Elumalai et al. 2019). Piscidin contains amino acid sequences in length between 18 and 46. The first cationic AMP of piscidin was isolated from hybrid striped bass (Morone saxatilis × M. chrysops) (Chinchar et al. 2004; Campagna et al. 2007; Lauth et al. 2001). It exhibits antibacterial, antifungal and antiviral properties. This peptide is also showed an innate immune response against parasitic infections (Dezfuli et al. 2010; Niu et al. 2013). It was reported that piscidin was sensitive towards fish and human pathogens and multidrug-resistant bacteria such as MRSA ¼ methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci sp., etc., (Lauth et al. 2001; Noga et al. 2009; Dezfuli et al. 2010). This review is especially dealing with the structure, types, mode of action, properties including cytotoxicity and membrane permeabilization and therapeutical properties of fish-derived piscidin.
Distribution of Piscidin
Piscidins are present in different taxa of teleosts including the families of Moronidae, Siganidae, Sciaenidae, Percichthyidae, Belontidae, Latridae (Andrews et al. 2010), Cichlidae, Sygnathidae (Sun et al. 2012) and Sparidae (Corrales et al. 2009). There is an evidence that piscidin is also present in families including Gasterosteidae, Sebastidae, Adrianichthyidae, Fundulidae, Cyprinodontidae and Anoplopomatidae, which was confirmed by Expressed Sequence Tag (EST) databases (Salger et al. 2017).
The piscidin gene transcript was present in striped bass (M. saxatilis), white bass (M. chrysops), mandarin fish (Siniperca chuatsi), European seabass (Dicentrarchus labrax), Nile tilapia (Oreochromis niloticus), Gadus morhua (Salger et al. 2016), hybrid striped bass (Salger et al. 2011) and Dicentrarchus labrax belongs to Moronidae (Salerno et al. 2007). The presence of piscidin gene transcript was also observed in Siganus rivulatus, Leiostomus xanthurus and Micropogonias undulates belongs to Siganidae family. There is an evidence for the presence of the same in Siniperca chuatsi and O. niloticus belongs to Percichthyidae and Cichlidae family, respectively (Acosta et al. 2013; Sun et al. 2007; Peng et al. 2012b). Trichogoster leeri, a Belontidae family also has a piscidin amino acid sequence in common. Epinephelus niveatus, which belongs to Serranidae family has the piscidin sequence in it (Silphaduang et al. 2006). The sequence of chrysopsins isolated from Chrysophrys major, a member of Sparidae family, which is similar to piscidin (Iijima et al. 2003). The expressed sequence tag (EST) databases showed evidence that piscidin was also isolated from Gasterosteus aculeatus, belongs to Gasterosteidae family (Brown et al. 2008). It is reported that piscidin was isolated from Sebastidae family which includes Sebastes caurinus and S. schlegelii (Heras et al. 2011). Moreover, presence of piscidin was reported in Oryzias latipes, belongs to Adrianichthyidae and Fundulus heteroclitus, belongs to Fundulidae family. Cyprinodon variegatus contains a piscidin sequence that was belong to Cyprinodontidae family; a similar piscidin sequence was reported in Anoplopoma fimbria from Anoplopomatidae family (Iijima et al. 2003; Brown et al. 2008). The piscidin like peptide was also isolated from orange-spotted grouper (Epinephelus coioides), red-spotted grouper (E. akaara), brown-marbled grouper (E. fuscoguttatus), sablefish (Anoplopoma fimbria), spotted seahorse (Hippocampus kuda), yellow croaker (Larimichthys crocea) (Ruangsri et al. 2012b) and Gadus morhua (Noga et al. 2009). Until now, it is reported that piscidin is only peptide present in superorder Acanthopterygii.
Other AMPs of Piscidin Family
Considering the piscidin family, it consists of many other peptides under its family including pleurocidin, moronecidin, dicentracin, epinecidin, chrysophsin, myxindin, misgurin and gaduscidin (Salerno et al. 2007; Sun et al. 2007; Peng et al. 2012b; Acosta et al. 2013; Katzenback 2015). Table 1 shows the amino acid sequence, charge, expression pattern, presence and properties of different AMPs of piscidin family.
Pleurocidin is a highly basic molecule and an amphipathic α-helical cationic peptide found in skin, gill and gut tissues of winter flounder (Pseudopleuronectes americanus) (Browne et al. 2011) and its antimicrobial activities was examined (Patrzykat et al. 2002). Piscidin has a similarity with pleurocidin as well as a close genetic relationship which suggests that pleurocidin are the members of piscidin family. Pleurocidin and pleurocidin like peptides were also isolated from Atlantic halibut (Hippoglossus hippoglossus), witch flounder (Glyptocephalus cynoglossus), American plaice (Hippoglossoïdes platessoïdes) and yellowtail flounder (Limanda ferruginea) (Heras et al. 2011; Ruangsri et al. 2012b). It has a broad spectrum of activity against Gram-positive and Gram-negative bacteria. The sequence has been arranged as H−Gly-Trp-Gly-Ser-Phe-Phe-Lys-LysAla-Ala-His-Val-Gly-Lys-His-Val-Gly-Lys-Ala-Ala-Leu-Thr-His-Tyr-Leu−OH (Yoshida et al. 2001). Pleurocidin has homology with dermaseptins (from skin of hylid frog) and ceratatoxins (from mediterrenean fruit fly) and is showing high antifungal activity (Jung et al. 2007). It is capable of causing dye leakage from liposomes, translocate across model membranes and pore-forming activity in planar lipid bilayers (Mason et al. 2006). The mode of action against the microbes was developed by membrane disruption mechanism by binding pleurocidin on microbial membrane (Jung et al. 2007). It is reported to show membrane disruption and oxidative stress with no hemolysis against human erythrocytes. The therapeutic application of pleurocidin against infectious diseases was reported by Choi and Lee (2012) with combination of other drugs and along with adjuvants. The pleurocidin were used for cancer treatment in zebrafish model (Morash et al. 2011). Thus, it was suggested to use in aquaculture as therapeutic agent and it is also used as a potential food preservative (Burrowes et al. 2006).
Moronecidin was isolated from gill, skin, intestine, spleen, head kidney and blood of hybrid striped bass and white bass (M. chrysops) (Browne et al. 2011); it is function against various bacterial pathogen. It is a novel helical AMP with 22 amino acid residue, which is C-terminally amidated (Lauth et al. 2001). This peptide have low toxicity and high salt tolerance (Shin et al. 2017). The moronecidin mRNA was upregulated in skin, heart, brain, gill, head kidney, kidney, intestine and spleen due to the induction of Escherichia coli LPS as well as Streptococcus iniae (Browne et al. 2011). The membrane disruption of moronecidin on microbes is due to the formation of pores. As it has salt tolerance, it can be used for therapeutic applications in marine organisms as well as human medicines (Lauth et al. 2001). The toxicity of moronecidin were analysed in murine and human hepatic cells, which resulted no cytotoxicity, thus it can be used as a therapeutic agent at safe concentration (Bo et al. 2019).
Dicentracin belongs to the piscidin family derived from European seabass (Dicentrarchus labrax) (Family: Moronidae) (Andrews et al. 2010; Rondeau et al. 2013). The novel amphipathic alpha helical peptide isolated from sea bass contains 22 amino acid residues and is having antibacterial, antiviral and antiparasitic activities (Meloni et al. 2015). It was also reported that dicentracin possessed antimicrobial activity in mandarin fish against microbial pathogen (Sun et al. 2007). Its expression was observed in macrophages, granulocytes and monocytes from head kidney, peripheral blood and peritoneal cavity (Salerno et al. 2007). As reported by Terova et al. (2009) Bio-Mos induced changes in the gene expression of dicentracin in seabass.
Epinecidin, cationic peptide with α-helical structure isolated from grouper (E. coioides) which contains 67 amino acid residues; it shows growth inhibitory activities against a range of Gram-positive and Gram-negative bacteria and fungus (Pan et al. 2009). It interacts with phospholipids in bacterial membranes through membrane disrupting mechanism (Chen et al. 2009a, b). It is responsible for pore formation in membranes of bacteria leading to lysis and their subsequent death (Pan et al. 2007). It has an anionic prodomain COOH terminal with RRRH amino acid residue that forms a non-helical hydrophilic domain (Yin et al. 2006). It has multifunctional properties including antisepsis, antitumor, antivirus and immunomodulatory activities (Narayana et al. 2015). The mature peptide showed sequence similarities with chrysophsin, moronecidin, pleurocidin and piscidin 3 (Pan et al. 2007). The major advantage is that it has the ability to target cancer cells with minimum cytotoxicity (Lin et al. 2009a, b). It can also be used as cleaning agent to prevent pathogenic infections (Pan et al. 2010) due to tis antimicrobial properties (Chee et al. 2019).
Chrysophsin was isolated from red sea bream (Pagrus major) which is similar to piscidin, pleurocidins and epinecidin that present in various fishes (Brown et al. 2008; Salger et al. 2016). Chrysophsin-1, chrysophsin‐2 and chrysophsin‐3 are present in the eosinophilic granule cells in gills; and has potent bactericidal activity against Gram‐negative and Gram‐positive pathogens of fish as well as crustaceans. The C-terminal region forms RRRH with a non-helical hydrophilic domain similar to epinecidin (Iijima et al. 2003). Costa et al. (2018) staed that chrysophin-1 peptide were covalently immobilized which result in higher antimicrobial activity than when the peptide is simply adsorbed. The disruption process of Chrysophin-3 were analyzed using Quartz Crystal Microbalance with Dissipation monitoring (QCM-D) by membrane permeabilization through pore formation (Michel et al. 2017).
Myxindin was isolated from the mucus layer of Myxine sp. (Ebbensgaard et al. 2015). It has activity against both Gram-positive and Gram-negative bacteria and even multidrug-resistant strains. It has the ability to disrupt bacterial membrane by pore formation and can be used for therapeutic agent development. It is not toxic even at high concentrations, thus has no significant hemolysis activity (Han et al. 2016). Some of the report contradict the activities of chrysophin peptide too (Ebbensgaard et al. 2015).
Misgurin, a 21 amino acid peptide found in loach (Misgurnus anguillicaudatus) with a broad spectrum of antimicrobial activity and has no significant hemolytic properties. It has activity against Gram positive bacteria, Gram negative bacteria and fungi species (Shabir et al. 2018). The sequence did not show any homology with other known AMPs. It acts as non-specific defense substance in fish skin. This peptide have a strong cationic tetrapeptide sequence ‘RRRK’ at the C-terminal region ‘Arg-Gln-Arg-Val-Glu-Glu-Leu-Ser-Lys-Phe-Ser-Lys-Lys-Gly-Ala–Ala-Ala-Arg-Arg-Arg-Lys’ (Park et al. 1997; Iijima et al. 2003).
Gaduscidin has two isomers namely Gad-1 and Gad-2 which was identified from the expressed sequence tag database of an G. morhua; both isomers have potential antimicrobial properties (McDonald et al. 2015). Its expression was observed in head, spleen, kidney, gill, peripheral blood, pyloric caecum and brain. The high level of gene expression of gaduscidin are consistent with the immune functions of these tissues in teleosts. However, it was hardly induced in spleen due to intraperitoneal injection of bacterial antigens (Brown et al. 2008). The crude protein extracts of gaduscidin also contains high antimicrobial activities. This peptides has several histidine residues, for example, Gad-1 has five and Gad-2 has four (McDonald et al. 2015).
Isoforms and Classes of Piscidin
Piscidin was present as different isomers in various fish species. Though they are structurally similar, they are functionally different. Piscidin 1 was first isolated from mast cells of the hybrid striped bass (Lee et al. 2014); it contains a potent AMP of 22 amino acid sequence which is conserved with other isoforms of piscidin in the amino terminus, where histidine and phenylalanine are rich. It is active against fungi, yeast, viruses, parasites, Gram-positive and Gram-negative bacteria and even active against antibiotic-resistant bacteria. As it has some hemolytic and cytotoxic effects, its therapeutic usage has been limited (Lee et al. 2007). It has the highest antibacterial activity among other piscidins (Lauth et al. 2001; Noga and Silphaduang 2003). Piscidin 1 was reported to have potent antimicrobial activity against Methicillin Resistant S. aureus (Menousek et al. 2012). It has the ability to permeabilize cancer cell membranes by interacting with a negative charge on their membranes. As lipids get through the alpha-helix of piscidin, it forms a toroidal hole lined by peptides and lipid groups (Lin et al. 2012).
Piscidin 2, a 22 amino acid residue with a conserved amino-terminal, rich in histidine and phenylalanine (Sung et al. 2008). Piscidin 2 differs to piscidin 1 only by single amino acid substitution at its 18th position and has an identical antimicrobial property (Colorni et al. 2008). It showed activity against viruses, fungi and bacteria, even against antibiotic-resistant bacteria (Lauth et al. 2001; Sung et al. 2008). Piscidin 2 is active against infective stages of parasites and is a potent component of antimicrobial defense in various fish species (Lee et al. 2014). Piscidin 2 is a lytic peptide which disrupts the permeability of microbial membrane leading to lysis of the cell. The parasite, Ichthyophthirius multifiliis was sensitive towards the exposure of piscidin 2 showing a lethal effect. Due to low cation concentration in freshwater, the sensitivity is more. As seawater has high concentrations of monovalent and divalent cations, they are inhibitory to most of the antibiotics. Comparatively, the peptide is active in salt and it is active against marine parasites as well. It was also reported that piscidin 1 and 2 are highly active against S. aureus even in the presence of high concentration of monovalent and divalent cations (Colorni et al. 2008). Piscidin 1 and 2 were also derived from skin and gill tissues of striped bass (Campagna et al. 2007).
Piscidin 3 is 22 amino acid in length; also, it is a cationic, amphipathic and membrane disruptive peptide with high histidine residues. It has shown anti-inflammatory properties both in in vitro and in vivo condition and can also be used as an anesthetic compound (Hayden et al. 2015). Piscidin 3 has an antiparasitic activity in which the mechanism is undetermined, and it is inhibitory to all bacterial pathogens as like piscidin 1 and 2 (Colorni et al. 2008). Compared to piscidin 2 and piscidin 1, piscidin 3 showed low minimum inhibitory concentration and minimum bacterial concentration (Moon et al. 2007). It is also showed low amphipathicity as it has a glycine at its 17th position (Lee et al. 2007). The piscidin 1, 2 and 3 are highly basic with isoelectric points ranged between 11.27 and 12.3.
Piscidin 4 is a 44 amino acid length peptide (Salger et al. 2011) with a molecular weight of 5329 Da and its N-terminus was highly homologous to other piscidins like Pis-1, Pis-2 and Pis-3. Piscidin 4 has pI of 11.23, which is highly basic in nature and they form a large hydrophobic region due to the presence of Phe2, Leu5, Phe6, Ala9, Ile12, Phe13, Ala16, Trp20 and Val34 in the sequence; altogether these residues formed a helix and loop regions (Noga et al. 2009). It has antibacterial activity against both Gram-negative and Gram-positive bacteria including pathogens causing pasteurellosis and lactococciosis (Salger et al. 2011; Peng et al. 2012b) caused by Photobacterium damselae subsp., piscicida and S. iniae. They are also active against multi-antibiotic resistant strains of L. garviae and even against some human pathogens. Piscidin 4 has a coil at the C-terminal end which separates two β-sheet (Lauth et al. 2001) and alpha-helix at the N-terminal, and it was present both in white bass and striped bass. This peptide has a modified amino acid on its 20th position which is a hydroxylated tryptophan based on mass spectrometry analysis and the Schiffer-Edmunson plot; both the reports suggest that piscidin 4 has an amphipathic α-helix structure (Park et al. 2011). As piscidin 4 has more glycine and proline residues than piscidin 1, it shows more α-helicity that causes flexibility and bending (Lee et al. 2007).
Piscidin 5 was reported from M. chrysops and it was highly expressed in the intestine. The β-sheets present in piscidin 4 and 5 are similar to carbohydrate and lipopolysaccharide-binding motifs which helps in pattern recognition function in innate immunity (Yoshida et al. 2001; Lauth et al. 2001; Patrzykat et al. 2002; Mason et al. 2006). Piscidin 5 is a mature peptide with 44 amino acids that contains a signal peptide with 22 amino acid at N terminal and 4 amino acid as prodomain at C-terminal (Pan et al. 2018). Piscidin 5 like peptide plays an immune response in large yellow croaker (Larimichthys crocea). It was showing 86% similarity with other homologous species (Zhou et al. 2014).
Piscidin 6 and 7 belongs to Class III family based on their structure as well as function. Piscidin 6 gene expression was predominantly observed in gill, intestine and kidney of M. saxatilis and M. chrysops. Also, piscidin 7 was expressed in the intestine of M. saxatilis (Campoverde et al. 2017). The different isomers of piscidin, their amino acid sequence and physico-chemical properties including isolelctric point and molecular weight are listed in Table 2.
Piscidin family were classified into three major classes based on the number of amino acid residues present in it. Piscidin 1, 2 and 3 belong to Class I while piscidin 4 and 5 belong to Class II and piscidin 6 and 7 belong to Class III family. Class I has 22 amino acid residues, whereas Class II and Class III has 44–46 and 55 amino acid residues, respectively (Salger et al. 2016). Class I piscidins have the highest activity against Gram-positive bacteria compared to Class II and class III piscidins. Class I piscidin showed higher activity against prokaryotes and ciliated protozoans compared to class II and class III (Campoverde et al. 2017). Class II piscidins have the highest activity against Gram-negative bacteria and Class III piscidins have very little activity against bacteria, whereas high activity was reported against protozoan (Salger et al. 2016; Shin et al. 2017). Class III piscidins has coil-β sheet-coil-α helix structure which is different from that of Class I and Class II (Salger et al. 2016). The seven piscidins isolated from different fish sources and the classification was listed in Table 3.
Structure of Piscidin
The structure of piscidin was determined by Nuclear Magnetic Resonance (NMR) spectroscopy; it shows that piscidin 1 formed an α-helical structure in SDS micelles (Jeong et al. 2016). Utilizing dodecyl phosphocholine (DPC) micelles, a zwitterionic lipid surface the three-dimensional structure was determined (Campagna et al. 2007). Piscidin-1 has an amphipathic α-helical structure, as it contains hydrophobic and hydrophilic amino acids in opposite sides which was determined by solid-state NMR (Lee et al. 2007). The solid-state NMR study shows that Pis-1 is parallelly oriented to membrane surface. The peptide-lipid interactions are enhanced by water-bilayer interface of amphipathic cationic AMPs (Lee et al. 2007). As reported Perrin et al. (2014) utilizing 3:1 ratio of 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC)/1,2-dimyristoyl-sn-glycero-3-phosphatidylglycerol (DMPG) and 1-palmitoyl-2-oleoyl-sn-glycerophosphatidylethanolamine (POPE)/1-palmitoyl-2-oleoyl-sn-glycero-phosphoglycerol (POPG) lipid bilayers, different high-resolution structures of piscidin 1 and piscidin 3 were determined (Perrin et al. 2014). In a circular dichroism analysis, Piscidin 2 was unstructured in the water while in trifluoroethanol showed α helical structure (Lauth et al. 2001; Sung et al. 2008). The prediction of the secondary structure of piscidin 4 suggested an alpha-helix in N-terminal and a random coil in the C-terminal of the sequence (Noga et al. 2009). Piscidin 4 has high activity towards DPPC and EYPC liposome which contain low alpha-helical regions (Lee et al. 2007). The piscidin 5 like peptide is more similar to piscidin 4; and piscidin 5 has a major role in immune response (Zhou et al. 2014).
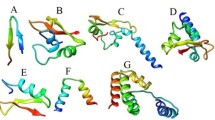
The structural diversity of piscidin is due to the adaptation of microbes in different environments (De Angelis et al. 2011). Kumaresan et al. (2018) have identified two isoforms of piscidin, which has conserved as well as variable regions with three distinct α-helices. The three-dimensional structure of piscidin was depicted in Fig. 1 (Kumaresan et al. 2019).
Gene Regulation of Piscidin
Piscidin was found as conserved among teleosts and it was upregulated by various pathogenic responses. This regulation towards pathogenic challenge is species and gene-specific; the regulation of piscidin was recognized by pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs), such as the action of an intracellular signaling cascade which includes MyD88, Toll-like receptors (TLRs), TRAF6, IRAK1 and IKK. The signaling cascade helps in the activation of NF-κB which translocates to the nucleus, where AMPs and effector molecules transcriptionally activated (Campoverde et al. 2017). Piscidin expression was observed in Nile tilapia, rodlet cells of mesentery organs, phagocytic granular cells of head kidney and gills of gilthead seabream (Sparus aurata) (Mulero et al. 2008) and in alimentary tract and gill of pearl gourami (Trichogaster leeri) (Silphaduang et al. 2006; Ruangsri et al. 2012b). The up and down-regulation of the different classes of piscidins against various pathogens in fish species are presented in Table 4. In fish, piscidins are received by a pathogen that contains phagosomes, thus the peptides act as an antimicrobial agent for bactericidal activity through phagocytosis due to the granules present in the phagocytic granulocytes of piscidin (Iijima et al. 2003; Andrews et al. 2010). The positively charged sites present in the piscidin gene codes for mature peptide which provide protection against evolving host pathogens (Peng et al. 2012a). Piscidin was introduced by some stimuli like Gram-positive and Gram-negative bacteria, bacterial cell components like LPS, bacterial antigen like ASAL and also by parasites, viruses and poly I:C (Masso-Silva and Diamond 2014).
Most piscidin genomic organization have four-exon and three-intron (Cole et al. 2000), whereas piscidin from Nile tilapia has a three-exon and two-intron in structure (Peng et al. 2012a). The epinecidin from yellow croaker and grouper has five-exon and four-introns (Pan et al. 2008). The proteolytic cleavage at N-terminal and prepropeptide of C-terminal leads to the removal of the signal peptide and prodomain, respectively; hence, releasing a short-matured peptide, piscidin. Piscidin has an amphipathic structure with hydrophilic cationic amino acids and can, therefore, interact with pathogenic membranes (Bae et al. 2014). During fish development, the piscidin expression was upregulated. The levels of gene expression of piscidin are noticed in skin mucus, skin, gill, head kidney, intestine/gastrointestinal tract, spleen, blood, liver, rectum, gall bladder, stomach, pyloric caeca, heart and muscle as high to low (Iijima et al. 2003; Salger et al. 2011). Most commonly piscidins are present in mast cells of gills and rodlet cells of skin, intestine, gill, epithelial mucous cells, eosinophilic granular cells, phagocytic granulocytes and intestinal goblet cells of intestinal mucosa (Katzenback 2015). The promoter regions of piscidin contains binding sites namely γ-IRE, α-IRE, C/EBPβ, NF-IL-6, AP-1 and hepatocyte nuclear factor (HNF-1) transcriptional factors; these receptors involved in intracellular signaling pathway and transcriptional factors in piscidin regulation (Lee et al. 2014; McDonald et al. 2015; Pan et al. 2007; Han et al. 2016; Campoverde et al. 2017).
Piscidin has an immune-modulatory role that expresses both pro-inflammatory genes and immune-related genes such as IL-10, IL-1β, TNF-α, NOS2, NF-kb, Myd88, TLR3, TLR1 and TLR4a by releasing NF-κB via IKB down-regulation; also, there is a down-regulation of some anti-inflammatory signals. In addition, a reduction of inflammatory cytokines by pathogen expression was observed in fishes (Menousek et al. 2012; Noga et al. 2009; Sun et al. 2007). Immunomodulatory activity of pleurocidin belongs to piscidin family in which expression of IL-1β and COX1 was induced by RTS11 macrophage cell line, where no effect was observed on antigen presentation or Mx gene expressions by JAK/STAT (Chiou et al. 2006); Mx protein is the interferon-induced antiviral protein, a product of the Mx gene (Jensen and Robertsen 2000). The Mx proteins have a role in resistance to negative-stranded RNA viruses, thus function as antiviral defense (Trobridge and Leong 1995). Pleurocidin does not affect the LPS induced responses, while cured barramundi brain (cBB) fish cell line induced Mx transcripts (Wang et al. 2010). In response to piscidin transcripts, Mx2 and Mx3 were down-regulated in grouper (Wang et al. 2010). In transgenic zebrafish epinecidin-1 expression was induced due to pathogenic microbes (Peng et al. 2010). When the fish is expressing epinecidin-1 due to E. coli, the expression of TNF, IL-1β, TLR4, NF-κB and nitric oxide synthase 2 were also observed (Katzenback 2015). The immunomodulatory activity of piscidin in fish enabled the expression of pro-inflammatory and other immune-related genes such as IL-1β, IL-22, TNF-α, IL-26, IFN-γ, lysozyme, IL-10, NOS2, NF-κB, MyD88, TLR1, TLR4a and TLR3 (Baumann 1991; Larrick et al. 1995; Akira et al. 2006; Lee et al. 2007; Noga et al. 2009; Hayden et al. 2015).
Piscidin was expressed in fish during embryonic development and the amount of expression was increased between day 8 and day 40 (Noga et al. 2009; Shin et al. 2017). However, a reduction was observed in 40 day old larvae, it was due to the dilution effect because of the rapid growth occurred after metamorphosis. In the developed juveniles, piscidin was highly expressed in gill, moderately in kidney and spleen and lower in gut (Noga et al. 2009; Dezfuli et al. 2010).
The gene expression of piscidin 1 was only found in striped bass and piscidin 2 were only found in white bass. While Piscidin 4 gene is inherited from both or any one of the parental species of hybrid striped bass. Piscidin 4 is one of the major components of host antibacterial defenses (Noga et al. 2009). The gene expression of piscidin 4 shown in both species; mainly the highest expression was noticed in gills and the lowest was observed in the foregut of intestine (Park et al. 2011). The striped bass and white bass piscidin orthologs contributed to the alleles of the hybrid striped bass genome as six different piscidin from 4 loci (piscidin 1, 3, 4, and 6), another 2 alleles from 2 loci (piscidin 5 and 7) 1 allele from 1 loci (piscidin 2) which are all derived from parental species (Salger et al. 2016).
Mechanism of Piscidin as Antimicrobial
The AMP has a net positive charge, a high isoelectric point which interacts with negatively charged bacterial membrane, partial or complete insertion of the lipid bilayer, flexible in structure and active biological conformation on binding with membrane (De Angelis et al. 2011). The mode of action of a peptide depends on the charge, length, amphipathicity, concentration of peptide (Hayden et al. 2015) and lipid composition of the membrane (Campagna et al. 2007). The amphipathic nature of these peptides was important for their mechanism of action against bacteria as it can alter antimicrobial activity (Haney and Hancock 2013). The interfacial activity of AMPs depends on the balance of hydrophobic and electrostatic interactions of peptides with water and lipids (Ii 2010). The proteoglycan and lipopolysaccharide outer membrane of Gram-positive and Gram-negative, respectively were crossed easily by AMPs while the permeation of large marker took time (Campagna et al. 2007; Perrin et al. 2014). After the contact of AMPs with the membrane, there is a chance of disruption, destruction and fragmentation (Ii 2010).
In low peptide-lipid ratio, the peptide binds parallel to the lipid bilayer, as peptide-lipid ratio increases, the peptide starts to be perpendicular to the membrane (Park and Hahm 2005). Thus, the membrane permeabilization was explained in different models including barrel-stave, carpet-like, toroidal pore models (Campagna et al. 2007) and detergent model (Ii 2010). In the barrel-stave model, the transmembrane pore formation leads to membrane permeabilization which was made of helices (Campagna et al. 2007). In the carpet-like mechanism, the peptides act as a detergent by forming pore by disrupting the bacterial membranes (Campagna et al. 2007). The correct mode of action of peptides was not established; however, it was suggested that the bacterial cells were lysed by disruption of the bacterial cell membrane (Moon et al. 2007) and disrupt the intercellular components (Hayden et al. 2015). The piscidin peptide disrupts the bacterial cell membrane by toroidal pore mechanism; lipids of the membrane were inserted between the α-helices of peptide (Corrales et al. 2010). The piscidin AMP showed similar characteristic features based on the mechanism involved in the membrane permeabilization compared to cationic AMPs (Rahmanpour et al. 2013).
AMPs are believed to kill microorganisms in two methods of mode of action, which are non-receptor mediated process and receptor-mediated process. The Gram-positive and Gram-negative bacteria attain a negative charge as they contain teichoic acids and lipopolysaccharides, respectively on their surfaces; they also contained negatively charged phospholipids. As antimicrobial peptide has a net positive charge, it binds to the negatively charged outer surface of bacteria. The lysis of bacterial cells occurs due to the following two processes: (i) the peptide lyses the bacterial membrane by pore formation which results in transmembrane electrochemical gradient damage that leads to loss of energy and ultimately further leads to cell swelling and osmosis (Mulero et al. 2008; Peng et al. 2012b; Masso-Silva and Diamond 2014) and (ii) the peptide act as a multi-hit mechanism, which includes more than one anionic targets (Shai 2002).
The piscidin peptide forms toroidal pores in the membrane as it interacts with acidic phospholipids (Pan et al. 2008; Bae et al. 2014). Their anti-fungal and anti-tumor activity occurs through pore formation, membrane permeabilization and by inducing Reactive Oxygen Species (ROS) and apoptotic pathways (Ebbensgaard et al. 2015; Pan et al. 2007; Dezfuli et al. 2011; Meloni et al. 2015). The peptide has a direct defense mechanism against a wide range of pathogens (Katzenback 2015). The disruption of cell membrane depends on the membrane composition, for example piscidin 1 and 3 by the formation of parallel α helical membrane, they induce a membrane antimicrobial interaction (Masso-Silva and Diamond 2014). The mode of action was through permeabilizing the plasma membrane of the pathogen (Campoverde et al. 2017). The piscidin peptide binds on the bacterial surface based on charge and formed toroidal formation as their mode of action depicted in Fig. 2.
AMPs are interacting as a direct antibacterial mechanism that leads to membrane perturbation, disruption of membrane-associated physiological events to interact with cytoplasmic targets. The positively charged AMP interact with negatively charged lipids in the outer cytoplasmic membrane. The alteration in membrane structure and localized perturbation results in the reorientation of peptide molecules. The peptides diffuse into the cytoplasm through the membrane and reach intracellular targets. The physicochemical and structural characteristic features depend on the interaction of AMPs with membrane (Fjell et al. 2012). The mechanism of piscidin peptide suggests that it targets bacterial and fungal membranes; the replacement of Pro for Gly exhibited bacterial cell selectivity. Pro gives a bent structure in two helices which are for bacterial cell selectivity (Jeong et al. 2016). Also, piscidin functions as an anti-bacterial activity which interacts with acidic phospholipids, thus formed toroidal pores in the membrane (Katzenback 2015).
Piscidin exhibits an antimicrobial activity by forming toroidal pores in bacterial membrane like that of cationic AMPs involved in permeabilization (Mehrnejad and Zarei 2010). The binding and disrupting properties of piscidin 1 and 3 helps in translocation of bacterial cell membranes and attach to the target sites as the mechanism of cell death. The physicochemical properties including cationic, amphipathicity, number of cationic residues and histidine present in piscidin 1 and 3 help in translocating across membranes and synthesis of macromolecules (Hayden et al. 2015).
Other Properties of Piscidin
Piscidins showed antibacterial, antifungal and antiviral properties (Ii, 2007; Dezfuli et al. 2010); recently it was found to have anti-parasitic properties (Colorni et al. 2008). Along with antimicrobial activity, piscidin has anti-tumor activity against cancer-derived cell lines including A549, U937, HT1080,U937, HeLa, HL60,MDA-MB-468, SKBR3, MCF7, T47-D, MDA-MB-231, MCF7-TX400 (paclitaxel-resistant MCF7) and 4T1 (Masso-Silva and Diamond 2014). Piscidins are hemolytic than magainin 2 (Hicks et al. 2003) and less hemolytic than mellitin (Moon et al. 2007). Piscidin 1 can be used as anticancer drug by modifying their sequence form so that other mammalian cells do not damage. The single or multiple modifications in their amino acid sequence may lead to the reduction in hydrophobicity, amphipathicity and helicity, which help to reduce hemolytic action (Lin et al. 2012). Piscidin 1 shown HIV inhibition activity which suggests the importance of cationic arginine side chain. It also shows anti-cancer properties against HeLa and HT1080 cells. Piscidin 2 also has a similar kind of antimicrobial properties (Chen and Cotten 2014).
Among piscidins, piscidin 3 was the least hemolytic peptide (Park et al. 2011). The cytotoxicity and antimicrobial activity increase with the hydrophobicity of peptides (Jeong et al. 2016) and which can be reduced by replacing with positively charged amino acids such as lysine. Piscidin 1 is highly cytotoxic due to the presence of hydrophobic amino acids. Comparatively, piscidin 3 has weaker antimicrobial activity than piscidin 1 and 2 (Colorni et al. 2008). When we compare the peptide treated with untreated samples, the protoplast cell wall was regenerated in treated samples. The antifungal property of piscidin 2 is due to the interaction of the peptide with plasma membrane rather than cell wall, which suggests that peptide act on plasma membrane of Candida albicans for exhibiting fungicidal activity (Sung et al. 2008). Piscidin 2 was non-hemolytic against both sheep and human erythrocytes at a concentration lesser than 2.5 µM (Campagna et al. 2007). Piscidin 4 exhibits less hemolytic activity than piscidin 1 in which the C-terminal region prevents the insertion into hydrophobic erythrocyte membrane (Lee et al. 2007).
Therapeutic Applications
The AMPs are most important in pharmaceutics as they are not as resistant as antibiotics. It is involved in chemotaxis, wound healing and can also be used against cancer (Li et al. 2012). At present, the AMPs are the therapeutically important compound with low toxicity and high efficacy in treating bacterial pathogens (Schuerholz et al. 2012). Piscidin 2 disrupts the fungal membrane (Rajanbabu and Chen 2011) and exhibits antifungal activity against human pathogenic fungi (Rakers et al. 2013). This fungicidal properties against C. albicans, Malassezia furfur and Trichosporon beigelii are at MIC values of 1.25–6.25 µM. The antimicrobial activities of piscidin 1 and 2 were found against Gram-positive and Gram-negative bacteria at 0.04–10 µM concentration. These were also found active against Tetrahymena pyriformes with PCmin value of 1.25 µM. These peptides were potent against V. vulnificus, V. alginolyticus, P. aeruginosa, S. agalactiae 819 and S. agalactiae BCRC 10787 strains (Peng et al. 2012b). The piscidin derived from tilapia can be used for wound healing due to bacterial infection. Piscidin AMPs showed activity against 11 strains of Acinetobacter baumanni and 6 strains of P. aeruginosa; and shows low hemolytic activity with human red blood (Jiang et al. 2014). Piscidin showed inhibitory activity against porcine epidemic diarrhea virus (PEDV), pseudorabies virus (PRV), transmissible gastroenteritis virus (TGEV), porcine reproductive and respiratory syndrome virus (PRRSV) and rotavirus (RV) (Hu et al. 2019). Piscidins can be used instead of antibiotics which has no immunotoxic effect (Huang et al. 2015). There is a high demand for AMPs in the food industry to invent the effectiveness of some of the inhibitory compounds, hence these can be used as potential food preservatives too (Li et al. 2012).
Conclusions
The AMPs have great importance in fish as well as human environments as the resistance of different bacterial and other organisms are very common. Day by day the antibiotic resistance is increasing which leads to the necessity of derived AMPs from natural resources. The piscidin peptides from a lower vertebrate group have both commercial as well as medicinal importance. The details related to the structure, classes, gene expression, mechanism and therapeutical importance discussed in this review provided a way to consider its importance in aquaculture industry as well as for human purpose. Apart from the antimicrobial nature of piscidin peptide, it can also be used as an anti-neuropathic, anti-nociceptive, anti-endotoxic and anesthetic compound. Furthermore, there is a need to have research on its designing for the large-scale therapeutics approach. So that, these peptides in humans as well as in aquaculture organisms play a variety of application roles.
Abbreviations
- AMPs:
-
Antimicrobial peptides (AMPs)
- AMPPs:
-
Antimicrobial peptides and proteins
- SGIV:
-
Singapore grouper iridovirus
- VNNV:
-
Viral nervous necrosis virus
- MHC:
-
Major histocompatibility complex
- TLR:
-
Toll-like receptor
- EST:
-
Expressed sequence tag (EST)
- LPS:
-
Lipo poly saccharide
- NMR:
-
Nuclear magnetic resonance
- DPC:
-
Dodecyl phosphocholine
- DMPC:
-
1,2-Dimyristoyl-sn-glycero-3-phosphatidylcholine
- DMPG:
-
1,2-Dimyristoyl-sn-glycero-3-phosphatidylglycerol
- POPE:
-
1-Palmitoyl-2-oleoyl-sn-glycerophosphatidylethanolamine
- POPG:
-
1-Palmitoyl-2-oleoyl-sn-glycero-phosphoglycerol
- PAMPs:
-
Pathogen-associated molecular patterns
- PRRs:
-
Pattern recognition receptors
- HNF:
-
Hepatocyte nuclear factor
- cBB:
-
Cured barramundi brain
- PEDV:
-
Porcine epidemic diarrhea virus
- PRV:
-
Pseudorabies virus
- TGEV:
-
Transmissible gastroenteritis virus
- PRRSV:
-
Porcine reproductive and respiratory syndrome virus
- RV:
-
Rotavirus
- QCM-D:
-
Quartz crystal microbalance with dissipation monitoring
References
Acosta J, Montero V, Carpio Y, Velázquez J (2013) Cloning and functional characterization of three novel antimicrobial peptides from tilapia (Oreochromis niloticus). Aquaculture 372:9–18. https://doi.org/10.1016/j.aquaculture.2012.07.032
Akila S, Rajesh P, Arasu MV, Al-Dhabi NA, Mukesh P, Arockiaraj J (2018) Fish heat shock cognate 70 derived AMPs CsHSC70 A1 and CsHSC70 A2. Int J Pept Res Ther 24:143–155. https://doi.org/10.1007/s10989-017-9599-z
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4):783–801. https://doi.org/10.1016/j.cell.2006.02.015
Andreu D, Rivas L (1998) Animal antimicrobial peptides: an overview. Biopolym Pept Sci Sect 47:415–433. https://doi.org/10.1002/(SICI)1097-0282
Andrews M, Battaglene S, Cobcroft J, Adams M, Noga E, Nowak B (2010) Host response to the chondracanthid copepod Chondracanthus goldsmidi, a gill parasite of the striped trumpeter, Latris lineata (Forster), in Tasmania. J Fish Dis 33:211–220. https://doi.org/10.1111/j.1365-2761.2009.01107.x
Arasu A, Kumaresan V, Sathyamoorthi A, Chaurasia MK, Bhatt P, Gnanam AJ, Palanisamy R, Marimuthu K, Pasupuleti M, Arockiaraj J (2014) Molecular characterization of a novel proto-type antimicrobial protein galectin-1 from striped murrel. Microbiol Res 169(11):824–834. https://doi.org/10.1016/j.micres.2014.03.005
Arasu A, Kumaresan V, Palanisamy R, Arasu MV, Al-Dhabi NA, Ganesh MR, Arockiaraj A (2017) Bacterial membrane binding and pore formation abilities of carbohydrate recognition domain of fish lectin. Dev Comp Immunol 67:202–212. https://doi.org/10.1016/j.dci.2016.10.001
Arasu A, Kumaresan V, Ganesh MR, Pasupuleti M, Arasu MV, Al-Dhabi NA, Arockiaraj J (2017) Bactericidal activity of fish galectin 4 derived membrane-binding peptide tagged with oligotryptophan. Dev Comp Immunol 71:37–48. https://doi.org/10.1016/j.dci.2016.10.001
Arockiaraj J, Gnanam AJ, Dhanaraj M, Ranganath G, Milton J, Singh A, Saravanan M, Marimuthu K, Bhassu S (2012) Crustin, a WAP domain containing antimicrobial peptide from freshwater prawn M. rosenbergii: immune characterization. Fish Shellfish Immunol 34:109–118. https://doi.org/10.1016/j.fsi.2012.10.009
Arockiaraj J, Gnanam AJ, Kumaresan V, Palanisamy R, Bhatt P, Thirumalai MK, Roy A, Pasupuleti M, Kasi M (2013) An unconventional antimicrobial protein histone from freshwater prawn Macrobrachium rosenbergii: analysis of immune properties. Fish Shellfish Immunol 35(5):1511–1522. https://doi.org/10.1016/j.fsi.2013.08.018
Arockiaraj J, Kumaresan V, Bhatt P, Palanisamy R, Gnanam AJ, Pasupuleti M, Kasi M, Chaurasia MK (2014) A novel single-domain peptide, anti-LPS factor from prawn: synthesis of peptide, antimicrobial properties and complete molecular characterization. Peptides 53:79–88. https://doi.org/10.1016/j.peptides.2013.11.008
Arockiaraj J, Kumeresan V, Chaurasia MK, Bhatt P, Palanisamy R, Pasupuleti M, Gnanam AJ, Kasi M (2014) Molecular characterization of a novel cathepsin B from striped murrel Channa striatus: bioinformatics analysis, gene expression, synthesis of peptide and antimicrobial property. Turk J Fish Aquat Sci 14:379–389. https://doi.org/10.4194/1303-2712-v14_2_08
Arockiaraj J, Chaurasia MK, Kumaresan V, Palanisamy R, Harikrishnan R, Pasupuleti M, Kasi M (2015) Macrobrachium rosenbergii mannose binding lectin: synthesis of MrMBL-N20 and MrMBL-C16 peptides and their antimicrobial characterization, bioinformatics and relative gene expression analysis. Fish Shellfish Immunol 43:364–374. https://doi.org/10.1016/j.fsi.2014.12.036
Bae JS, Shim SH, Hwang SD, Park MA, Jee BY, An CM, Kim YO, Kim JW, Park CI (2014) Expression analysis and biological activity of moronecidin from rock bream, Oplegnathus fasciatus. Fish Shellfish Immunol 40:345–353. https://doi.org/10.1016/j.fsi.2014.07.023
Baumann M (1991) A method for identifying a proposed carbohydrate-binding motif of proteins. Glycobiology 1(5):537–542. https://doi.org/10.1093/glycob/1.5.537
Bo J, Yang Y, Zheng R, Fang C, Jiang Y, Liu J, Wang K (2019) Antimicrobial activity and mechanisms of multiple antimicrobial peptides isolated from rockfish Sebastiscus marmoratus. Fish Shellfish Immunol 93:1007–1017. https://doi.org/10.1016/j.fsi.2019.08.054
Brown T, Chipman J, Katsiadaki I, Sanders M, Craft JA (2008) Construction of subtracted EST and normalised cDNA libraries from liver of chemical-exposed three-spined stickleback (Gasterosteus aculeatus) containing pollutant-responsive genes as a resource for transcriptome analysis. Mar Environ Res 66(1):127–130. https://doi.org/10.1016/j.marenvres.2008.02.043
Browne M, Feng C, Booth V (2011) Characterization and expression studies of Gaduscidin-1 and Gaduscidin-2; paralogous antimicrobial peptide-like transcripts from Atlantic cod (Gadus morhua). Dev Comp Immunol 35(3):399–408. https://doi.org/10.1016/j.dci.2010.11.010
Bulet P, Stöcklin R, Menin L (2004) Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev 198:169–184. https://doi.org/10.1111/j.0105-2896.2004.0124.x
Burrowes OJ, Hadjicharalambous C, Diamond G, Lee TC (2006) Evaluation of antimicrobial spectrum and cytotoxic activity of pleurocidin for food applications. J Food Sci 69(3):66–71. https://doi.org/10.1111/j.1365-2621.2004.tb13373.x
Campagna S, Saint N, Molle G, Aumelas A (2007) Structure and mechanism of action of the antimicrobial peptide piscidin. Biochemistry 46:1771–1778. https://doi.org/10.1021/bi0620297
Campoverde C, Milne DJ, Estévez A, Duncan N, Secombes CJ, Andree KB (2017) Ontogeny and modulation after PAMPs stimulation of β-defensin, hepcidin, and piscidin antimicrobial peptides in meagre (Argyrosomus regius). Fish Shellfish Immunol 69:200–210. https://doi.org/10.1016/j.fsi.2017.08.026
Chaithanya ER, Philip R, Sathyan N, Anil Kumar PR (2013) Molecular characterization and phylogenetic analysis of a histone-derived antimicrobial peptide teleostin from the marine teleost fishes, Tachysurus jella and Cynoglossus semifasciatus. ISRN Mol Biol 13:1–7. https://doi.org/10.1155/2013/185807
Chaurasia MK, Palanisamy R, Bhatt P, Kumaresan V, Gnanam AJ, Pasupuleti M, Kasi M, Harikrishnan R, Arockiaraj J (2014) A prawn core histone 4: derivation of N and C terminal peptides and their antimicrobial properties, molecular characterization and mRNA transcription. Microbiol Res 170:78–86. https://doi.org/10.1016/j.micres.2014.08.011
Chee PY, Mang M, Lau ES, Tan LTH, He YW, Lee WL, Goh BH (2019) Epinecidin-1, an antimicrobial peptide derived from grouper (Epinephelus coioides): pharmacological activities and applications. Front Microbiol. https://doi.org/10.3389/fmicb.2019.02631
Chen J, Lin W, Wu J, Her G, Hui CF (2009a) Epinecidin-1 peptide induces apoptosis which enhances antitumor effects in human leukemia U937 cells. Peptides 30(12):2365–2373. https://doi.org/10.1016/j.peptides.2009.08.019
Chen JY, Lin WJ, Lin TL (2009b) A fish antimicrobial peptide, tilapia hepcidin TH2-3, shows potent antitumor activity against human fibrosarcoma cells. Peptides 30(9):1636–1642. https://doi.org/10.1016/j.peptides.2009.06.009
Chen W, Cotten ML (2014) Expression, purification, and micelle reconstitution of antimicrobial piscidin 1 and piscidin 3 for NMR studies. Protein Expr Purif 102:63–68. https://doi.org/10.1016/j.pep.2014.08.001
Chinchar VG, Bryan L, Silphadaung U, Noga E, Wade D, Rollins-Smith L (2004) Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology 323:268–275. https://doi.org/10.1016/j.virol.2004.02.029
Choi H, Lee DG (1820) Antimicrobial peptide pleurocidin synergizes with antibiotics through hydroxyl radical formation and membrane damage, and exerts antibiofilm activity. Biochim Biophys Acta (BBA) 12:1831–1838. https://doi.org/10.1016/j.bbagen.2012.08.012
Cole AM, Darouiche RO, Legarda D, Connell N, Diamond G (2000) Characterization of a fish antimicrobial peptide: gene expression, subcellular localization, and spectrum of activity. Antimicrob Agents Chemother 44:2039–2045. https://doi.org/10.1128/AAC.44.8.2039-2045.2000
Colorni A, Ullal A, Heinisch G, Noga EJ (2008) Activity of the antimicrobial polypeptide piscidin 2 against fish ectoparasites. J Fish Dis 31:423–432. https://doi.org/10.1111/j.1365-2761.2008.00922.x
Conlon J, Mechkarska M, Lukic M, Flatt PR (2014) Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 57:67–77. https://doi.org/10.1016/j.peptides.2014.04.019
Corrales J, Gordon WL, Noga EJ (2009) Development of an ELISA for quantification of the antimicrobial peptide piscidin 4 and its application to assess stress in fish. Fish Shellfish Immunol 27:154–163. https://doi.org/10.1016/j.fsi.2009.02.023
Corrales J, Mulero I, Mulero V, Noga EJ (2010) Detection of antimicrobial peptides related to piscidin 4 in important aquacultured fish. Dev Comp Immunol 34:331–343. https://doi.org/10.1016/j.dci.2009.11.004
Costa F, Gomes P, Martins MCL (2018) Antimicrobial peptides (AMP) biomaterial coatings for tissue repair. Peptides and proteins as biomaterials for tissue regeneration and repair. Woodhead Publishing, Cambridge, pp 329–345. https://doi.org/10.1016/b978-0-08-100803-4.00013-9
Cuesta A, Meseguer J, Esteban M (2008) The antimicrobial peptide hepcidin exerts an important role in the innate immunity against bacteria in the bony fish gilthead seabream. Mol Immunol 45(8):2333–2342. https://doi.org/10.1016/j.molimm.2007.11.007
De Angelis AA, Grant CV, Baxter MK, McGavin JA, Opella SJ, Cotton ML (2011) Amphipathic antimicrobial piscidin in magnetically aligned lipid bilayers. Biophys J 101:1086–1094. https://doi.org/10.1016/j.bpj.2011.07.015
De Zoysa M, Nikapitiya C, Whang I, Lee JS, Lee J (2009) Abhisin: a potential antimicrobial peptide derived from histone H2A of disk abalone (Haliotis discus discus). Fish Shellfish Immunol 27(5):639–646. https://doi.org/10.1016/j.fsi.2009.08.007
Dezfuli BS, Pironi F, Giari L, Noga EJ (2010) Immunocytochemical localization of piscidin in mast cells of infected seabass gill. Fish Shellfish Immunol 28:476–482. https://doi.org/10.1016/j.fsi.2009.12.012
Dezfuli BS, Giari L, Lui A, Lorenzoni M, Noga EJ (2011) Mast cell responses to Ergasilus (Copepoda), a gill ectoparasite of sea bream. Fish Shellfish Immunol 30:1087–1094. https://doi.org/10.1016/j.fsi.2011.02.005
Diamond G, Beckloff N, Weinberg A (2009) The roles of antimicrobial peptides in innate host defense. Curr Pharm Des 15(21):2377–2392. https://doi.org/10.2174/138161209788682325
Ebbensgaard A, Mordhorst H, Overgaard MT, Nielsen CG, Aarestrup FM, Hansen EB (2015) Comparative evaluation of the antimicrobial activity of different antimicrobial peptides against a range of pathogenic bacteria. PLoS ONE 10:1–18. https://doi.org/10.1371/journal.pone.0144611
Elumalai P, Rubeena AS, Arockiaraj J, Wongpanya R, Cammarata M, Ringø E, Vaseeharan B (2019) The role of lectins in finfish: a review. Rev Fish Sci Aquacult 27:152–169. https://doi.org/10.1080/23308249.2018.1520191
Fernandes JMO, Ruangsri J, Kiron V (2010) Atlantic cod piscidin and its diversification through positive selection. PLoS ONE 5(3):e9501. https://doi.org/10.1371/journal.pone.0009501
Fjell CD, Hiss JA, Hancock REW, Schneider G (2012) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11:37–51. https://doi.org/10.1038/nrd3591
Gordon YJ, Romanowski EG, McDermott AM (2005) Mini review: a review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 30:505–515. https://doi.org/10.1080/02713680590968637
Guo M, Wei J, Huang X, Huang Y, Qin Q (2012) Antiviral effects of β-defensin derived from orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol 32(5):828–838. https://doi.org/10.1016/j.fsi.2012.02.005
Han H, Gopal R, Park Y (2016) Design and membrane-disruption mechanism of charge-enriched AMPs exhibiting cell selectivity, high-salt resistance, and anti-biofilm properties. Amino Acids 48(2):505–522. https://doi.org/10.1007/s00726-015-2104-0
Hancock R (2000) The role of antimicrobial peptides in animal defenses. PNAS 97(16):8856–8861. https://doi.org/10.1073/pnas.97.16.8856
Haney EF, Hancock REW (2013) Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 100:572–583. https://doi.org/10.1002/bip.22250
Hayden RM, Goldberg GK, Ferguson BM, Schoeneck MW, Libardo MDJ, Mayeux SE, Shrestha A, Bogardus KA, Hammer J, Pryshchep S, Lehman HK, McCormick ML, Blazyk J, Angeles-Boza AM, Fu R, Cotton ML (2015) Complementary effects of host defense peptides piscidin 1 and piscidin 3 on DNA and lipid membranes: biophysical insights into contrasting biological activities. J Phys Chem B 119:15235–15246. https://doi.org/10.1021/acs.jpcb.5b09685
Heras J, Koop B, Aguilar A (2011) A transcriptomic scan for positively selected genes in two closely related marine fishes: Sebastes caurinus and S. rastrelliger. Marine genomes 4(2):93–98. https://doi.org/10.1016/j.margen.2011.02.001
Hicks RP, Mones E, Kim H, Koser BW, Nichols DA, Bhattacharjee AK (2003) Comparison of the conformation and electrostatic surface properties of magainin peptides bound to sodium dodecyl sulfate and dodecylphosphocholine micelles. Biopolymers 68:459–470. https://doi.org/10.1002/bip.10325
Hiemstra PS, Amatngalim GD, Van Der Does AM, Taube C (2016) Antimicrobial peptides and innate lung defenses: role in infectious and noninfectious lung diseases and therapeutic applications. Chest 149:545–551. https://doi.org/10.1378/chest.15-1353
Hu Y, Yang Y, You QD, Liu W, Gu HY, Zhao L, Zhang K, Wang W, Wang XT, Guo QL (2006) Oroxylin A induced apoptosis of human hepatocellular carcinoma cell line HepG2 was involved in its antitumor activity. Biochem Biophys Res Commun 351:521–527. https://doi.org/10.1016/j.bbrc.2006.10.064
Hu H, Guo N, Chen S, Guo X, Liu X (2019) Antiviral activity of Piscidin 1 against pseudorabies virus both in vitro and in vivo. Virol J. https://doi.org/10.1186/s12985-019-1199-4
Huang HN, Chan YL, Wu CJ, Chen JY (2015) Tilapia Piscidin 4 (TP4) stimulates cell proliferation and wound closure in MRSA-infected wounds in mice. Mar Drugs 13:2813–2833. https://doi.org/10.3390/md13052813
Ii MV (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5(7):905–917. https://doi.org/10.1021/cb1001558
Iijima N, Tanimoto N, Emoto Y, Morita Y, Uematsu K, Murakami T, Nakai T (2003) Purification and characterization of three isoforms of chrysophsin, a novel antimicrobial peptide in the gills of the red sea bream, Chrysophrys major. Eur J Biochem 270:675–686. https://doi.org/10.1046/j.1432-1033.2003.03419.x
Jensen V, Robertsen B (2000) Cloning of an Mx cDNA from atlantic halibut (Hippoglossus hippoglossus) and characterization of Mx mRNA expression in response to double-stranded RNA or infectious pancreatic necrosis virus. J Interf Cytokine Res 20(8):701–710. https://doi.org/10.1089/10799900050116408
Jenssen H, Hamill P, Hancock REW (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511. https://doi.org/10.1128/CMR.00056-05
Jeong MC, Jeon D, Shin A, Jin S, Shin SY, Park YS, Kim Y (2016) Effects of hydrophobic peptoid substitutions on the bacterial cell selectivity and antimicrobial activity of Piscidin 1. Bull Korean Chem Soc 37:1545–1551. https://doi.org/10.1002/bkcs.10959
Jiang Z, Vasil AI, Vasil ML, Hodges RS (2014) “Specificity determinants” improve therapeutic indices of two antimicrobial peptides piscidin 1 and dermaseptin S4 against the gram-negative pathogens Acinetobacter baumannii and Pseudomonas aeruginosa. Pharmaceuticals 7:366–391. https://doi.org/10.3390/ph7040366
Jin JY, Zhou L, Wang Y, Li Z, Zhao JG, Zhang QY, Gui JF (2010) Antibacterial and antiviral roles of a fish β-defensin expressed both in pituitary and testis. PLoS ONE 5(12):e12883. https://doi.org/10.1371/journal.pone.0012883
Jung HJ, Park Y, Sung WS, Suh BK, Lee J, Hahm KS, Lee DG (2007) Fungicidal effect of pleurocidin by membrane-active mechanism and design of enantiomeric analogue for proteolytic resistance. Biochim Biophys Acta (BBA) 1768(6):1400–1405. https://doi.org/10.1016/j.bbamem.2007.02.024
Katzenback B (2015) Antimicrobial peptides as mediators of innate immunity in teleosts. Biology (Basel) 4:607–639. https://doi.org/10.3390/biology4040607
Kumaresan V, Bhatt P, Ganesh MR, Harikrishnan R, Arasu MV, Al-Dhabi NA, Pasupuleti M, Marimuthu K, Arockiaraj J (2015) A novel antimicrobial peptide derived from fish goose type lysozyme disrupts the membrane of Salmonella enterica. Mol Immunol 68:421–433. https://doi.org/10.1016/j.molimm.2015.10.001
Kumaresan V, Mukesh P, Arasu MV, Al-Dhabi NA, Arshad A, Amin SMN, Yusoff FM, Arockiaraj J (2018) A comparative transcriptome approach for identification of molecular changes in Aphanomyces invadans infected Channa striatus. Mol Biol Rep 45(6):2511–2523. https://doi.org/10.1007/s11033-018-4418-y
Kumaresan V, Pasupuleti M, Paray BA, Al-Sadoon MK, Arockiaraj J (2019) Gene profiling of antimicrobial peptides, complement factors and MHC molecules from the skin transcriptome of Channa striatus and its expression pattern during Aeromonas hydrophila infection. Fish Shellfish Immunol 84:48–55. https://doi.org/10.1016/j.fsi.2018.09.061
Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC (1995) Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. ASM 63(4):1291–1297
Lauth X, Shike H, Burns JC, Westerman ME, Ostland VE, Carlberg JM, Van Olst JC, Nizet V, Taylor SW, Shimizu C, Bulet P (2001) Discovery and characterization of two isoforms of moronecidin, a novel antimicrobial peptide from hybrid striped bass. JBC 277:5030–5039. https://doi.org/10.1074/jbc.M109173200
Lee SA, Kim YK, Lim SS, Zhu WL, Ko H, Shin SY, Hahm KS, Kim Y (2007) Solution structure and cell selectivity of piscidin 1 and its analogues. Biochemistry 46:3653–3663. https://doi.org/10.1021/bi062233u
Lee E, Shin A, Jeong KW, Jin B, Jnawali HN, Shin S, Shin SY, Kim Y (2014) Role of phenylalanine and valine10 residues in the antimicrobial activity and cytotoxicity of piscidin-1. PLoS ONE. https://doi.org/10.1371/journal.pone.0114453
Li Y, Xiang Q, Zhang Q, Huang Y, Su Z (2012) Overview on the recent study of antimicrobial peptides: origins, functions, relative mechanisms and application. Peptides 37:207–215. https://doi.org/10.1016/j.peptides.2012.07.001
Lin S, Fan T, Wu J, Hui C, Chen J (2009a) Immune response and inhibition of bacterial growth by electrotransfer of plasmid DNA containing the antimicrobial peptide, epinecidin-1, into zebrafish muscle. Fish Shellfish Immunol 26:451–458. https://doi.org/10.1016/j.fsi.2009.01.008
Lin WJ, Chien YL, Pan CY, Lin TL, Chen JY, Chiu SJ, Hui CF (2009b) Epinecidin-1, an antimicrobial peptide from fish (Epinephelus coioides) which has an antitumor effect like lytic peptides in human fibrosarcoma cells. Peptides 30(2):283–290. https://doi.org/10.1016/j.peptides.2008.10.007
Lin HJ, Huang TC, Muthusamy S, Lee JF, Duann YF, Lin CH (2012) Piscidin-1, an antimicrobial peptide from fish (hybrid striped bass Morone saxatilis × M. chrysops), Induces apoptotic and necrotic activity in HT1080 cells. Zool Sci 29:327–332. https://doi.org/10.2108/zsj.29.327
Lu XJ, Chen J, Huang ZA, Shi YH, Lυ JN (2011) Identification and characterization of a novel cathelicidin from ayu, Plecoglossus altivelis. Fish Shellfish Immunol 31(1):52–57. https://doi.org/10.1016/j.fsi.2011.03.005
Maier VH, Dorn KV, Gudmundsdottir BK, Gudmundsson GH (2008) Characterisation of cathelicidin gene family members in divergent fish species. Mol Immunol 45(14):3723–3730. https://doi.org/10.1016/j.molimm.2008.06.002
Marimuthu K, Gunaselvam P, Rahman MA, Xavier R, Arockiaraj J, Subramanian S, Yusoff FM, Arshad A (2015) Antibacterial activity of ovary extract from sea urchin Diadema setosum. Eur Rev Med Pharmacol Sci 19:1895–1899
Mason A, Chotimah I, Bertani P (2006) A spectroscopic study of the membrane interaction of the antimicrobial peptide Pleurocidin. Mol Membr Biol 23(2):185–194. https://doi.org/10.1080/09687860500485303
Masso-Silva JA, Diamond G (2014) Antimicrobial peptides from fish. Pharmaceuticals 7:265–310. https://doi.org/10.3390/ph7030265
McDonald M, Mannion M, Pike D, Let K (2015) Structure–function relationships in histidine-rich antimicrobial peptides from atlantic cod. BBA 1848(7):1451–1461. https://doi.org/10.1016/j.bbamem.2015.03.030
Mehrnejad F, Zarei M (2010) Molecular dynamics simulation study of the interaction of piscidin 1 with dppc bilayers: structure-activity relationship. J Biomol Struct Dyn 27:551–559. https://doi.org/10.1080/07391102.2010.10507338
Meloni M, Candusso S, Galeotti M, Volpatti D (2015) Preliminary study on expression of antimicrobial peptides in European sea bass (Dicentrarchus labrax) following in vivo infection with Vibrio anguillarum A time course experiment. Fish Shellfish Immunol 43:82–90. https://doi.org/10.1016/j.fsi.2014.12.016
Menousek J, Mishra B, Hanke M (2012) Database screening and in vivo efficacy of antimicrobial peptides against methicillin-resistant Staphylococcus aureus USA300. Int J Antimicrob Agents 39(5):402–406. https://doi.org/10.1016/j.ijantimicag.2012.02.003
Michel JP, Wang YX, Kiesel I, Gerelli Y, Rosilio V (2017) Disruption of asymmetric lipid bilayer models mimicking the outer membrane of Gram-negative bacteria by an active plasticin. Langmuir 33(41):11028–11039. https://doi.org/10.1021/acs.langmuir.7b02864
Moon WJ, Hwang DK, Park EJ, Kim YM, Chae YK (2007) Recombinant expression, isotope labeling, refolding, and purification of an antimicrobial peptide, piscidin. Protein Expr Purif 51:141–146. https://doi.org/10.1016/j.pep.2006.07.010
Morash MG, Douglas SE, Robotham A, Ridley CM, Gallant JW, Soanes KH (2011) The zebrafish embryo as a tool for screening and characterizing pleurocidin host-defense peptides as anti-cancer agents. Dis Model Mech 4:622–633. https://doi.org/10.1242/dmm.007310
Mulero I, Noga EJ, Meseguer J, García-Ayala A, Mulero V (2008) The antimicrobial peptides piscidins are stored in the granules of professional phagocytic granulocytes of fish and are delivered to the bacteria-containing phagosome upon phagocytosis. Dev Comp Immunol 32:1531–1538. https://doi.org/10.1016/j.dci.2008.05.015
Narayana J, Huang H, Wu C (2015) Epinecidin-1 antimicrobial activity: in vitro membrane lysis and In vivo efficacy against Helicobacter pylori infection in a mouse model. Biomaterials 61:41–51. https://doi.org/10.1016/j.biomaterials.2015.05.014
Niu SF, Jin Y, Xu X, Qiao Y, Wu Y, Mao Y, Su YQ, Wang J (2013) Characterization of a novel piscidin-like antimicrobial peptide from Pseudosciaena crocea and its immune response to Cryptocaryon irritans. Fish Shellfish Immunol 35:513–524. https://doi.org/10.1016/j.fsi.2013.05.007
Noga E, Silaphaduang U (2003) Piscidins: a novel family of peptide antibiotics from fish. Drug News Perspect 16(2):87–92. https://doi.org/10.1358/dnp.2003.16.2.829325
Noga EJ, Silphaduang U, Park NG, Seo JK, Stephenson J, Kozlowicz S (2009) Piscidin 4, a novel member of the piscidin family of antimicrobial peptides. Comp Biochem Physiol B Biochem Mol Biol 152:299–305. https://doi.org/10.1016/j.cbpb.2008.12.018
Pálffy R, Gardlík R, Behuliak M, Kadasi L, Turna J, Celec P (2009) On the physiology and pathophysiology of antimicrobial peptides. Mol Med 15:51–59. https://doi.org/10.2119/molmed.2008.00087
Pan CY, Chen JY, Cheng YSE, Chen CY, Ni IH, Sheen JF, Pan YL, Kuo CM (2007) Gene expression and localization of the epinecidin-1 antimicrobial peptide in the grouper (Epinephelus coioides), and its role in protecting fish against pathogenic infection. DNA Cell Biol 26:403–413. https://doi.org/10.1089/dna.2006.0564
Pan CY, Chen JY, Ni IH, Wu JL, Kuo CM (2008) Organization and promoter analysis of the grouper (Epinephelus coioides) epinecidin-1 gene. Comp Biochem Physiol B Biochem Mol Biol 150:358–367. https://doi.org/10.1016/j.cbpb.2008.04.006
Pan C, Chen J, Lin T (2009) In vitro activities of three synthetic peptides derived from epinecidin-1 and an anti-lipopolysaccharide factor against Propionibacterium acnes, Candida albicans. Peptides 30(6):1058–1068. https://doi.org/10.1016/j.peptides.2009.02.006
Pan CY, Rajanbabu V, Chen JY, Her GM, Nan FH (2010) Evaluation of the epinecidin-1 peptide as an active ingredient in cleaning solutions against pathogens. Peptides 31(8):1449–1458. https://doi.org/10.1016/j.peptides.2010.05.011
Pan Y, Zheng L, Mao Y, Wang J, Lin L, Su Y, Li Y (2018) The antibacterial activity and mechanism analysis of piscidin 5 like from Larimichthys crocea. Dev Comp Immunol. https://doi.org/10.1016/j.dci.2018.10.008
Park CB, Lee JH, Park IY, Kim MS, Kim SC (1997) A novel antimicrobial peptide from the loach, Misgurnus anguillicaudatus. FEBS Lett 411(2–3):173–178. https://doi.org/10.1016/s0014-5793(97)00684-4
Park Y, Hahm KS (2005) Antimicrobial peptides. J Biochem Mol Biol 38:507–516. https://doi.org/10.3390/ph6121543
Park NG, Silphaduang U, Moon HS, Seo JK, Corrales J, Noga EJ (2011) Structure-activity relationships of piscidin 4, a piscine antimicrobial peptide. Biochemistry 50:3288–3299. https://doi.org/10.1021/bi101395j
Patrzykat A, Friedrich CL, Zhang L, Mendoza V, Hancock REW (2002) Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob Agents Chemother 46:605–614. https://doi.org/10.1128/AAC.46.3.605-614.2002
Peng KC, Pan CY, Chou HN, Chen JY (2010) Using an improved Tol2 transposon system to produce transgenic zebrafish with epinecidin-1 which enhanced resistance to bacterial infection. Fish Shellfish Immunol 28:905–917. https://doi.org/10.1016/j.fsi.2010.02.003
Peng K, Lee S, Hour A, Pan C, Lee L (2012) Five different piscidins from Nile tilapia, Oreochromis niloticus: analysis of their expressions and biological functions. PLoS ONE 7(11):e50263. https://doi.org/10.1371/journal.pone.0050263
Peng KC, Lee SH, Hour AL, Pan CY, Lee LH, Chen JY (2012) Five different piscidins from Nile tilapia, Oreochromis niloticus: analysis of their expressions and biological functions. PLoS ONE 7(11):e50263. https://doi.org/10.1371/journal.pone.0050263
Perrin BS, Tian Y, Fu R, Grant CV, Chekmenev EY, Wieczorek WE, Dao AE, Hayden RM, Burzynski CM, Venable RM, Sharma M, Opella SJ, Pastor RW, Cotton ML (2014) High-resolution structures and orientations of antimicrobial peptides piscidin 1 and piscidin 3 in fluid bilayers reveal tilting, kinking, and bilayer immersion. J Am Chem Soc 136:3491–3504. https://doi.org/10.1021/ja411119m
Peter Chiou P, Khoo J, Bols NC, Douglas S, Chen TT (2006) Effects of linear cationic α-helical antimicrobial peptides on immune-relevant genes in trout macrophages. Dev Comp Immunol 30:797–806. https://doi.org/10.1016/j.dci.2005.10.011
Prabha N, Sannasimuthu A, Kumaresan V, Elumalai P, Arockiaraj J (2019) Intensifying the anticancer potential of cationic peptide derived from serine threonine protein kinase of teleost by tagging with oligo tryptophan. Int J Pept Res Ther. https://doi.org/10.1007/s10989-019-09817-3
Purabi S, Stefi R, Mukesh P, Paray BA, Al-Sadoon MK, Arockiaraj J (2020) Antioxidant molecular mechanism of adenosyl homocysteinase from cyanobacteria and its wound healing process in fibroblast cells. Mol Biol Rep 47:1821–1834. https://doi.org/10.1007/s11033-020-05276-y
Rahmanpour A, Ghahremanpour MM, Mehrnejad F, Moghaddam ME (2013) Interaction of Piscidin-1 with zwitterionic versus anionic membranes: a comparative molecular dynamics study. J Biomol Struct Dyn 31:1393–1403. https://doi.org/10.1080/07391102.2012.737295
Rajanbabu V, Chen JY (2011) Applications of antimicrobial peptides from fish and perspectives for the future. Peptides 32:415–420. https://doi.org/10.1016/j.peptides.2010.11.005
Rajesh P, Prasanth B, Venkatesh K, Mukesh P, Arockiaraj J (2018) Innate and adaptive immune molecules of striped murrel Channa striatus. Rev Aquac 10:296–319. https://doi.org/10.1111/raq.12161
Raju VS, Sarkar P, Pachaiappan R, Paray BA, Al-Sadoon MK, Arockiaraj J (2020) Defense involvement of piscidin from striped murrel Channa striatus and its peptides CsRG12 and CsLC11 involvement in an antimicrobial and antibiofilm activity. Fish Shellfish Immunol 99:368–378. https://doi.org/10.1016/j.fsi.2020.02.027
Rakers S, Niklasson L, Steinhagen D, Kruse C, Schauber J, Sundell K, Paus R (2013) Antimicrobial peptides (AMPs) from fish epidermis: perspectives for investigative dermatology. J Invest Dermatol 133:1140–1149. https://doi.org/10.1038/jid.2012.503
Rathinakumar R, Walkenhorst WF, Wimley WC (2009) Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: the importance of interfacial activity. J Am Chem Soc 131:7609–7617. https://doi.org/10.1021/ja8093247
Ravichandran G, Kumaresan V, Arasu MV, Al-Dhabi NA, Ganesh MR, Mahesh A, Dhayalan A, Pasupuleti M, Arockiaraj J (2016) Pellino-1 derived cationic antimicrobial prawn peptide: bactericidal activity, toxicity and mode of action. Mol Immunol 78:171–182. https://doi.org/10.1016/j.molimm.2016.09.015
Ravichandran G, Kumaresan V, Bhatt P, Arasu MV, Al-Dhabi NA, Arockiaraj J (2017) A cumulative strategy to predict and characterize antimicrobial peptides (AMPs) from protein database. Int J Pept Res Ther 23:281–290. https://doi.org/10.1007/s10989-016-9559-z
Ravichandran G, Kumaresan V, Mahesh A, Dhayalan A, Arshad A, Arasu MV, Al-Dhabi NA, Pasupuleti M, Arockiaraj J (2018) Bactericidal and fungistatic activity of peptide derived from GH18 domain of prawn chitinase 3 and its immunological functions during biological stress. Int J Biol Macromol 106:1014–1022. https://doi.org/10.1016/j.ijbiomac.2017.08.098
Rodrigues PNS, Vázquez-Dorado S, Neves JV, Wilson JM (2006) Dual function of fish hepcidin: response to experimental iron overload and bacterial infection in sea bass (Dicentrarchus labrax). Dev Comp Immunol 30(12):1156–1167. https://doi.org/10.1016/j.dci.2006.02.005
Rondeau EB, Messmer AM, Sanderson DS, Jantzen SG, Von Schalburg KR, Minkley DR, Leong JS, Macdonald GM, Davidsen AE, Parker WA, Mazzola RS, Campbell B, Koop BF (2013) Genomics of sablefish (Anoplopoma fimbria): expressed genes, mitochondrial phylogeny, linkage map and identification of a putative sex gene. BMC Genomics 14:452. https://doi.org/10.1186/1471-2164-14-452
Ruangsri J, Fernandes JMO, Rombout JHWM, Brinchmann MF, Kiron V (2012) Ubiquitous presence of piscidin-1 in Atlantic cod as evidenced by immunolocalisation. BMC Vet Res 8:1–13. https://doi.org/10.1186/1746-6148-8-46
Ruangsri J, Salger SA, Caipang CMA, Kiron V, Fernandes JMO (2012) Differential expression and biological activity of two piscidin paralogues and a novel splice variant in Atlantic cod (Gadus morhua L.). Fish Shellfish Immunol 32:396–406. https://doi.org/10.1016/j.fsi.2011.11.022
Ruangsri J, Kitani Y, Kiron V, Lokesh J, Brinchmann MF, Karlsen BO, Fernandes JMO (2013) A novel beta-defensin antimicrobial peptide in Atlantic cod with stimulatory effect on phagocytic activity. PLoS ONE 8(4):e62302. https://doi.org/10.1371/journal.pone.0062302
Salerno G, Parrinello N, Salerno G, Parrinello N, Roch P (2007) cDNA sequence and tissue expression of an antimicrobial peptide, dicentracin; a new component of the moronecidin family isolated from head kidney leukocytes of sea bass, Dicentrarchus labrax. Comp Biochem Physiol B Biochem Mol Biol 146(4):521–529. https://doi.org/10.1016/j.cbpb.2006.12.007
Salger SA, Reading BJ, Baltzegar DA, Sullivan CV, Noga EJ (2011) Molecular characterization of two isoforms of piscidin 4 from the hybrid striped bass (Morone chrysops × Morone saxatilis). Fish Shellfish Immunol 30:420–424. https://doi.org/10.1016/j.fsi.2010.10.009
Salger SA, Cassady KR, Reading BJ, Noga EJ (2016) A diverse family of host-defense peptides (piscidins) exhibit specialized anti-bacterial and anti-protozoal activities in fishes. PLoS ONE 11:1–25. https://doi.org/10.1371/journal.pone.0159423
Salger SA, Reading BJ, Noga EJ (2017) Tissue localization of piscidin host-defense peptides during striped bass (Morone saxatilis) development. Fish Shellfish Immunol 61:173–180. https://doi.org/10.1016/j.fsi.2016.12.034
Sannasimuthu A, Kumaresan V, Pasupuleti M, Paray BA, Al-Sadoon MK, Arockiaraj J (2018) Radical scavenging property of a novel peptide derived from C-terminal SOD domain of superoxide dismutase enzyme in Arthrospira platensis. Algal Res 35:519–529. https://doi.org/10.1016/j.algal.2018.09.028
Sannasimuthu A, Arockiaraj J (2019) Intracellular free radical scavenging activity and protective role of mammalian cells by antioxidant peptide from thioredoxin disulfide reductase of Arthrospira platensis. J Funct Foods 61:103513. https://doi.org/10.1016/j.jff.2019.103513
Sannasimuthu A, Kumaresan V, Anilkumar S, Pasupuleti M, Ganesh M, Mala K, Paray BA, Al-Sadoon MK, Albeshr MF, Arockiaraj J (2019) Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free Radic Biol Med 135:198–209. https://doi.org/10.1016/j.freeradbiomed.2019.03.006
Sathyamoorthi A, Bhatt P, Ravichandran G, Kumaresan V, Arasu MV, Al-Dhabi NA, Arockiaraj J (2017) Gene expression and in silico analysis of snakehead murrel interleukin 8 and antimicrobial activity of C-terminal derived peptide WS12. Vet Immunol Immunopathol 190:1–9. https://doi.org/10.1016/j.micres.2014.03.005
Sathyamoorthi A, Kumaresan V, Palanisamy R, Pasupuleti M, Arasu MV, Al-Dhabi NA, Marimuthu K, Amin SMN, Arshad A, Yusoff FM, Arockiaraj J (2019) Therapeutic cationic antimicrobial peptide (CAP) derived from fish aspartic proteinase cathepsin D and its antimicrobial mechanism. Int J Pept Res Ther 25:93–105. https://doi.org/10.1007/s10989-017-9652-y
Schuerholz T, Brandenburg K, Marx G (2012) Annual update in intensive care and emergency medicine. In: Vincent JL (ed) Annual update in intensive care and emergency medicine. Springer, Berlin. https://doi.org/10.1007/978-3-642-25716-2
Shabir U, Ali S, Magray AR, Ganai BA, Firdous P, Hassan T, Nazir R (2018) Fish antimicrobial peptides (AMP’s) as essential and promising molecular therapeutic agents: a review. Microb Pathog 114:50–56. https://doi.org/10.1016/j.micpath.2017.11.039
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolym Pept Sci Sect 66:236–248. https://doi.org/10.1002/bip.10260
Shin SC, Ahn IH, Ahn DH, Lee YM, Lee J, Lee JH, Kim HW, Park H (2017) Characterization of two antimicrobial peptides from antarctic fishes (Notothenia coriiceps and Parachaenichthys charcoti). PLoS ONE 12(1):e0170821. https://doi.org/10.1371/journal.pone.0170821
Silphaduang U, Colorni A, Noga EJ (2006) Evidence for widespread distribution of piscidin antimicrobial peptides in teleost fish. Dis Aquat Organ 72:241–252. https://doi.org/10.3354/dao072241
Star B, Nederbragt A, Jentoft S (2011) The genome sequence of Atlantic cod reveals a unique immune system. Nature 477:207–210. https://doi.org/10.1038/nature10342
Sun BJ, Xie HX, Song Y, Nie P (2007) Gene structure of an antimicrobial peptide from mandarin fish, Siniperca chuatsi (Basilewsky), suggests that moronecidins and pleurocidins belong in one family: the piscidins. J Fish Dis 30:335–343. https://doi.org/10.1111/j.1365-2761.2007.00789.x
Sun D, Wu S, Jing C, Zhang N (2012) Identification, synthesis and characterization of a novel antimicrobial peptide HKPLP derived from Hippocampus kuda Bleeker. J Antibiot 65:117–121. https://doi.org/10.1038/ja.2011.120
Sung WS, Lee J, Lee DG (2008) Fungicidal effect and the mode of action of piscidin 2 derived from hybrid striped bass. Biochem Biophys Res Commun 371:551–555. https://doi.org/10.1016/j.bbrc.2008.04.107
Terova G, Forchino A, Rimoldi S, Brambilla F, Antonini M, Saroglia M (2009) Bio-Mos®: an effective inducer of dicentracin gene expression in European sea bass (Dicentrarchus labrax). Comp Biochem Physiol B Biochem Mol Biol 153(4):372–377. https://doi.org/10.1016/j.cbpb.2009.04.008
Thirumalai MK, Roy A, Sanikommu S, Arockiaraj J, Pasupuleti M (2014) A simple, robust enzymatic-based high-throughput screening method for antimicrobial peptides discovery against Escherichia coli. J Pept Sci 20:341–348. https://doi.org/10.1002/psc.2619
Timalata K, Marimuthu K, Vengkades R, Xavier R, Rahman MA, Sreeramanan S, Arasu MV, Al-Dhabi NA, Arockiaraj J (2015) Elucidation of innate immune components in the epidermal mucus of different freshwater fish species. Acta Ichthyol Piscat 45(3):221–230. https://doi.org/10.3750/AIP2015.45.3.01
Trobridge GD, Leong JAC (1995) Characterization of a Rainbow Trout Mx gene. J Interf Cytokine Res 15(8):691–702. https://doi.org/10.1089/jir.1995.15.691
Wang KJ, Cai JJ, Cai L, Qu HD, Yang M, Zhang M (2009) Cloning and expression of a hepcidin gene from a marine fish (Pseudosciaena crocea) and the antimicrobial activity of its synthetic peptide. Peptides 30(4):638–646. https://doi.org/10.1016/j.peptides.2008.12.014.
Wang YD, Kung CW, Chen JY (2010) Antiviral activity by fish antimicrobial peptides of epinecidin-1 and hepcidin 1–5 against nervous necrosis virus in medaka. Peptides 31(6):1026–1033. https://doi.org/10.1016/j.peptides.2010.02.025
Yan S, Wu G (2012) Structure, undefined function, and bioinformatics, analysis on folding of misgurin using two-dimensional HP model. Proteins 80(3):764–773. https://doi.org/10.1002/prot.23233
Yang J, Lu XJ, Chai FC, Chen J (2016) Molecular characterization and functional analysis of a piscidin gene in large yellow croaker (Larimichthys crocea). Zool Res 37:347–355. https://doi.org/10.13918/j.issn.2095-8137.2016.6.347
Yin Z, He W, Chen W, Yan J, Yang J (2006) Cloning, expression and antimicrobial activity of an antimicrobial peptide, epinecidin-1, from the orange-spotted grouper, Epinephelus coioides. Aquaculture 253(1–4):204–211. https://doi.org/10.1016/j.aquaculture.2005.10.002
Yoshida K, Mukai Y, Niidome T, Takashi C, Tokunaga Y, Hatakeyama T, Aoyagi H (2001) Interaction of pleurocidin and its analogs with phospholipid membrane and their antibacterial activity. J Pept Res 57:119–126. https://doi.org/10.1034/j.1399-3011.2001.00802.x
Zhou QJ, Su YQ, Niu SF, Liu M, Qiao Y, Wang J (2014) Discovery and molecular cloning of piscidin-5-like gene from the large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol 41(2):417–420. https://doi.org/10.1016/j.fsi.2014.09.023
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395. https://doi.org/10.1038/415389a
Funding
No funding was received to perform this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research Involving Human and Animal Rights
No human sample or animal sample used in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raju, S.V., Sarkar, P., Kumar, P. et al. Piscidin, Fish Antimicrobial Peptide: Structure, Classification, Properties, Mechanism, Gene Regulation and Therapeutical Importance. Int J Pept Res Ther 27, 91–107 (2021). https://doi.org/10.1007/s10989-020-10068-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10068-w