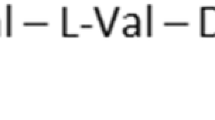Abstract
Multi drug resistance is a major problem of the twenty first century. In order to combat this issue, there is an urgent need in the pharmaceutical industry, for novel therapeutic agents. Antimicrobial peptides such as protegrins which exhibit non-specific membranolytic action can be viewed as probable therapeutic agents and replace conventional antibiotics. Protegrin-1 (PG-1) is a peptide isolated from porcine leucocytes. Its primary role is its antimicrobial activity against a broad-spectrum of gram-positive as well as gram-negative bacteria and fungi. Its antagonistic activity can be accounted by its pore formation mechanism in microbial membranes. In addition, PG-1 has multiple roles viz., anticancer and antiviral activity, immunomodulatory functions and numerous applications which increase its suitability as a potential therapeutic agent. This review paper presents a comprehensive overview of biological roles, lytic mechanism of action and applications of PG-1, thus providing a thorough understanding of this β-sheet peptide, which structurally resembles defensin peptides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multi-drug resistance has been reported as one of the major threats to human health by the World Health Organization (WHO) (“WHO | Antimicrobial resistance” 2014). An estimated 10 million deaths of people per year may occur by the year 2050 (Cordes et al. 2014). Thus, there is an urgent requirement for therapeutic agents to combat this problem.
Antimicrobial peptides (AMPs) majorly lyse microbial cells via non-specific membranolytic mechanism, which reduces their susceptibility to antimicrobial resistance. Therefore, antimicrobial peptides have been viewed as probable therapeutics which can replace conventional antibiotics. Protegrin-1 (PG-1), is an AMP which has been viewed as a probable therapeutic agent by the pharmaceutical industry to combat the issue of antimicrobial resistance. However, the authors found a limited number of review articles which were specifically focused on topics viz., understanding pore formation mechanisms of PG-1 through computational approaches or gene expression, structure and applications of PG-1 (Sun and Zhang 2007; Bolintineanu et al. 2012; Yan et al. 2013). This review presents the reader with an overview of the membranolytic antimicrobial peptide, protegrin-1, highlighting its role as an antimicrobial agent, its antagonistic mechanism of action in addition to its other biological roles and applications (Fig. 1).
Origin and Structure of Protegrin-1 (PG-1)
Protegrin-1 is a peptide isolated from swine leucocytes (Kokryakov et al. 1993). It was observed to exhibit antagonistic activity towards Escherichia coli, Listeria monocytogenes and Candida albicans and; it was named after a Latin word ‘protegere’, which means ‘to protect’. It forms a part of the non-specific first line of defense in the immune system of pigs. The sequence of PG-1 was elucidated using a combination of electrospray mass ion spectrometry and Edman degradation comprised of 18 amino acids with an amidated C-terminal (Mirgorodskaya et al. 1993). In vivo, PG-1 acquires an amide group at its C-terminal, post-translation and; it is then known as mature PG-1. Its peptide sequence constitutes RGGRLCYCRRRFCVCVGR. It comprises 33% arginine which imparts a cationic nature to PG-1.
NMR studies of PG-1revealed that its amino acid residues from 5th to 9th positions and the amino acid residues from 12th to 16th positions adopted β-sheet secondary structures in antiparallel orientation, connected by a hair-pin loop structure assumed by amino acids at 9th to 12th positions. Also, the monomer of PG-1is stabilized by two disulphide bonds between the cysteines viz., CYS (6)–CYS (15) and CYS (8)–CYS (13) (Aumelas et al. 1996). It was observed that these porcine peptides resembled defensins, especially rabbit defensin, NP-3a and tachyplesins—AMPs isolated from hemocytes of horseshoe crab (Kokryakov et al. 1993). The 3D structure of PG-1 is illustrated in Fig. 2.
Antimicrobial Activity of PG-1
In vitro antimicrobial activity of PG-1 was first reported by Kokryakov et al. (1993). Its disulphide bridges and cationic nature are essential for its antibacterial activity. Also, its cationic charge is responsible for the differential mechanism of action and antibacterial activity towards gram-positive and gram-negative bacteria (Aumelas et al. 1996; Su et al. 2011). PG-1 was also found to display antimicrobial activity towards Neisseria gonorrhea. It was observed that PG-1 formed 80–100 nm wide circular plaques in its outer membrane (Fahrner et al. 1996).
Another study that demonstrated in vitro antibacterial action of PG-1 towards Mycobacterium tuberculosis was found to be comparable to that of human and rabbit defensins (Miyakawa et al. 1996). In vivo study in mice demonstrated the antimicrobial activity of PG-1towards P. aeruginosa, methicillin resistant Staphylococcus aureusand methicillin sensitive Staphylococcus aureus. Additionally, intraperitoneal administration of PG-1 in the infected mice reduced their mortality to 0% (Steinberg et al. 1997). In 2017, a study demonstrated that mature PG-1 produced in Pichia pastoris after cleavage of matrix-metalloprotease site (inserted in its gene sequence) displayed higher efficiency in combating methicillin—resistant Staphylococcus aureus than the mature PG-1 produced normally (proform PG-1) (Hill and Li 2017). A study reported that PG-1 showed antimicrobial activity towards epidemiologically unrelated clinical isolates of Haemophilus ducreyi—a pathogen causing sexually transmitted disease, chancroid. This study found that PG-1 showed higher activity towards Haemophilus ducreyi in comparison to antibiotics viz., erythromycin, kanamycin and chloramphenicol. Additionally, PG-1 lysed Haemophilus ducreyi at very low doses. Thus PG-1 was proposed as an ingredient of topical applicants for treatment of chanchroid (Fortney et al. 1998). In addition to imparting antimicrobial activity, PG-1 was found to display synergistic behavior with conventional antibiotics towards Escherichia coli (GuoDong 2013). A study reported the synergistic antimicrobial activity of combination of PG-1 and colistin towards Acinetobacter baumanii, isolated from surgical wounds (Morroni et al. 2019). Another interesting study by Zharkova et al. (2019), demonstrated that the combination of PG-1 with individual antibiotics viz., rifampicin, polymyxin, gentamycin, ofloxacin and oxacillin displayed higher antimicrobial activity towards Escherichia coli ML-35p and methicillin resistant Staphylococcus aureus ATCC 33,591 compared to the antimicrobial activity exhibited by antibiotics alone. However, one study mentioned that though PG-1 effectively treated bacterial sepsis, it could significantly alter the host innate immune response to infection (Steinstraesser et al. 2003). Additionally, a study by Soundrarajan et al. (2019) reported the differential cytotoxicity of PG-1 to various types of mammalian cell lines.
Mechanism of Action of PG-1: Pore Formation
The antimicrobial activity exhibited by PG-1 towards the aforementioned microbial flora can be attributed to its pore formation in their membranes. This section about PG-1 pore formation mechanism is further divided into four parts, as follows:
-
1.
Interaction of PG-1with model microbial membranes
-
2.
Membrane thinning effect of PG-1
-
3.
Dimerization of PG-1 and the formation of pores
-
4.
PG-1 oligomers form its pore
Interaction of PG-1 with Model Microbial Membranes
Interaction of PG-1 with microbial membranes can be attributed mainly to the electrostatic interactions between them. These electrostatic interactions can be attributed to the positively charged residues in PG-1. Additionally, a study carried out by NMR spectroscopy demonstrated that the side chains of amino acid residues viz., LEU (5), PHE (12), VAL (14) and VAL (16) formed a hydrophobic cluster which aided the interaction of PG-1 with dodecylphosphocholine (DPC) micelles (Kolosova et al. 2016).Thus, it can be inferred that both hydrophobic and positively charged residues of PG-1 mediate the interaction with model microbial membranes.
Interaction of PG-1 with microbial membranes can be also be understood by application of biophysical techniques like potential mean force and adaptive biasing force to molecular dynamic simulations of PG-1 in model microbial membranes (Rui and Im 2010; Vivcharuk and Kaznessis 2011).
Potential mean force is the average force on particles along a particular coordinate. It is related to the radial distribution function of particles and it can be used to calculate and understand biomolecular interactions. Calculation of potential mean forces from molecular dynamics (MD) simulations was initially used to understand the interaction of PG-1 with biological membranes. The binding free energy of PG-1 to phosphatidylethanolamine/phosphatidylcholine (PE/PC) membranes was computed as − 2.4 ± 0.8 kcal/mol (Vivcharuk and Kaznessis 2010a). Further, the energy of PG-1 when possessing a transmembrane orientation was calculated as − 20.0 kcal /mol. The transfer of PG-1 from aqueous phase to transmembrane state was determined using adaptive biasing force (Vivcharuk and Kaznessis 2011). Additionally, a study conducted using multistep molecular dynamics simulations revealed that the guanidium group of arginine in PG-1, initially interacted with the polar phosphate headgroups of membranes, aiding its translocation across the membrane (Lai and Kaznessis 2018).
Membrane Thinning Effect of PG-1
Antimicrobial activity of PG-1 can be attributed to its membrane thinning effect. Using oriented circular dichroism (OCD), Heller et al. (1998) showed the two states of PG-1- surface adsorbed (S) and inserted (I) in a phospholipid bilayer. These two states of PG-1 support its membrane thinning effect. Further, the pore formation and membrane thinning effect of PG-1 was confirmed with lamellar X-ray diffraction experiments and NMR studies (Heller 1999; Wi and Kim 2008).
Pore formation in diphytanoyl phosphatidylcholine (DPhPC) by PG-1 revealed that membrane thinning effect increased with PG-1 concentration. The binding of PG-1 to DPhPC caused DPhPC to fold onto itself, increasing its thickness. This mechanism of membrane thinning by PG-1 led to the proposal of torroidal pore model mechanism of action (Heller et al. 2000).
During MD simulations, it was observed that the turn region of PG-1 occupied by ARG (9), ARG (10) and ARG (11) forms electrostatic interactions with the phosphate headgroups of the 1,2 dilauroyl-sn-glycero-3-phosphocholine (DLPC) bilayer and is pulled towards the core of DLPC bilayer (Khandelia and Kaznessis 2007). Since amino acids at positions 9–11 form the turn region of PG-1, the membrane thinning effect of the upper layer of DLPC membrane can be attributed to this region of PG-1. Additionally, Jang et al. (2006) stated through MD simulations that the β-turn region of PG-1 played a significant role in the membrane thinning by PG-1 in DLPC bilayers.
PG-1 ultimately formed transmembrane pores in membrane monolayers (mimicking Gram-positive bacterial membranes) by initially increasing the surface pressure on membrane surface to + 8.5 mN/m followed by formation of small clusters of 5 µm in the monolayer. This formation of clusters due to increasing surface pressure on biological monolayers confirms the membrane thinning effect of PG-1 (Knyght et al. 2016).
Dimerization of PG-1 and Formation of Pores
Using atomic force microscopy (AFM), Lam et al. (2006) demonstrated the complete disruption of supportive DLPC membranes via pore formation by PG-1at a concentration of 20 µg/ml. Yang et al. (2000) successfully crystallized hexagonal pores formed by PG-1 in completely hydrated fluid membranes using neutron diffraction experiments.
PG-1 dimerizes upon binding to DPC micelles. Its interaction is mainly due to its arginine residues (Rui et al. 2009). PG-1 dimers then oligomerize to form pores in the DPC micelles. Aggregation of PG-1 dimers can be accounted for by the C-terminal amide group of PG-1. This mechanism of pore formation by oligomerization by PG-1 dimers was found similar to that of human defensin HNP-3.PG-1 was found to have similar orientation to that of the 21 residue α-helical peptide alamethicin (Roumestand et al. 1998). Jang et al. (2007) demonstrated through MD simulations that the dimers of PG-1 exist in both parallel and anti-parallel orientations, while interacting with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) membranes. These orientations were maintained with the help of two intramolecular disulphide bonds between the cysteine residues of the PG-1 monomers. It was also observed that the dimer orientation was dependent on the surrounding environment (Jang et al. 2007).
Drin and Temsamani (2002) confirmed that the formation of pores in the membrane was dependent on the presence of disulphide bridges. The significance of disulphide bridges in pore formation by PG-1 was demonstrated using a fluorescent PG-1 analog ([12 W]PG-1) with intact disulphide bridges and a linearized PG-1 with alanine/leucine residues replacing the cysteine residues in PG-1. Pore formation was exhibited only by the fluorescent PG-1 analog. However, a study conducted by Mangoni et al. (1996) established that disulphide bridges in protegrins were essential for pore formation but not for antimicrobial activity.
Orientations of PG-1 in the membrane were confirmed by X-ray diffraction methods and NMR studies (Buffy et al. 2003a; Gidalevitz et al. 2003). Study by Buffy et al. (2003b) stated that the orientation of PG-1 in DLPC bilayers was such that GLY (2) and PHE (12) were located at the surface of the bilayer whereas LEU (5) and VAL (16) interacted with the acyl chains in the bilayer. The depth of PG-1in DLPC bilayer increased in the following order: GLY (2) > PHE (12) > LEU (5) > VAL (16). Study by Kandasamy and Larson (2007) using model lipid bilayers with hydrophobic lengths revealed that PG-1 displayed two different orientations when it formed transmembrane pores. This altered orientation was attributed to the flexible ‘RGGR’ region in the N’terminal of PG-1.Further, the two leucine residues helped stabilize the β-sheet structure of protegrin during pore formation in the membrane (Gottler et al. 2008).
Pore formation of PG-1 in zwitterionic lipid bilayers followed a particular order as PG-1 concentration increased viz. (i) structural deformation of the bilayer at the edges (ii) formation of nanopores in the membrane (iii) formation of a network of stripe like structures in the membrane. A similar pattern was followed by PG-1 upon interaction with anionic lipid bilayers, but with a lower concentration of PG-1 (Lam 2007).
PG-1 Oligomers Form Its Pore
A study by Langham et al. (2008) stated that the formation mechanism of octamer pore of PG-1 resembled that of the barrel stave model than the torroidal pore model. Additionally, this study examined the transportation of ions through the octamer pore of PG-1 and established that one chloride ions passes through them every two seconds. Passage of ions through a pore disabled the bacterial membrane potential, which then allowed water ions to pass through the pore-ultimately lysing the bacteria. Interestingly, only one pore was required for complete osmolysis of bacteria. Bolintineanu et al. (2009) carried out ion conductance studies-using Poisson Nernst Planck models which investigated the diffusion of ions through octameric pores of PG-1 in membrane bilayers. In its octameric pores, PG-1 monomers displayed an oblique orientation-a tilt angle of 19° with respect to the membrane normal (Sayyed-Ahmad and Kaznessis 2009). Further, these octameric pores may comprise of either antiparallel NCCN or parallel NCNC orientation of PG-1 dimers, as they are more energetically favorable (Lazaridis et al. 2013).
Jang et al. (2008) observed that PG-1 octamer pores formed in phosphatidylethanolamine/ phosphatidylglycerol (PE/PG) membranes shared a common subunit organization with that of the four to six-unit β-amyloid ion channels formed during Alzheimer’s disease (Jang et al. 2008). A study by Jang et al. (2011) revealed that amyloid forming β-peptides shared common motif with PG-1 which formed fibrillar structures on a hydrophilic mica surface. However, a study demonstrated that the optimal oligomeric state for PG-1 pores was the nonamer (pore formed by 9 PG-1 monomers) (Lipkin and Lazaridis 2017).
Differential Activity of PG-1 Towards Bacterial and Mammalian Membranes and the Protective Role of Cholesterol Present in Mammalian Membranes
The charge of PG-1 and the overall charge of membrane determine the electrostatic interactions between them. PG-1 shows higher electrostatic interactions with anionic membranes compared to zwitterionic—neutral membranes. The interaction of PG-1 with membrane is affected by its charge, the overall charge of the membrane, its secondary structure, its amphiphilicity, the structural features of phospholipid headgroups and lipid packing (Jing et al. 2005; Bolintineanu et al. 2007). An MD study showed that altering the charge of C-terminal of PG-1 from positive to negative significantly affected its penetration in DPC micelles (Langham and Kaznessis 2006).
It was observed that PG-1 formed channel like structures in membranes composed of PE, Phosphatidyl serine (PS) or PG, but not in membranes composed of PC (Vivcharuk and Kaznessis 2010a). Besides, a study conducted using grazing incidence X-ray diffraction (GIXD) showed the differential activity of PG-1—enhanced interaction with lipid A monolayers (Gram-negative membrane mimics) than 1,2 -dimyristoyl-sn-glycero-3-phosphocholine (DMPC) monolayers (mammalian erythrocyte membrane mimics). The selectivity of PG-1 interaction towards anionic membranes in comparsion to DMPC membranes (erythrocytic membrane mimics) was confirmed by the decreasing interaction of PG-1 with DMPC bilayers with increase in percentage of cholesterol from 0 to 30% (Maldonado et al. 2011). Further, a study by Neville et al. (2008) revealed that the peptide-lipid interactions were dependent on the composition as well as the packing density of the lipids in membrane monolayers. Henderson et al. (2019) showed that the interaction of PG-1 with phosphatidylcholine (PC) membranes reduced with increasing amounts of monounsaturated fatty acyl chains.
Mammalian cells are primarily composed of PC and cholesterol whereas, bacterial membranes are composed of PE and PG. Cholesterol is a neutral molecule and its interspersion in mammalian membranes reduces their overall charge, thereby decreasing the electrostatic interactions with PG-1. Reduced electrostatic interaction might account for its protective role from damaging effects of PG-1 (Mani et al. 2004; Henderson et al. 2012, 2014, 2015; Iyengar et al. 2016).
Reducing Toxicity of PG-1
A molecular dynamics study by Langham et al. (2006) showed that the two strands of PG-1—RGGRLCYCRR (strand 1) and RFCVCVGR (strand 2) interacted differently with all-atom sodium dodecyl sulphate (SDS) and dodecylphosphocholine (DPC) micelles. Strand 2 (more hydrophobic than strand 1) is embedded in the core of DPC micelles while strand 1 embedded itself in the core of the SDS micelles. From this observation the authors concluded that the amino acid substitutions in PHE (12) or VAL (14) can alter the hemolytic activity of PG-1 while retaining its antibacterial activity.
Physical Resistance to PG-1
PG-1 was identified as a promising therapeutic scaffold among its peptide family based on computational techniques such as interaction with representative gram-negative bacterial membrane, mammalian cell toxicity and static-dynamic structural stability (Shruti and Rajasekaran 2019). This study assessed PG-1 stability based on hydrogen bonds (H-bonds). Vivcharuk and Kaznessis (2010b) also emphasized the role of hydrogen and ionic bonds in pore formation efficacy of PG-1. A study by Bolintineanu et al. (2010) established that the antimicrobial activity of PG-1 towards E. coli occurred due to the formation of 10–100 pores, which led to leakage of potassium ions and complete loss of its potential across the membrane- ultimately leading to bacterial cell lysis.
The antimicrobial activity of PG-1 is also dependent on the fluidity of microbial membrane. Alteration of membrane fluidity by bacteria can be a physical mechanism of resistance to PG-1 (Chapman et al. 2009).
Other Biological Roles and Applications of PG-1
Immunomodulatory Function of PG-1 and Its Aapplication in Immune Disorders
PG-1 caused human mast cell degranulation, which is essential for the healing of wounds (Gupta et al. 2015). A study by Penney and Li (2018), decoded the immunomodulatory mechanism of PG-1. This study found that mature PG-1 interacts with Insulin-like growth factor 1, which in turn modulates other immune activity as well as brings about migration of intestinal cells. This study therefore proposed the application of PG-1 for combating immune disorders related to the GI tract, such as irritable bowel syndrome.
Anticancer and Antiviral Activity of PG-1
A study confirmed the strong time and dose-dependent anticancer activity of PG-1 expressed in Pichia pastoris, towards HepG2 cells (Niu et al. 2015). Additionally, PG-1 exhibits apoptosis in lymphoma cells. A study conducted by Sugawara et al. (2016), used PG-1, an apoptosis inducing agent, as part of a peptide probe for the electrochemical detection of lymphoma cells.
Another study demonstrated the in vivo antiviral activity of fusion protein, PG-1-MAP30-PLSN in mice infected with dengue virus. The fusion protein prevented viral replication by inhibiting the NS2B-NS3 protease (Rothan et al. 2014). Another study demonstrated that PG-1 exhibited antiviral activity in swine infected with porcine reproductive and respiratory syndrome virus by blocking the virus from attaching to Marc-145 cells, thus preventing their replication in Marc-145 (Macrophages) (Guo et al. 2015).
Therapeutic Potential of PG-1 to Treat Murine Colitis
Huynh et al. (2019) showed that PG-1 brought about a reduction in murine colitis induced by dextran sodium sulphate by influencing the expression of tissue repair factors such as trefoil factor 3 and mucin. This study thus proposed the therapeutic potential of PG-1 in treatment of intestinal damage and inflammation. Another study conducted in 2018 demonstrated the in-vivo antimicrobial activity of PG-1 towards Citrobacter rodentium, which causes infection in the intestine of mice (colitis). Additionally, PG-1 altered the expression of various inflammatory mediators during infection and helped in the re-establishment of intestinal homeostasis (Osakowicz 2018).
PG-1 as Topical Applicant for Wound Healing and for Treatment of Cystic Fibrosis
The potency of PG-1 as a topical applicant for treatment of wounds was confirmed by in vivo experiments of PG-1 on adult male rats infected by Pseudomonas aeruginosa (Steinstraesser et al. 2001). Also, PG-1 was used as a topical applicant on tooth-mimetic surfaces to treat oral infections caused by pathogen, Streptococcus mutans (Liu et al. 2016).
Another interesting application of PG-1 was discovered after its antimicrobial activity against clinical isolates of Pseudomonas aeruginosa commonly found in cystic fibrosis patients. It was reported that the PG-1 effectively killed Pseudomonas cells by binding to its lipopolysaccharide molecules on its surface (Albrecht et al. 2002).
PG-1 Combats Plant Pathogens
A study proposed the application of PG-1 in preventing bacterial infections in plant by demonstrating the antagonistic activity of PG-1 towards plant pathogen Erwinia carotovora by enabling the expression of PG-1 in tobacco chloroplast genomic (Lee et al. 2011). Another study showed that the expression of PG-1 in Nicotiana tabacum plant displayed antibacterial as well as antifungal activity towards a broad spectrum of bacteria and Candida spp. (Patiño-Rodríguez et al. 2013).
PG-1 as an Applicant for Livestock
A study demonstrated that ectopic expression of PG-1 in transgenic mice enhanced their resistance to bacterial infection such as those of Actinobacillus suis. Thus, this study proposed the application of PG-1 to livestock (transgenic livestock) to increase their resistance to bacterial infections (Cheung et al. 2008). Further in order to enhance the expression of PG-1 in livestock such as pigs, research has proposed the supplementation of diet to livestock with lactoferrin, Achyranthes bidentata polysaccharides, micron Astragulas copper. Supplementation with the aforementioned substances were shown to increase PG-1 expression, enhancing the non-specific immune response to bacterial and viral infections (Wang et al. 2006; Qing-hua et al. 2008; Guo et al. 2015; Yan et al. 2015; Tu et al. 2006).
Conclusion
A review of PG-1 is necessary to determine its therapeutic potency. This review represents a thorough overview of the multifaceted biological roles of PG-1 in addition to its primary role as an antimicrobial peptide. Its mechanism of lysing bacterial cells has also been focused upon. Thus, several biological roles and applications of PG-1 render it a promising therapeutic agent for the global pharmaceutical industry.
Abbreviations
- AMP:
-
Antimicrobial peptide
- AFM:
-
Atomic force microscopy
- DMPC:
-
1,2-Dimyristoyl-sn-glycero-3-phosphocholine
- DLPC:
-
Dilauroyl-sn-glycero-3-phosphocholine
- DPC:
-
Dodecylphosphocholine
- DPhPC:
-
Diphytanoylphosphatidylcholine
- GIXD:
-
Grazing incidence X-ray diffraction
- MD:
-
Molecular dynamics
- NMR:
-
Nuclear magnetic resonance
- OCD:
-
Oriented circular dichroism
- PG-1:
-
Protegrin-1
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PG:
-
Phosphatidylglycerol
- PS:
-
Phosphatidylserine
- POPC:
-
1-Palmitoyl-2-oleoyl-glycero-3-phosphocholine
- WHO:
-
World Health Organization
References
Albrecht MT, Wang W, Shamova O, Lehrer RI, Schiller NL (2002) Binding of protegrin-1 to Pseudomonas aeruginosa and Burkholderia cepacian. Respir Res 3:18
Aumelas A, Mangoni M, Roumestand C, Chiche L, Despaux E, Grassy G, Calas B, Chavanieu A (1996) Synthesis and solution structure of the antimicrobial peptide protegrin-1. Eur J Biochem 237:575–583
Bolintineanu DS, Langham AA, Davis HT, Kaznessis YN (2007) Molecular dynamics simulations of three protegrin-type antimicrobial peptides: interplay between charges at the termini β-sheet structure and amphiphilic interactions. Mol Simul 33:809–819
Bolintineanu D, Hazrati E, Davis HT, Lehrer RI, Kaznessis YN (2010) Antimicrobial mechanism of pore-forming protegrin peptides: 100 pores to kill E. coli. Peptides 31:1–8
Bolintineanu DS, Sayyed-Ahmad A, Kaznessis DHT (2009) Poisson-Nernst-Planck models of nonequilibrium ion electrodiffusion through a protegrin transmembrane pore. PLoS Comput Biol 5:e1000277
Bolintineanu DS, Vivcharuk V, Kaznessis YN (2012) Multiscale models of the antimicrobial peptide protegrin-1 on gram-negative bacteria membranes. Int J Mol Sci 13:11000–11011
Buffy JJ, Waring AJ, Lehrer RI, Hong M (2003a) Immobilization and aggregation of the antimicrobial peptide Protegrin-1 in lipid bilayers investigated by solid-State NMR. Biochemistry 42:13725–13734
Buffy JJ, Hong T, Yamaguchi S, Waring AJ, Lehrer RI, Hong M (2003b) Solid-state NMR investigation of the depth of insertion of protegrin-1 in lipid bilayers using paramagnetic Mn2+. Biophys J 85:2363–2373
Chapman MR, Lam KLH, Waring AJ, Lehrer RI, Lee KYC (2009) The origin of antimicrobial resistance and fluidity dependent membrane structural transformation by antimicrobial peptide Protegrin-1. Biophys J 96:550a
Cheung QCK, Turner PV, Song C, Wu D, Cai HY, MacInnes JI, Li J (2008) Enhanced resistance to bacterial infection in Protegrin-1 transgenic mice. Antimicrob Agents Chemother 52:1812–1819
Cordes J, Wittersheim M, Harder J, Gläser R (2014) The skin's own antibiotics. Important features of antimicrobial peptides for clinical practice. Der Hautarzt 65:50–55
Drin G, Temsamani J (2002) Translocation of protegrin I through phospholipid membranes: role of peptide folding. Biochim Biophys Acta 1559:160–170
Fahrner RL, Dieckmann T, Harwig SS, Lehrer RI, Eisenberg D, Feigon J (1996) Solution structure of protegrin-1 a broad-spectrum antimicrobial peptide from porcine leukocytes. Chem Biol 3:543–550
Fortney K, Totten PA, Lehrer RI, Spinola SM (1998) Haemophilus ducreyi is susceptible to protegrin. Antimicrob Agents Chemother 42:2690–2693
Gidalevitz D, Ishitsuka Y, Muresan AS, Konovalov O, Waring AJ, Lehrer RI, Lee KYC (2003) Interaction of antimicrobial peptide protegrin with biomembranes. Proc Natl Yang Acad Sci 100:6302–6307
Gottler LM, de la Salud BR, Shelburne CE, Ramamoorthy A, Marsh ENG (2008) Using fluorous amino acids to probe the effects of changing hydrophobicity on the physical and biological properties of the beta-hairpin antimicrobial peptide protegrin-1. Biochemistry 47:9243–9250
Guo C, Cong P, He Z, Mo D, Zhang W, Chen Y, Liu X (2015) Inhibitory activity and molecular mechanism of protegrin-1 against porcine reproductive and respiratory syndrome virus in vitro. Antivir Ther 20:573–582
GuoDong W (2013) Interaction in vitro between antibacterial peptide protegrin-1 and antibiotics to pathogenic E. coli. J Henan Agric Sci 42:124–127
Gupta K, Kotian A, Subramanian H, Daniell H, Ali H (2015) Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget 6:28573–28587. https://doi.org/10.18632/oncotarget.5611
Heller WT (1999) A comparative study of the membrane-active beta-sheet peptide protegrin with the alpha-helical peptide alamethicin (Thesis). https://scholarship.rice.edu/handle/1911/19391
Heller WT, Waring AJ, Lehrer RI, Huang HW (1998) Multiple states of beta-sheet peptide protegrin in lipid bilayers. Biochemistry 37:17331–17338
Heller WT, Waring AJ, Lehrer RI, Harroun TA, Weiss TM, Yang L, Huang HW (2000) Membrane thinning effect of the β-Sheet antimicrobial protegrin. Biochemistry 39:139–145
Henderson JM, Burck J, Lehrer R, Waring AJ, Majewski J, Ulrich AS, Lee KYC (2014) Cholesterol incorporation in membranes attenuates the disruption ability of antimicrobial peptide Protegrin-1. Biophys J 106:85a
Henderson JM, Cao KD, Gong ZL, Tietjen GT, Heffern CTR, Kerr D, Nishanth I, Indroneil R, Alan JW, Mati M, Binhua L, Sushil S, Jaroslaw M, Lee KYC(2015) Activity of antimicrobial peptide Protegrin-1 is tuned by membrane cholesterol content. Biophys J 108:550a–551a. https://doi.org/10.1016/j.bpj.2014.11.3020
Henderson JM, Iyengar NS, Lam KLH, Maldonado E, Suwatthee T, Roy I, Waring AJ, Lee KYC (2019) Beyond electrostatics: Antimicrobial peptide selectivity and the influence of cholesterol-mediated fluidity and lipid chain length on protegrin-1 activity. Biochim Biophys Acta. https://doi.org/10.1016/j.bbamem.2019.04.011
Henderson JM, Maldonado E, Lehrer R, Waring AJ, Lee KYC (2012) Membrane disruption by antimicrobial peptide Protegrin-1 is tuned by incorporation of cholesterol and phosphoethanolamine lipids. Biophys J 102:89a
Hill EK, Li J (2017) Production of protegrin-1 with a matrix metalloproteinase/elastase cleavage site and its therapeutic potential for skin wound infections. Ann Biol Sci. https://doi.org/10.21767/2348-1927-C1-002
Huynh E, Penney J, Caswell J, Li J (2019) Protective effects of protegrin in dextran sodium sulfate-induced murine colitis. Front Pharmacol 10:156. https://doi.org/10.3389/fphar.2019.00156
Iyengar NS, Henderson JM, Suwatthee T, Roy I, Waring AJ, Lee KYC (2016) A thermodynamic study of the effects of cholesterol on the activity of antimicrobial peptide Protegrin-1. Biophys J 110:355a–356a
Jang H, Arce FT, Mustata M, Ramachandran S, Capone R, Nussinov R, Lal R (2011) Antimicrobial protegrin-1 forms amyloid-like fibrils with rapid kinetics suggesting a functional link. Biophys J 100:1775–1783. https://doi.org/10.1016/j.bpj.2011.01.072
Jang H, Ma B, Woolf TB, Nussinov R (2006) Interaction of Protegrin-1 with lipid bilayers: membrane thinning effect. Biophys J 91:2848–2859
Jang H, Ma B, Nussinov R (2007) Conformational study of the protegrin-1 (PG-1) dimer interaction with lipid bilayers and its effect. BMC Struct Biol 7:21
Jang H, Ma B, Lal R, Nussinov R (2008) Models of toxic β-sheet channels of Protegrin-1 suggest a common subunit organization motif shared with toxic Alzheimer β-amyloid ion channels. Biophys J 95(10):4631–4642
Jing W, Prenner EJ, Vogel HJ, Waring AJ, Lehrer RI, Lohner K (2005) Headgroup structure and fatty acid chain length of the acidic phospholipids modulate the interaction of membrane mimetic vesicles with the antimicrobial peptide protegrin-1. J Pept Sci 11:735–743
Kandasamy SK, Larson RG (2007) Binding modes of protegrin-1 a beta-strand antimicrobial peptide in lipid bilayers. Mol Simul 33:799–807
Khandelia H, Kaznessis YN (2007) Structure of the antimicrobial β-hairpin peptide protegrin-1 in a DLPC lipid bilayer investigated by molecular dynamics simulation. BBA Biomembranes 1768:509–520. https://doi.org/10.1016/j.bbamem.2006.11.015
Kolosova OA, Usachev KS, Aganov AV, Klochkov VV (2016) Antimicrobial peptide protegrins interact with DPC micelles by apolar hydrophobic cluster: structural studies by high-resolution NMR spectroscopy. Bionanoscience 6:317–319
Kokryakov VN, Harwig SSL, Panyutich EA, Shevchenko AA, Aleshina GM, Shamova OV, Korneva HA, Lehrer RI (1993) Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett 327:231–236
Knyght I, Clifton L, Saaka Y, Lawrence MJ, Barlow DJ (2016) Interaction of the antimicrobial peptides rhesus θ-defensin and porcine Protegrin-1 with anionic phospholipid monolayers. Langmuir 32:7403–7410
Lai PK, Kaznessis YN (2018) Insights into membrane translocation of protegrin antimicrobial peptides by multistep molecular dynamics simulations. ACS Omega 3:6056–6065
Lam KLH (2007) Mechanism of membrane disruption by antimicrobial peptide Protegrin-1 (p. K1.013). Presented at the APS March Meeting Abstracts. https://adsabs.harvard.edu/abs/2007APS.MAR.K1013L
Lam KLH, Ishitsuka Y, Cheng Y, Chien K, Waring AJ, Lehrer RI, Lee KYC (2006) Mechanism of supported membrane disruption by antimicrobial peptide protegrin-1. J Phys Chem B 110:21282–21286
Langham AA, Kaznessis YN (2006) Mol Simul 32:193–201
Langham AA, Khandelia H, Kaznessis YN (2006) How can a beta-sheet peptide be both a potent antimicrobial and harmfully toxic? Molecular dynamics simulations of protegrin-1 in micelles. Biopolymers 84:219–231
Langham AA, Ahmad AS, Kaznessis YN (2008) On the nature of antimicrobial activity: a model for Protegrin-1 pores. J Am Chem Soc 130:4338–4346
Lazaridis T, He Y, Prieto L (2013) Membrane interactions and pore formation by the antimicrobial peptide protegrin. Biophys J 104:633–642. https://doi.org/10.1016/j.bpj.2012.12.038
Lee SB, Li B, Jin S, Daniell H (2011) Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol J 9–100–115. https://doi.org/10.1111/j.1467-7652.2010.00538.x
Lipkin R, Lazaridis T (2017) Computational prediction of the optimal oligomeric state for membrane-inserted β-barrels of protegrin-1 and related mutants. J Pept Sci 23:334–345
Liu Y, Kamesh AC, Xiao Y, Sun V, Hayes M, Daniell H, Koo H (2016) Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials 105:156–166
Mangoni ME, Aumelas A, Charnet P, Roumestand C, Chiche L, Despaux E, Grassy G, Calas B, Chavanieu A (1996) Change in membrane permeability induced by protegrin 1: implication of disulphide bridges for pore formation. FEBS Lett 383:93–98. https://doi.org/10.1016/0014-5793(96)00236-0
Mani R, Buffy JJ, Waring AJ, Lehrer RI, Hong M (2004) Solid-state NMR investigation of the selective disruption of lipid membranes by Protegrin-1. Biochemistry 43:13839–13848
Morroni G, Simonetti O, Brenciani A, Brescini L, Kamysz W, Kamysz E, Neubauer D, Caffarini M, Orciani M, Giovanetti E, OffidaniA GA, Cirioni O (2019) In vitro activity of Protegrin-1, alone and in combination with clinically useful antibiotics, against Acinetobacter baumannii strains isolated from surgical wounds. Med Microbiol Immunol 208:877–883
Mirgorodskaya OA, Shevchenko AA, Abdalla KO, Chernushevich IV, Egorov TA, Musoliamov AX, Kokryakov VN, Shamova OV (1993) Primary structure of three cationic peptides from porcine neutrophils. Sequence determination by the combined usage of electrospray ionization mass spectrometry and Edman degradation. FEBS Lett 330:339–342
Miyakawa Y, Ratnakar P, Rao AG, Costello ML, Mathieu-Costello O, Lehrer RI, Catanzaro A (1996) In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect Immun 64:926–932
Maldonado E, Henderson M, Lee KY, Lehrer R, Waring AJ (2011) Assessing pore forming capability of Protegrin-1 in lipid bilayers of varying cholesterol content. Biophys J 100:336
Neville F, Ishitsuka Y, Hodges CS, Konovalov O, Waring AJ, Lehrer R, Lee KY, Gidalevitz D (2008) Protegrin interaction with lipid monolayers: grazing incidence X-ray diffraction and X-ray reflectivity study. Soft Matter 4:1665–1674
Niu M, Chai S, You X, Wang W, Qin C, Gong Q, Zhang T, Wan P (2015) Expression of porcine protegrin-1 in Pichia pastoris and its anticancer activity in vitro. Exp Ther Med 9:1075–1079
Osakowicz C (2018) Protective and anti-inflammatory effects of Protegrin-1 on Citrobacter rodentium intestinal infection in mice (Doctoral dissertation). https://hdl.handle.net/10214/13002
Patiño-Rodríguez O, Ortega-Berlanga B, Llamas-González YY, Flores-Valdez MA, Herrera-Díaz A, Montes-de-Oca-Luna R, Korban SS, Alpuche-Solís ÁG (2013) Transient expression and characterization of the antimicrobial peptide protegrin-1 in Nicotiana tabacum for control of bacterial and fungal mammalian pathogens. Plant Cell Tissue Organ Cul 115:99–106
Penney J, Li J(2018). Protegrin 1 enhances innate cellular defense via the insulin-like growth factor 1 receptor pathway. Front Cell Infect Microbiol 8:330. https://doi.org/10.3389/fcimb.2018.00331
Qing-hua ZYRC, Zhi-yong HJHF, Zhu-ying ZCL (2008). The effect of achyranthes bidentata polysaccharides on Protegrin-1 mRNA expression in weaned piglets. Chin J Anim Nutr
Rui H, Lee J, Im W (2009) Comparative molecular dynamics simulation studies of protegrin-1 monomer and dimer in two different lipid bilayers. Biophys J 97:787–795
Rui H, Im W (2010) Protegrin-1 orientation and physicochemical properties in membrane bilayers studied by potential of mean force calculations. J Comput Chem 31:2859–2867
Roumestand C, Louis V, Aumelas A, Grassy G, Calas B, Chavanieu A (1998) Oligomerization of protegrin-1 in the presence of DPC micelles. A proton high-resolution NMR study. FEBS Lett 421:263–267
Sayyed-Ahmad A, Kaznessis YN (2009) Determining the orientation of protegrin-1 in DLPC bilayers using an implicit solvent-membrane model. PLoS ONE 4:e4799
Shruti SR, Rajasekaran R (2019) Identification of protegrin-1 as a stable and nontoxic scaffold among protegrin family—a computational approach. J Biomol Struct Dyn 37:2430–2439
Soundrarajan N, Park S, Le Van CQ, Cho HS, Raghunathan G, Ahn B, Song H, Kim JK, Park C (2019) Protegrin-1 cytotoxicity towards mammalian cells positively correlates with the magnitude of conformational changes of the unfolded form upon cell interaction. Sci Rep 9:1–12
Sugawara K, Shinohara H, Kadoya T, Kuramitz H (2016) Sensing lymphoma cells based on a cell-penetrating/apoptosis-inducing/electron-transfer peptide probe. Anal Chim Acta 924:106–113
Steinberg DA, Hurst MA, Fujii CA, Kung AH, Ho JF, Cheng FC, Loury DJ, Fiddes JC (1997) Protegrin-1: a broad-spectrum rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother 41:1738–1742
Steinstraesser L, Klein RD, Aminlari A, Fan MH, Khilanani V, Remick DG, Su GL, Wang SC (2001) Protegrin-1 enhances bacterial killing in thermally injured skin. Crit Care Med 29:1431–1437
Steinstraesser L, Burghard O, Nemzek J, Fan MH, Merry A, Remick DI, Su GL, Steinau HU, Wang SC (2003) Protegrin-1 increases bacterial clearance in sepsis but decreases survival. Crit Care Med 31:221–226
Su Y, Waring AJ, Ruchala P, Hong M (2011) Structures of β-Hairpin antimicrobial protegrin peptides in lipopolysaccharide membranes: mechanism of gram selectivity obtained from solid-state nuclear magnetic resonance. Biochemistry 50:2072–2083
Sun YH, Zhang N (2007) Developing process and activity mechanism of protegrin. Chin J Mod Appl Pharm 5:6
Rothan HA, Mohamed Z, Sasikumar PG, Reddy KA, Rahman NA, Yusof R (2014) In vitro characterization of novel Protegrin-1 analogues against neoplastic cells. Int J Pept Res Ther 20:259–267
Tu Y, Wang Y, Xu C, Yao G, Shan T (2006) Effect of astragalus and astragalus polysaccharide on antimicrobial peptides PR-39 and protegrin-1 gene expression in pigs. Turk J Vet Anim Sci 30:325–330
Vivcharuk V, Kaznessis Y (2010) Free energy profile of the interaction between a monomer or a dimer of Protegrin-1 in a specific binding orientation to a model lipid bilayer. J Phys Chem B 114:2790–2797
Vivcharuk V, Kaznessis YN (2010) Dimerization of protegrin-1 in different environments. Int J Mol Sci 11:3177–3194
Vivcharuk V, Kaznessis YN (2011) Thermodynamic analysis of protegrin-1 insertion and permeation through a lipid bilayer. J Phys Chem B 115:14704–14712
Wang Y, Shan T, Xu Z, Liu J, Feng J (2006) Effect of lactoferrin on the growth performance intestinal morphology and expression of PR-39 and protegrin-1 genes in weaned piglets. J Anim Sci 84:2636–2641
Wi S, Kim C (2008) Pore structure thinning effect and lateral diffusive dynamics of oriented lipid membranes interacting with antimicrobial peptide protegrin-1: 31P and 2H solid-state NMR study. J Phys Chem B 112:11402–11414
WHO | Antimicrobial resistance: global report on surveillance 2014. (n.d.). WHO website. Accessed 19 Jul 2019. https://www.who.int/drugresistance/documents/surveillancereport/en/
Yan J, Zhang C, Tang L, Kuang S (2015) Effect of dietary copper sources and concentrations on serum lysozyme concentration and Protegrin-1 gene expression in weaning piglets. Ital J Anim Sci 14:3709
Yan JY, Zhang C, Tang L, Kuang SY (2013) Research progress on antibacterial peptide protegrin. China Anim Husb Vet Med 7:54
Yang L, Weiss TM, Lehrer RI, Huang HW (2000) Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys J 79:2002–2009
Zharkova MS, Orlov DS, Golubeva OY, Chakchir OB, Eliseev IE, Grinchuk TM, Shamova OV (2019) Application of antimicrobial peptides of the innate immune system in combination with conventional antibiotics—a novel way to combat antibiotic resistance? Front Cell Infect Microbiol 9:128
Acknowledgements
The authors thank Vellore Institute of Technology (Deemed to be University) for providing ‘VIT SEED GRANT’ for carrying out this review on protegrin-1.
Author information
Authors and Affiliations
Contributions
Conceptualization: [RR]; Literature survey, data analysis and drafting the article: [SSR]; Critical revision of article: [RR].
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranade, S.S., Ramalingam, R. A Review on Bioactive Porcine Peptide, Protegrin-1. Int J Pept Res Ther 26, 1493–1501 (2020). https://doi.org/10.1007/s10989-019-09955-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09955-8