Abstract
Bioactive peptides are functional agents encrypted in food proteins with several potential health benefits. Food-derived proteins when hydrolysed release large variety of bioactive peptides which are similar in structure to peptide sequences acting in the organism as endogenous signals, or hormones and alter their physiological functions. Moreover, these bioactive peptides owing to their high tissue affinity, specificity and efficiency can interact with receptors, enzymes and certain biomolecules in organism thereby confer health promoting effects. In addition, several studies have revealed that these peptides exhibit beneficial effects for the treatment and management of chronic and several degenerative diseases including hypertension, diabetes, obesity and cancer. Therefore, this review mainly used ISI, SCOPUS and PubMed indexed journals containing various experimental reports on in vitro and in vivo studies from humans and animals to elucidate the potential health promoting effects of food-derived bioactive peptides with emphasis on antihypertensive peptides, antidiabetic peptides, cholesterol-lowering peptides, anticancer peptides, and antimicrobial peptides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is increase in epidemiological evidences linking the prevalence of lifestyle-related diseases including hypertension, obesity, diabetes, and cancer to dietary factors (Hernández-Ledesma et al. 2011; Gul et al. 2015; Daliri et al. 2017). Consequently, functional foods are emerging in response to the increased perception about the relation of food and health benefits. The protein in foods serves both nutritional and physiological roles (Daliri et al. 2017). Food protein can be hydrolysed to produce several bioactive peptides with different potential health benefits. Bioactive peptides are active fragments, but they remain inactive as long as they remain locked in the parent protein (Hernández-Ledesma et al. 2014). These bioactive peptides are produced by enzymatic hydrolysis of the parent proteins as well as during food processing such as cooking, fermentation and ripening (Palaniswamy et al. 2012; Yahya et al. 2017; Daliri et al. 2017). Bioactive peptides are known to specifically interact with biomolecules and certain receptors thereby modulate their physiological functions (Daliri et al. 2017; Hayes 2018). The production of bioactive peptides and their possible incorporation into food is gaining interest particularly owing to their health promoting effects and safety (Reddi et al. 2016; Daliri et al. 2017; Lin et al. 2018). The classical approach involves enzymatic proteolysis with food-grade proteolytic enzymes to release numerous peptide fragments in hydrolysate (Ugwu et al. 2019; Abdel-Hamid et al. 2017; Babini et al. 2017; Wang et al. 2017). Also, bioactive peptides can be produced from parent proteins by the use of proteolytic system of microorganism during fermentation (Hernández-Ledesma et al. 2014; Koyama et al. 2014; García-Tejedor et al. 2015; Aguilar-Toalá et al. 2017). The biologically active peptides generated can be subjected to purification, sequence synthesis and then formulated as functional foods or nutraceuticals (Daliri et al. 2017). Moreover, in silico proteolysis has been employed by several researchers to identify potential bioactive peptide sequences and also elucidate their mechanism of actions via molecular docking analysis (Schneidman-Duhovny et al. 2005; Masuyer et al. 2012; Lin et al. 2018; Ugwu et al. 2019). Therefore, this review mainly used ISI, SCOPUS and PubMed indexed journals containing various experimental reports on in vitro and in vivo studies on humans and animals to elucidate the potential health promoting effects of foods-derived bioactive peptides with emphasis on antihypertensive peptides, antidiabetic peptides, cholesterol-lowering peptides, anticancer peptides, and antimicrobial peptides.
Production of Bioactive Peptides
Food-derived bioactive peptides are usually produced through enzymatic hydrolysis of their parent protein by enzymatic proteolysis (Mirzaei et al. 2018; Kumar et al. 2016), gastrointestinal digestion (Mohanty et al. 2016), microbial fermentation (Yahya et al. 2017) and in silico proteolysis (Ugwu et al. 2019; Lin et al. 2018). Besides, if the peptide sequence is known, it is also possible to synthesize the peptide via chemical or enzymatic synthesis or by genetic recombination using bacteria (Park et al. 1998; Lv et al. 2003; Jeong et al. 2007; Hernández-Ledesma et al. 2011).
Enzymatic Hydrolysis
In enzymatic hydrolysis, biologically active peptides can be produced through hydrolysis of the whole protein molecule using analytical-grade proteinases such as alcalase, chymotrypsin, pepsin, trypsin, elastase, flavourzyme, savinase, thermolysin, and pancreatin individually or combined (Mohanty et al. 2016; Kumar et al. 2016; Bamdad et al. 2017; Chaudhari et al. 2017; Ugwu et al. 2019). The process involves reconstitution of protein sample in appropriate buffer of different pH (the optimum pH of the hydrolytic enzymes) for optimum enzymatic action. The reconstituted protein solution has to be heated in water bath (95 °C) for 5 min to kill microorganisms, which may produce proteolytic enzymes during hydrolysis and also to denature the indigenous enzymes of proteins, if present. The optimum pH and temperature for the hydrolysis has to be standardized (Kumar et al. 2016). The enzyme/substrate ratio (E:S) should be kept at 1:100 (Kumar et al. 2016), 1:10 (Mirzaei et al. 2018), 3:1 (Lin et al. 2017) and 1:50 (Bamdad et al. 2017) for 4 h, 5 h, 4 h and 1 h respectively. Each hydrolyzed sample is immediately heated to 85 °C for 15 min in water bath to inactivate the residual enzyme left in hydrolysates (Kumar et al. 2016). The hydrolysate is allowed to cool, centrifuged in refrigerated centrifuge and then the supernatant obtained is subjected to further purification techniques (Bamdad et al. 2017) (Table 1).
Gastrointestinal Digestion
Biologically active peptides can also be released from food-derived proteins during gastrointestinal digestion by the action of digestive enzymes such as pepsin, trypsin, chymotrypsin and peptidases (Hernandez-Ledesma et al. 2011; Mohanty et al. 2016). Proteins from food sources are usually denatured in the presence of hydrochloric acid (HCl) secreted by the parietal cells of the stomach. This low pH mediates the activation of pepsinogen and its subsequent conversion to its active form called pepsin (Mohanty et al. 2016). These enzymes occur at the surface of epithelial cells where they release several peptides of different lengths. Some of these peptides exert direct function at the gastrointestinal tract while other peptides are absorbed via systemic circulation to reach target organs and tissues (Shimizu, 2004). Scientists have employed simulated digestion using different proteins such as milk proteins (Hernandez-Ledesma et al. 2004, 2007; Gomez-Ruiz et al. 2004; Lignitto et al. 2010; Egger et al. 2017; Sanchón et al. 2018; Basilicata et al. 2018), soybean seeds (Capriotti et al. 2015; González-Montoya et al. 2018), soy milk proteins (Capriotti et al. 2015), egg proteins (Wang et al. 2018), meat protein (Anna et al. 2016; Wang et al. 2018), fish proteins (Borawska et al. 2016; Mora et al. 2017; Polona et al. 2017; Korczek et al. 2018; Zhang et al. 2018), plant protein (Pachaiappan et al. 2018) in order to examine how these gastrointestinal proteases mediate the proteolytic digestion of food proteins and release bioactive peptides which in addition to nutritional benefits may exert many physiological and health beneficial functions.
Fermentation
An alternative strategy for production of biologically active peptides uses the proteolytic system of microorganism (Hernández-Ledesma et al. 2014). For instance, during fermentation process, microorganisms hydrolyse proteins into peptides and amino acids which serve as nitrogen source necessary for their growth (Juillard et al. 1998). These bioactive peptides also can be isolated by centrifugation (Palaniswamy et al. 2012) and purified through ultrafiltration or molecular sieve (Mirzaei et al. 2018), and the amino acid sequences of bioactive peptide are identified by chromatographic methods (Lin et al. 2017; Mirzaei et al. 2018). For example, IPP and VPPP are antihypertensive peptides produced by fermented milk protein using Lactobacillus helveticus and Saccharomyces cerevisiae (Nakamura et al. 1995). Also, fermentation of milk with Enterococcus faecalis, produced bioactive peptides (LHLPLP and HLPLP) which have demonstrated antihypertensive effect in rat model (Quirós et al. 2007).
The results of animal experiment and human trials suggest that fermented milk products may exhibit antioxidant effect associated with cardiovascular benefits (Hernández-Ledesma et al. 2014). Furthermore, the consumption of fermented goat milk by healthy individuals improved the total plasma antioxidant activity (Hernández-Ledesma et al. 2014). Although the compounds responsible for these effects have not yet been identified, however the peptides released in fermentated milk might have a key role (Hernández-Ledesma et al. 2014).
According to Yahya et al. bioactive peptides in milk are released by fermentation of skim milk using L.helveticus and S. thermophilus and these peptides have shown antihypertensive effects on spontaneously hypertensive rats (Yahya et al. (2017). Furthermore, Palaniswamy et al. used the proteolytic system of Lactobacillus plantarum isolated from commercially available dairy products to generate milk hydrolysates from goat milk. The hydrolysates obtained exhibited ACE-inhibitory and antioxidant properties (Palaniswamy et al. 2012). Although, the amino acid sequences of the bioactive peptides present in the hydrolysates responsible for ACE-inhibitory and antioxidant activity were not identified yet, but the bioactive peptide released in the fermentation product could be responsible.
Genetic Engineering
Recombinant DNA technology is being exploited for mass production of biologically active peptides (Schrimpf et al. 2018; De Brito et al. 2018) especially for the synthesis of long chain peptides and proteins (Chahardoli et al. 2018; Boga et al. 2018). This method involves the construction of peptides coding region and its subsequent cloning into a prokaryotic expression vector using E. coli cells as host. This allows the production of several peptides, simultaneously (Espita et al. 2009). However, one of the challenges of this technique is that the expression products may be harmful to the host (Hernández-Ledesma et al. 2011). Moreover, antibacterial peptides possess strong antibacterial activity against the expression vector cells and relative sensitivity to proteolytic enzymes (Espita et al. 2009). Interestingly, these shortcomings have been overcomed by expression of these peptides in the forms of a fusion protein or a tandem gene to neutralize their inherent toxic properties and improve their expression levels (Espita et al. 2009; Hernández-Ledesma et al. 2011). For example, ACE-inhibitory peptides with amino acid sequences HHL, HVLPVP, FFVAPFPEVFGK, and GHIATFQER have been expressed successfully in E. coli (Park et al. 1998; Lv et al. 2003; Jeong et al. 2007; Liu et al. 2007). Although promising results are being obtained, notwithstanding, the use of genetically modified microorganisms in food products is still controversial.
Purification and Characterization of Bioactive Peptides
The techniques mostly employed in production of bioactive peptides usually generate crude peptides which required further purification processes. This is because crude peptide consists of a mixture of peptides, residues of reagents and products of side reactions (Espitia et al. 2012). Thus, different separation techniques are used for purification of these crude peptides. In the initial stage of the purification process, the crude hydrolysates are subjected to centrifugation (Palaniswamy et al. 2012) followed by ultrafiltration using molecular weight cut off membranes (Bamdad et al. 2017; Chaudhari et al. 2017). Subsequently, the partially purified peptides are put through any of the reversed-phase high-performance liquid chromatography (RP-HPLC), ion-exchange chromatography, size exclusion chromatography, affinity chromatography, or capillary electrophoresis (Table 2).
The purity of a peptide is usually verified by a method different from the one used for purification process. Thus, the characterization is carried out by different methods of mass spectrometry such as electrospray ionization mass spectrometry (ESI–MS), fast atom bombardment mass spectrometry (FAB-MS) or matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) (Espitia et al. 2012; Bamdad et al. 2017) (Fig. 1).
Production, purification and characterization of bioactive peptides: RP-HPLC reversed phase- high performance liquid chromatography, IEC ion exchange chromatography, SEC size exclusion chromatography, AC affinity chromatography, CE capillary electrophoresis, ESI–MS electrospray ionization mass spectrometry, FAB-MS fast atom bombardment mass spectrometry, MALDI-MS matrix-assisted laser desorption/ionization mass spectrometry
Health Beneficial Effects of Bioactive Peptides
Bioactive peptides are 2 to 20 amino acid residues present in the parent proteins, they act as endogenous signals, or hormones in the organism (Hernández-Ledesma et al. 2014) by interacting with certain receptors and biomolecules to modulates their biological functions (Hernández-Ledesma et al. 2014). The inherent characteristic of the amino acid residues present in the peptide sequences are mainly responsible for the biological activities including ACE-inhibitory, antidiabetic, cholesterol lowering, antibacterial, Immunomodulatory, and antioxidative effects (Pihlanto 2006; Zou et al. 2016; Daliri et al. 2017; Khan et al. 2018). Therefore, bioactive peptides when exploited could be beneficial for treatment and management of numerous life style-related diseases.
Antihypertensive Peptides
ACE-Inhibitory Peptides
Hypertension is one of the major risk factors for cardiovascular disease (Erdmann et al. 2008). Angiotensin converting enzyme (EC 3.4.15.1) is one of the main regulators of blood pressure and it is a key component of the renin–angiotensin–aldosterone system (RAAS). The RAAS pathway starts with renin converting angiotensinogen into angiotensin I. Angiotensin I is then converted to angiotensin II via angiotensin converting enzyme (ACE) activity. Angiotensin II mediates vasoconstriction and activates aldosterone release from adrenal gland (Nawaz et al. 2017). Aldosterone is a steroid hormone that increases the expression of epithelial sodium channels which leads to sodium and water reabsorption resulting in hypertension (Nawaz et al. 2017). Yet in another mechanism, the kinin–kallikrein system (KKS), indicated that ACE cleaves the terminal dipeptide (Phe–Arg) of the vasodilator, bradykinin to its inactive form bradykinin 1–7 (Imig 2004). Thus, inhibition of this enzyme is considered as one of the strategies for treatment of hypertension. Owing to the facts that many synthetic antihypertensive drugs have been reported to exhibit adverse effects such as dizziness, dysgeusia, headache, angioedema, and cough (Daliri et al. 2016), there is a rapid growing demand for food-derived bioactive peptides with health-promoting effect and safety (Daliri et al. 2017). In recent years, anti-hypertensive effects of food-derived bioactive peptides in vitro and in vivo have been reported (Hernandez-Ledesma et al. 2014; Capriotti et al. 2015; Korczek et al. 2018). In addition, antihypertensive peptides possess remarkable high tissue affinities and thus eliminated slowly from tissues compared to synthetic drugs (Koyama et al. 2014). Some of the antihypertensive peptides are presented in Table 3.
Cholesterol-Lowering Peptides
Cholesterol is required by the body for synthesis of vitamin D, steroid hormones, and bile acids. However, hypercholesterolemia leads to formation of plaques in arteries resulting in arteriosclerosis and subsequent hypertension (Daliri et al. 2017). Also, cholesterol plaques in the coronary artery may reduce oxygen supply to the heart leading to cardiovascular diseases. Synthetic drugs employed for treatment of hypercholesterolemia cause liver injury, myopathy and diabetes (Katsiki and Banach 2012; Carter et al. 2013; Mancini et al. 2016). Thus, search for bioactive peptides with cholesterol lowering ability has increased over the years and many have been discovered with potential hypercholesterolemic effect (Table 4).
Antioxidant Peptides
High blood pressure is characterized by increase in reactive oxygen species (ROS) production and dysfunctional endogenous antioxidant mechanisms (Lassegue and Griendling 2004). The inability of the antioxidant mechanisms of the body to scavenge free radicals produced due to pathophysiological conditions results in induction of oxidative stress which in turn leads to tissue injury (Panth et al. 2016). Some of the risk factors for cardiovascular diseases (CVDs) such as diabetes mellitus, aging, smoking, hypercholesterolemia, and nitrate intolerance can increase the production of ROS (Panth et al. 2016). In addition, these risk factors can also trigger apoptosis, activation of metalloproteinases, proliferation and migration of smooth muscle cells, lipid peroxidation and change in vasomotor functions, all resulting to CVDs (Panth et al. 2016). Reactive Oxygen Species with unpaired electrons, such as superoxide anion (O2·−), hydroxyl radical (OH·−) and lipid radicals, are referred to as free radicals. Others such as hydrogen peroxide (H2O2), peroxynitrite (ONOO−), and hypochlorous acid (HOCl), have oxidizing effects capable of causing oxidative stress (Mada et al. 2017). For instance, the reactions of free radicals with fatty acids such as polyunsaturated fatty acids (PUFAs) within the cytoplasmic membrane generate fatty acid peroxyl radical which can attack the adjacent side chain of the fatty acid and trigger the production of other lipid radicals. The lipid radicals generated target the plasma membrane and may have adverse effect on cell function, including alteration in cell membrane permeability and dysfunction of membrane bound receptors (Bayir 2005; Mada et al. 2018). Free radicals are implicated in hypertension because they oxidize low density lipoproteins (LDLs) thereby promoting atherosclerosis (Paudel et al. 2016). Oxidized-LDLs is available in the arterial wall and macrophages take up oxidized-LDLs through scavenger receptor pathways resulting in cholesterol ester-rich foam cells and endothelial cell dysfunction, in part, via the role of lectin-like oxidized-LDLs receptor-1 (Mitra et al. 2011; Paudel et al. 2016). The foam cells secret calcium-dependent zinc containing endopeptidase and matrix metalloproteinase which are activated during oxidative stress in part because of inflammation and non-laminar shear stress (Harrison, 1997).This is one of the important steps in the progression and development of atherosclerosis (Harrison et al. 2003). Atherosclerosis disrupts the flow of blood due to plaque buildup on the artery wall leading to more resistance in blood vessels thereby causes increase in blood pressure. Food-derived peptides exhibit antioxidant property without side effects (Shanmugam et al. 2015; Sarmadi and Ismail 2010). Consequently, many peptides with antioxidant properties have been discovered (Table 5).
Mechanism of Action of Angiotensin-Converting Enzyme (ACE) Inhibitory Peptides
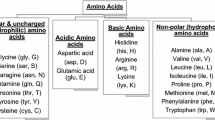
ACE inhibitory peptides usually consist of short amino acid sequence (He et al. 2013) with tyrosine, phenylalanine, tryptophan, lysine, leucine, isoleucine, valine and arginine as dominant amino acids (Murray and Fitzgerald 2007). In addition, peptides containing hydrophobic amino acids are the most effective ACE inhibitors especially those with proline in the C-terminal and positively charged amino acids (arginine and lysine) in the N-terminal (Lemes et al. 2016). Likewise, presence of branched chain amino acids such as valine and isoleucine in the peptide sequence promotes hydrophobic interaction at the ACE active site ensuing inhibition the enzyme (Daliri et al. 2017). Similarly, casein-derived peptides contain phosphorylated serine residue which are effective cation chelators that form complexes with Zn2+ in the active site of ACE to prevent metal ion catalysis (Daliri et al. 2017). Also, bioactive peptides with histidine and glutamate residues acts as Zn2+ chelators thereby enhances ACE inhibition (Fitzgerald et al. 2004; Pihlanto 2006; Daliri et al. 2017).Consequently, the ratio of hydrophilic-hydrophobic amino acids in the peptide sequence is a critical factor in ACE-inhibitory activity due to disruption of access of the peptides to ACE active site by hydrophilic amino acid residues (Mirzaei et al. 2018; Asoodeh et al. 2016; Li et al. 2004). Researchers have revealed that ACE prefers substrates or competitive inhibitors that contain aromatic amino acid residues such as tryptophan, phenylalanine and tyrosine at their C-terminal tripeptide sequence as well as branched and aliphatic amino acids such as glycine, valine, leucine, and isoleucine at the N-terminal (Kapel et al. 2006; Ondetti and Cushman 1984; Sharma et al. 2011). Besides, molecular docking analysis have shown that ACE-inhibitory peptides combine with ACE residues through the interaction forces of hydrogen bonds, hydrophobic, van der waals and electrostatic interactions that exist between amino acids residues of ACE and those of peptides (Li et al. 2014). However, hydrogen bonds interaction force plays crucial role for stabilizing the docking complex and enzyme catalytic reactions (Mirzaei et al. 2018). Wu et al. reported that ACE contained three main active site pockets viz: S1, S2, and S01 (Wu et al. 2015). The first pocket (S1) contained Ala354, Glu384 and Tyr523 residues, and S2 pocket consisted of Glu281, His353, Lys511, His513 and Tyr520 residues, while S01 included Glu162 residue. Also previous molecular docking study revealed that VLSTSFPPK, LPGSVHLAK peptides formed hydrogen bond with the S1, S2 and S01 pockets and inhibited ACE activity (Mirzaei et al. 2018) (Fig. 2).
Mechanism of Action of Cholesterol-Lowering Peptides
Biologically active peptides that possess hypocholesterolaemic effect are also crucial in the management of hypertension. For example, Cumin seed derived peptides have been shown to inhibit cholesterol micelle formation, inhibit lipase activity and bind strongly to bile acids and may therefore lower cholesterol level (Siow et al. 2016). Previous study also revealed that sericin-derived oligopeptides suppressed serum total cholesterol and inhibited cholesterol uptake by monolayer cells. These peptides also bound tightly to taurocholate, deoxytaurocholate, and glycodeoxycholate which lead to a reduced cholesterol absorption in the gut (Lapphanichayakool et al. 2017). Additionally, Lammi et al. reported that soybean peptides (LPYP, IAVPGEVA and IAVPTGVA) activated LDLR-SREBP-2 signaling pathway, improved LDL absorption and inhibit HMG-CoA reductase activity in HepG2 cells and this may account for their significant hypocholesterolaemic effect (Lammi et al. 2015). In a related studies, peptides derived from cowpea and rice bran protein hydrolysates exhibited HMG-CoA reductase-inhibitory effect and reduced cholesterol micellar solubilization in vitro (Marques, et al. 2015; Zhang et al. 2012). Moreover, black bean and cowpea-derived peptide (YAAAT) can bind tightly to the N-terminal domain of Niemann-Pick C1 (NPC1L1) and disrupt the interactions between NPC1L1 and membrane proteins thereby improve cholesterol absorption (Hernandez and de Mejia 2017) (Fig. 3).
Mechanism of Action of Antioxidative Peptides
Antioxidative activity of bioactive peptides is associated with the composition, sequence and hydrophobicity of amino acid residues present in the peptide sequence (Lassoued et al. 2015). Also presence of hydrophobic amino acids in peptides sequence enhances their solubility in lipid and facilitates accessibility to free radical species, thereby promoting antioxidant activity (Chen et al. 1996; Suetsuna et al. 2000; Qian et al. 2008). Previous study demonstrated that there exists a strong correlation between the antioxidant properties of peptides with hydrophobic and aromatic amino acid residues (Cheison et al. 2007). For instance, the presence of valine and leucine at the N-terminal and proline in the sequence of peptide contribute to its antioxidant activity (Chen et al. 1995). Additionally, the aliphatic groups in valine and leucine have high affinity to hydrophobic poly unsaturated fatty acids (Qian et al. 2008). Also, lysine-containing peptides possess antioxidant property, especially due to their ability to reduce Fe3+ to Fe2+ and to chelate Fe2+ and Cu2+ ions (Carrasco-Castilla et al. 2012; Huang et al. 2010). Phenylalanine acts as proton donor to electron deficient radicals and efficiently scavenge them (Duan et al. 2014) while bioactive peptide with histidine residue in the sequence act as oxygen quencher and hydroxyl radical scavenger due to its imidazole ring which act as proton donor (Pihlanto 2006; Zou et al. 2016). In addition, tyrosine delivers a proton to suppress free radicals (Wang et al. 2008). Furthermore, the presence of histidine, proline, cysteine, tyrosine, tryptophan, phenylalanine, and methionine in a peptide sequence promotes delay in lipid peroxidation, thus producing antioxidant effect (Li and Yu 2015). Thus it is important to mention that antioxidative effect of a single amino acid is far weaker than the additive effect of many amino acids in the sequence of a bioactive peptide (Zhu et al. 2012) (Fig. 4).
Antidiabetic Peptides
Diabetes mellitus (DM) is a metabolic disease characterized by increased blood glucose level due to inadequate insulin secretion or action, or both. DM is classified as type I and type II. The Type I diabetes (Insulin dependent diabetes mellitus) is an autoimmune disease characterized by beta cells dysfunction leading to little or no insulin secretion by pancreas. Type 2 diabetes mellitus (T2DM) also known as non-insulin dependent diabetes mellitus, there is an imbalance in the insulin secretion and blood sugar absorption (Chaudhury et al. 2017). Unfortunately, the current therapy for diabetes uses synthetic drugs that have been linked with adverse effects and increases the risks of obesity (Thulé and Umpierrez 2014), gastrointestinal disorder (Thong et al. 2015), pancreatitis (Meier and Nauck 2014) and intolerance and other metabolic disorders (Dujic et al. 2015). However, bioactive peptides present in functional foods have the potentials to regulate sugar absorption and insulin level in the body (González-Montoya et al. 2018). T2DM account for 90% of all diabetes cases (DeFronzo et al. 2015). Following consumption of a carbohydrate rich diet, insulin secretion is stimulated by the combined actions of Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-Like Peptide-1 (GLP-1) on the pancreatic cells (Silveira et al. 2013). These incretins hormones perform their physiological roles through activation of their receptors. GLP-1 exhibits both insulinotropic and glucagonostatic effects that can normalize blood glucose levels in patients with T2DM (Deacon, 2018). As a metabolic regulatory mechanism, the biological activity of incretins is significantly reduced upon degradation by dipeptidyl peptidase IV (DPP-IV). Interestingly, inhibition of DPP-IV is considered a novel therapeutic strategy for managing T2DM (Deacon 2018; Mulvihill 2018). Food-derived DPP-IV-inhibitory peptides have been identified as natural alternatives to DPP-IV inhibitory compound (Nongonierma and FitzGerald 2015). Also, α-amylase and α-glucosidase inhibitors have been employed for the control of glucose homeostasis in diabetic patients (González-Montoya et al. 2018). Food-derived bioactive peptides are gaining interest from researchers owing to their antidiabetic properties and safety (Daliri et al. 2017). Some of the bioactive peptides with antidiabetic effects have been summarized in Table 6.
Mechanism of Action of Antidiabetic Peptides
Many bioactive peptides have been demonstrated to exhibit antidiabetic effect through inhibition of DPP-IV as well as key enzymes linked to carbohydrates metabolism including α-amylase and α-glucosidase (Zhang et al. 2016). Previous study reported that fermented soybean protein contains low molecular weight peptides some of which induce insulin-stimulated glucose uptake in 3T3-L1 cells and antagonize PPAR-activities (Kwon et al. 2011). Similarly, AKSPLF, ATNPLF, FEELN, and LSVSVL peptides obtained from black bean protein hydrolysates possess glucose transporter-2 (GLUT-2) and sodium-dependent glucose transporter-1 (SGLT-1) inhibitory activity thereby reduce blood glucose level (Mojica et al. 2017). Salmon frame protein hydrolysates contain peptides promote glucose uptake in muscle cells (Roblet et al. 2016). Also, in related study, peptides (LPIIDI and APGPAGP) obtained from Silver carp protein hydrolysates effectively inhibited DPP-IV (Zhang et al. 2016) as presented in Table 6 (Fig. 5).
Antimicrobial Peptides and Their Mechanism of Action
Antimicrobial peptides are gaining interest because of their multifunctional properties including wound healing (Tomioka et al. 2014) and immunomodulation (Mansour et al. 2014) on a wide range of microorganisms (Memarpoor-Yazdi et al. 2012).These properties make antimicrobial peptides better alternatives against resistant pathogenic bacteria than conventional antibiotics (Daliri et al. 2017). Milk-derived peptides with antimicrobial property have been extensively studied (Piotto et al. 2012). Their antimicrobial activities are diverse, ranging from those that prevent the attachment or invasion of pathogen microorganisms, to those that inhibit microbial growth (Hernandez-Ledesma et al. 2014). In addition, peptide (ELLLNPTHQIYPVTQPLAPV) isolated from human colostrum inhibited bacterial growth by cell wall and cytoplasmic membrane destruction (Zhang et al. 2017). Also, AMPSSSEESII from β-s2–casein inhibit the growth of Listeria innocua, Micrococcus luteus, Salmonella enteritidis and E. coli. Milk-derived antimicrobial peptides contain more positively charged amino acid residues in their sequence (Guterstam et al. 2009). The net positive charge could aid binding to negatively charged bacterial membranes and arginine and lysine-containing peptides permeate cells by inducing ATP-dependent endocytic micropinocytosis (Guterstam et al. 2009). Besides, hydrolysis of bovine lactoferrin with pepsin and digestion of bovine whey proteins (β-lactoglobulin and α-Lactalbumin) produced antibacterial peptides with a broad spectrum against Gram-positive and Gram-negative bacteria (Pellegrini et al. 2001; Bellamy et al. 1992). Furthermore, biologically active peptide (SIFIQRFTT) has also been isolated from fish proteins with antimicrobial potentials against Listeria innocua and Escherichia coli (Guinane et al. 2015). Also, forage fish protein yielded GLSRLFTALK peptide which showed strong antimicrobial effects against S. aureus, B. subtilis, S. pneumoniae, E. coli, S. dysenteriae, P. aeruginosa and S. typhimurium (Ennaas et al. 2015). In some cultures and religion, consumption of blood is a taboo. However, in some countries, blood from domesticated animals is processed and consumed as food (Alan 2006). Hydrolysis of blood protein has also yielded several peptides with antimicrobial property. For examples, VNFKLLSHSLLVTLASHL peptide isolated from bovine haemoglobin effectively inhibited the growth of Candida albicans, Escherichia coli and Staphylococcus aureus (Aissaoui et al. 2016). In addition, hydrolysis of haemoglobin using pepsin enzyme yielded many peptides which effectively inhibited the growth of Salmonella enteritidis, Escherichia coli, Shigella sonnei, Micrococcus luteus, Enterococcus faecalis, Listeria innocua, Staphylococcus saprophyticus, Bacillus cereus and Staphylococcus simulants (Hu et al. 2011; Adje et al. 2013). Antimicrobial peptides possess both hydrophilic and hydrophobic amino acids at their terminals and this has been recognized as the major structural motifs through which they interact with microbes (Daliri et al. 2017). The mechanisms amongst which these bioactive peptides exert antibacterial effect can be either by making pores through the bacteria cell membrane or by interacting with macromolecules inside the microbial cells (Shah et al. 2016; Taniguchi et al. 2016). Researchers have discovered many antimicrobial peptides and these are available on database such as APD3 (Wang et al. 2016), CAMPR3 (Waghu et al. 2016) and YADAMP (Piotto et al. 2012). Antimicrobial peptides derived from food proteins are presented in Table 7.
Anticancer Peptides and Their Mechanism of Action
The orthodox drugs used for treatment and management of different forms of cancer are usually not effective and have adverse effects such as gonadotoxicity (Gutierrez et al. 2016), nephrotoxicity (Van Acker et al. 2016), neurotoxicity and cardiotoxicity (Oun et al. 2013). Consequently, researchers have intensified efforts towards investigation of food-derived bioactive peptides with anticancer properties with little or no side effects. Food protein-derived peptides possess inherent potentials for preventing different stages of cancer, including initiation, promotion, and progression (De Mejia and Dia 2010). The selectivity of action of anti-cancer peptides seems to be due to their strongly cationic character that allows them to interact with negatively charged structures on cancer cells, resulting in the destabilization of cancer cell membranes (Hoskin and Ramamoorthy 2008). Additionally, in vitro studies have shown that anti-cancer peptides act by inducing apoptosis, cell cycle arrest, modulating gene expression, and preventing angiogenesis (De Mejia and Dia 2010). In previous study, casein phosphopeptides (CPPs) inhibit proliferation of intestinal tumour HT-29 and AZ-97 cells and induce apoptosis by activating voltage-activated calcium channels, which mediate the calcium flux according to the depolarization state of the cell (Perego et al. 2012). Moreover, peptides from different sources have shown promising effects against cancer. For instance, oyster hydrolysate containing LANAK peptide and HVLSRAPR peptide isolated from S. platensis hydrolysate inhibited HT-29 cancer cell proliferation (Umayaparvathi et al. 2014; Wang and Zhang 2017). In addition, another peptide RQSHFANAQP from chickpea hydrolysate increased the level of p53 in breast cancer cell lines and inhibited their proliferation (Xue et al. 2015). Furthermore, setipinna taty-derived peptide (YALPAH) induced apoptosis in prostate cancer PC-3 cells (Song et al. 2014). Moreover, soybean protein hydrolysates contain many anticancer peptides exhibited strong antiproliferative effect against breast cancer cell line MCF-7 through induction of cell cycle arrest in S-phase and promote the expression of p21 and p27, decrease cyclin A expression, cleaved caspase 3, downregulate Bcl-2, PARP and caspase 9 expression with concomitant upregulation of p53 and Bax expression (Hung et al. 2014). In related study, peptides isolated from Dendrobium catenatum showed antiproliferative activity against HepG-2, SGC-7901 and MCF-7 cancer cells (Zheng et al. 2015) (Table 8).
Conclusion
Food-derived biologically active peptides have the inherent potentials to interact with tissues, cells, enzymes, reactive oxygen species and certain molecules to exert some therapeutic functions which are helpful in the management and treatment of many lifestyle-related diseases including hypertension, diabetes mellitus, obesity and cancer. In addition, several bioactive peptides possess antimicrobial effects owing to their multifunctional properties. However, the major challenge associated with bioactive peptides is retention of the activity after oral ingestion. Thus, the present review further highlighted the health promoting effects of food-derived bioactive peptides against chronic and degenerative diseases.
References
Abdel-Hamid M, Otte J, De Gobba C, Osman A, Hamad E (2017) Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int Dairy J 66:91–98
Adje EY, Balti R, Lecouturier D, Kouach M, Dhulster P, Guillochon D, Nedjar-Arroume N (2013) Controlled enzymatic hydrolysis: a new strategy for the discovery of antimicrobial peptides. Probiotics Antimicrob Proteins 5:176–186
Aguilar-Toalá J, Santiago-López L, Peres C, Peres C, Garcia H, Vallejo-Cordoba B, González-Córdova A, Hernández-Mendoza A (2017) Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J Dairy Sci 100:65–75
Aissaoui N, Chobert JM, Haertlé T, Marzouki MN, Abidi F (2016) Purification and biochemical characterization of a neutral serine protease from Trichoderma harzianum. Use in antibacterial peptide production from a fish by-product hydrolysate. Appl Biochem Biotech 182(2):831–845
Alan D (2006) The Oxford companion to food, 2nd edn. Oxford University Press, Oxford, pp 81–82
Anna T, Alexey K, Anna B, Vyacheslav K, Mikhail T, Ulia M (2016) Effect of in vitro gastrointestinal digestion on bioactivity of poultry protein. Curr Res Nutr Food Sci 4(SI.2):77–86
Arias M, Hilchie AL, Haney EF, Bolscher JG, Hyndman ME, Hancock RE (2017) Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem Cell Biol 95:91–98
Asoodeh A, Homayouni-Tabrizi M, Shabestarian H, Emtenani S (2016) Biochemical characterization of a novel antioxidant and angiotensin I-converting enzyme inhibitory peptide from Struthio camelus egg white protein hydrolysis. J Food Drug Anal 24(2):332–342
Babini E, Tagliazucchi D, Martini S, Dei Più L, Gianotti A (2017) LC-ESI-QTOF-MS identification of novel antioxidant peptides obtained by enzymatic and microbial hydrolysis of vegetable proteins. Food Chem 228:186–196
Bamdad F, Shin SH, Suh J, Nimalaratne C, Sunwoo H (2017) Anti-inflammatory and antioxidant properties of casein hydrolysates produced using high hydrostatic pressure combined with proteolytic enzymes. Molecules 22:609
Basilicata MJ, Pepe G, Adesso S, Ostacolo C, Sala M, Sommella E, Scala MC, Messore A, Autore G, Marzocco S, Campiglia P (2018) Antioxidant properties of buffalo-milk dairy products: a β-Lg peptide released after gastrointestinal digestion of buffalo ricotta cheese reduces oxidative stress in intestinal epithelial cells. Int J Mol Sci 19:1955
Bayir H (2005) Reactive oxygen species. Crit Care Med 33(12):S498–S501
Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M (1992) Identification of the bactericidal domain of lactoferrin. Biochin Biophys Acta 1121:130–136
Boga S, Bouzada D, Pena DG, Lopez MV, Vazquez ME (2018) Sequence-specific DNA recognition with designed peptides. Eur J Org Chem 3:249–261
Borawska J, Darewicz M, Pliszka M, Vegarud GE (2016) Antioxidant properties of salmon (Salmo salar L.) protein fraction hydrolysates revealed following their ex vivo digestion and in vitro hydrolysis. J Sci Food Agric 96:2764–2772
Capriotti AL, Caruso G, Cavaliere C, Samperi R, Ventura S, Chiozzi RZ, Lagana A (2015) Identification of potential bioactive peptides generated by simulated gastrointestinal digestion of soybean seeds and soy milk proteins. J Food Compost Anal 44:205–213
Carrasco-Castilla J, Hernandez-Alvarez AJ, Jimenez-Martínez C, Jacinto-Hernandez C, Alaiz M, Giron-Calle J (2012) Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem 131(4):1157–1164
Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM (2013) Risk of incident diabetes among patients treated with stains: population based study. Br Med J 346:2610
Castellano P, Aristoy MC, Sentandreu MÁ, Vignolo G, Toldrá F (2013) Peptides with angiotensin I converting enzyme (ACE) inhibitory activity generated from porcine skeletal muscle proteins by the action of meat-borne lactobacillus. J Proteomics 89:183–190
Chahardoli M, Fazeli A, Niazi A, Ghabooli M (2018) Recombinant expression of LFchimera antimicrobial Peptide in a plant-based expression systems and its antimicrobial activity against clinical and phytopathogenic bacteria. Biotechnol Biotechnol Equip 32:714–723
Chaudhari DD, Singh R, Mallappa RH, Rokana N, Kaushik JK, Bajaj R, Batish VK, Grover S (2017) Evaluation of casein and whey protein hydrolysates as well as milk fermentates from Lactobacillus helveticus for expression of gut hormones. Indian J Med Res 146:409–419
Chaudhury A, Duvoor C, Dendi VSR, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K (2017) Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol 8:6
Cheison SC, Wang Z, Xu SY (2007) Preparation of whey protein hydrolysates using a single-and two stage enzymatic membrane reactor by multivariate data analysis. J Agric Food Chem 55:3896–3904
Chen HM, Muramoto K, Yamauchi F (1995) Structural analysis of antioxidative peptides from soybean bet-conglycinin. J Agric Food Chem 43(3):574–578
Chen HM, Muramoto K, Yamauchi F, Nokihara K (1996) Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digested of a soybean protein. J Agric Food Chem 44(9):2619–2623
Chetwynd AJ, Guggenheim EJ, Briffa SM, Thorn JA, Lynch I, Valsami-Jones E (2018) Current application of capillary electrophoresis in nanomaterial characterisation and its potentials to characterise the protein and small molecules corona. Nanomaterials 8(2):99
Daliri EBM, Lee BH, Oh DH (2016) Current perspectives on antihypertensive probiotics. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-016-9241-y
Daliri EB, Deog HO, Byong HL (2017) Bioactive peptides. Foods 6:32
De Brito RCF, Cardoso JMDO, Reis LES, Vieira JF, Mathias FAS, Roatt BM, Aguiar-Soares RDDO, Ruiz JC, Resende DDM, Reis AB (2018) Peptide vaccines for leishmaniasis. Front Immunol 9:1043
De Mejia EG, Dia VP (2010) The role of nutraceutical proteins and peptides in apoptosis, angiogenesis, and metastasis of cancer cells. Cancer Metastasis Rev 29:511–528
Deacon CF (2018) Peptide degradation and the role of DPP-4 inhibitors in the treatment of type 2 diabetes. Peptides 100:150–157
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI (2015) Type 2 diabetes mellitus. Nat Rev Dis Primers 1:15019
Duan X, Ocen D, Wu F, Li M, Yang N, Xu J (2014) Purification and characterization of a natural antioxidant peptide from fertilized eggs. Food Res Int 56:18–24
Dujic T, Causevic A, Bego T, Malenica M, Velija-Asimi Z, Pearson E, Semiz S (2015) Organic cation transporter 1 variant and gastrointestinal side effects of metformin in patients with type 2 diabetes. Diabet Med 4:511–514
Egger L, Ménard O, Baumanna C, Duerra D, Schlegel P, Stoll P, Vergèresa G, Dupont D, Portmanna R (2017) Digestion of milk proteins: comparing static and dynamic in vitro digestion systems with in vivo data. Food Res Int 12:049
Ennaas N, Hammami R, Beaulieu L, Fliss I (2015) Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem Biophys Res Commun 462:195–200
Erdmann K, Cheung BWY, Schröder H (2008) The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J Nutr Biochem 19:643–654
Espita PJP, De Fátima NFS, Coimbra JSR, Andrade NJ, Cruz RS, Medeiros EAA (2009) Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol 5:1447–1464
Espitia PJP, Soares NF, Coimbra JS, Jose de Andrade N, Cruz NARS, Medeiros EAA (2012) Bioactive peptides: synthesis, properties, and applications in the packaging and preservation of food. Compr Rev Food Sci Food Saf 2012:11
Fernández-Tomé S, Sanchón J, Recio I, Hernández-Ledesma B (2017) Transepithelial transport of lunasin and derived peptides: inhibitory effects on the gastrointestinal cancer cells viability. J Food Compost Anal 68:101–110
FitzGerald RJ, Murray BA, Walsh DJ (2004) Hypotensive peptides from milk proteins. J Nutrition 134(4):980S–988S
García-Mora P, Martín-Martínez M, Bonache MA, González-Múniz R, Peñas E, Frias J, Martinez-Villaluenga C (2017) Identification, functional gastrointestinal stability and molecular docking studies of Lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chem 221:464–472
García-Tejedor A, Castelló-Ruiz M, Gimeno-Alcañíz JV, Manzanares P, Salom JB (2015) In vivo antihypertensive mechanism of lactoferrin-derived peptides: reversion of angiotensin-I and angiotensin II-induced hypertension in wister rats. J funct foods 15:294–300
Girgih AT, He R, Malomo S, Offengenden M, Wu J, Aluko RE (2014) Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J Funct Foods 6:384–394
Gomez-Ruiz JA, Ramos M, Recio I (2004) Angiotensin converting enzyme-inhibitory activity of peptide isolated from Manchego cheese. Stability under simulated gastrointestinal digestion. Int Dairy J 14:1075
González-Montoya M, Hernández-Ledesma B, Mora-Escobedo R, Martínez-Villaluenga C (2018) Bioactive peptides from germinated soybean with anti-diabetic potential by inhibition of dipeptidyl peptidase-IV, α-amylase, and α-glucosidase enzymes. Int J Mol Sci 19:2883
Guinane CM, Kent RM, Norberg S, O’Connor PM, Cotter PD, Hill C, Fitzgerald GF, Stanton C, Ross RP (2015) Generation of the antimicrobial peptide caseicin a from casein by hydrolysis with thermolysin enzymes. Int Dairy J 49:1–7
Gul W, Farooq N, Anees D, Khan U, Rehan F (2015) Camel milk: a boon to mankind. Int J Res Stud Biosci 3:23–29
Guterstam P, Madani F, Hirose H, Takeuchi T, Futaki S, Andaloussi SE, Gräslund A, Langel Ü (2009) Elucidating cell-penetrating peptide mechanisms of action for membrane interaction, cellular uptake, and translocation utilizing the hydrophobic counter-anion pyrenebutyrate. Biochim Biophys Acta 1788:2509–2517
Gutierrez K, Glanzner WG, Chemeris RO, Rigo ML, Comim FV, Bordignon V, Gonçalves PB (2016) Gonadotoxic effects of busulfan in two strains of mice. Reprod Toxicol 59:31–39
Harnedy PA, O’Keeffe MB, FitzGerald RJ (2015) Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria palmate. Food Chem 172:400–406
Harrison DG (1997) Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 100(9):2153–2157
Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H (2003) Role of oxidative stress in atherosclerosis. Am J Cardiol 91(3):7–11
Hayes M (2018) Food proteins and bioactive peptides: new and novel sources, characterisation strategies and applications. Foods 7:38
He R, Alashi A, Malomo SA, Girgih AT, Chao D, Ju X (2013) Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem 141:153–159
Hernandez LMR, de Mejia EG (2017) Bean peptides have higher in silico binding affinities than Ezetimibe for the N-terminal domain of cholesterol receptor Niemann-Pick C1 like-1. Peptides 17:30052–30059
Hernandez-Ledesma B, Amigo L, Ramos M, Recio I (2004) Release of angiotensin converting enzyme inhibitory peptides by simulated gastrointestinal digestion of infant formulas. Int Dairy J 14:889
Hernandez-Ledesma B, Contreras M, Recio I (2011) Antihypertensive peptides: production, bioavailability and incorporation into foods. Adv Colloid Interface Sci 165:23–35
Hernandez-Ledesma B, Garcia-Nebot MJ, Fernandez-Tome S, Amigo L, Recio I (2014) Dairy protein hydrolysates: peptides for health benefits. Int Dairy J 38:82–100
Hernández-Ledesma B, Quirós A, Amigo L, Recio I (2007) Identification of bioactive peptides after digestion of human milk and infant formula with pepsin and pancreatin. Int Dairy J 17:42
Hoskin DW, Ramamoorthy A (2008) Studies on anticancer activities of anti-microbial peptides. Biochim Biophy Acta 1778:357–385
Hu J, Xu M, Hang B, Wang L, Wang Q, Chen J, Song T, Fu D, Wang Z, Wang S (2011) Isolation and characterization of an antimicrobial peptide from bovine hemoglobin subunit. World J Microbiol Biotechnol 27:767–771
Huang SM, Chen KN, Chen YP, Hong WS, Chen MJ (2010) Immunomodulatory properties of the milk whey products obtained by enzymatic and microbial hydrolysis. Int J Food Sci Technol 45(5):1061–1067
Huang SL, Jao CL, Ho KP, Hsu KC (2012) Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 2012(35):114–121
Hung CC, Yang YH, Kuo PF, Hsu KC (2014) Protein hydrolysates from tuna cooking juice inhibit cell growth and induce apoptosis of human breast cancer cell line MCF-7. J Funct Foods 11:563–570
Imig JD (2004) ACE inhibition and bradykinin-mediated renal vascular responses: EDHF involvement. Hypertension 43(3):533–535
Jeong DW, Shin DS, Ahn CW, Song IS, Lee HJ (2007) Expression of antihypertensive peptide, His-His-Leu as tandem repeats in Escherichia coli. J Microbiol Biotechnol 17:952
Juillard V, Guillot A, Le Bars D, Gripon JC (1998) Specificity of milk peptide utilization by Lactococcus lactis. Appl Environ Microbiol 64:1230
Kamali AE, Ehsani M (2017) Antimicrobial peptides derived from milk: a review. J Food Biosci Technol 7:49–56
Kapel R, Rahhou E, Lecouturier D, Guillochon D, Dhulster P (2006) Characterization of an antihypertensive peptide from an alfalfa white protein hydrolysate produced by a continuous enzymatic membrane reactor. Process Biochem 41:1961–1966
Katsiki N, Banach M (2012) Statin use and risk of diabetes mellitus in postmenopausal women. Clin Lipidol 7:267–270
Khan MU, Pirzadeh M, Förster CY, Shityakov S, Shariati MA (2018) Role of milk-derived antibacterial peptides in modern food biotechnology: their synthesis, applications and future perspectives. Biomolecules 8:110
Korczek K, Tkaczewska J, Migdał W (2018) Antioxidant and antihypertensive protein hydrolysates in fish products—a review. Czech J Food Sci 36(3):195–207
Koyama M, Hattori S, Amano Y, Watanabe M, Nakamura K (2014) Blood pressure -lowering peptides from neo-fermented buckwheat sprouts: a new approach to estimating ACE-inhibitory activity. PLoS ONE 9:e105802
Kumar D, Chatli MK, Singh R, Mehta N, Kumar P (2016) Enzymatic hydrolysis of camel milk casein and its antioxidant properties. Dairy Sci Technol l96:391–404
Kwon DY, Hong SM, Ahn IS, Kim MJ, Yang HJ, Park S (2011) Isoflavonoids and peptides from meju long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effects in vitro. Nutrition 27:244–252
Lacroix IM, Chen XM, Kitts D, Li-Chan EC (2017) Investigation into the bioavailability of milk protein-derived peptides with dipeptidyl-peptidase IV inhibitory activity using Caco-2 cell monolayers. Funct Foods 8:701–709
Lafarga T, Hayes M (2016) Bioactive protein hydrolysates in the functional food ingredient industry: overcoming current challenges. Food Rev Int 33:217–246
Lammi C, Zanoni C, Arnoldi A (2015) IAVPGEVA, IAVPTGVA, and LPYP, three peptides from soy glycinin, modulate cholesterol metabolism in HepG2 cells through the activation of the LDLR-SREBP2 pathway. J Funct Foods 14:469–478
Lammi C, Zanoni C, Calabresi L, Arnoldi A (2016) Lupin protein exerts cholesterol-lowering effects targeting PCSK9: from clinical evidences to elucidation of the in vitro molecular mechanism using HepG2 cells. J Funct Foods 23:230–240
Lapphanichayakool P, Sutheerawattananonda M, Limpeanchob N (2017) Hypocholesterolemic effect of sericin-derived oligopeptides in high-cholesterol fed rats. J Nat Med 71:208–215
Lassegue B, Griendling KK (2004) Reactive oxygen species in hypertension; an update-review article. Am J Hypertens 17(9):852–860
Lassoued I, Mora L, Barkia A, Aristoy M, Nasr M, Toldra F (2015) Bioactive peptides identified in thorn-back ray skin’s gelatin hydrolysates by proteases from Bacillus subtilis and Bacillus amyloliquefaciens. J Proteomics 128:8–17
Lemes AC, Sala L, Ores Jda C, Braga AR, Eqea MB, Fernandes KF (2016) A review of the latest advances in encrypted bioactive peptides from protein-rich waste. Int J Mol Sci 17:950–974
Li Y, Yu J (2015) Research progress in structure–activity relationship of bioactive peptides. J Med Food 18:147–156
Li GH, Le GW, Shi YH, Shrestha S (2004) Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr Res 24(7):469–486
Li P, Jia J, Fang M, Zhang L, Guo M, Xie J (2014) In vitro and in vivo ACE inhibitory of pistachio hydrolysates and in silico mechanism of identified peptide binding with ACE. Process Biochem 49(5):898–904
Li-Chan EC, Hunag SL, Jao CL, Ho KP, Hsu KC (2012) Peptides derived from atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J Agric Food Chem 60:973–978
Lignitto L, Cavatorta V, Balzan S, Gabai G, Galaverna G, Novelli E (2010) Angiotensin-converting enzyme inhibitory activity of water-soluble extracts of Asiago d’allevo cheese. Int Dairy J 20:11
Lin S, Liang R, Xue P, Zhang S, Liu Z, Dong X (2017) Antioxidant activity improvement of identified Pine nut peptides by pulsed electric field (PEF) and the mechanism exploration. LWT-Food Sci Technol 75:366–372
Lin K, Zhang L, Han X, Xin L, Meng Z, Gong P, Cheng D (2018) Yak milk casein as potential precursor of angiotensin-I-converting enzyme inhibitory peptides based on in silico proteolysis. Food Chem 254:340–347
Liu D, Sun HY, Zhang LJ, Li SM (2007) High-level expression of milk-derived antihypertensive peptide in Escherichia coli and its bioactivity. J Agric Food Chem 55:5109
Lv GS, Huo GC, Fu XY (2003) Expression of milk derived antihypertensive peptide in Escherichia coli. J Dairy Sci 86:1927
Mada SB, Reddi S, Kumar N, Kumar R, Kapila S, Kapila R, Trivedi R, Karvande A, Ahmad N (2017) Antioxidative peptide from milk exhibits antiosteopenic effects through inhibition of oxidative damage and bone-resorbing cytokines in ovariectomized rats. Nutrition 43–44:21–31
Mada SB, Reddi S, Kumar N, Vij R, Yadav R, Kapila S, Kapila R (2018) Casein-derived antioxidative peptide prevents oxidative stress-induced dysfunction in osteoblast cells. PharmaNutrition 6:169–179
Mancini GJ, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, Gupta M, Hegele RA, Ng D, Pearson GJ (2016) Diagnosis, prevention, and management of stain adverse effects and intolerance: Canadian consensus working group update. Can J Cardiol 32:S35–S65
Mansour SC, Pena OM, Hancock RE (2014) Host defense peptides: front-line immunomodulators. Trends Immunol 35:443–450
Marques MR, Freitas RAMS, Carlos ACC, Siguemoto ÉS, Fontanari GG, Arêas JA (2015) Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem 168:288–293
Masuyer G, Schwager SLU, Sturrock ED, Isaac RE, Acharya KR (2012) Molecular recognition and regulation of human angiotensin-I converting enzyme (ACE) activity by natural inhibitory peptides. Sci Rep 2:717
McClean S, Beggs LB, Welch RW (2014) Antimicrobial activity of antihypertensive food-derived peptides and selected alanine analogue. Food Chem 146:443–447
Meier JJ, Nauck MA (2014) Risk of pancreatitis in patients treated with incretin-based therapies. Diabetologia 57:1320–1324
Memarpoor-Yazdi M, Asoodeh A, Chamani J (2012) A novel antioxidant and antimicrobial peptides from egg white lysozyme hydrolysates. J Funct Foods 4:278–286
Mirzaei M, Mirdamadi S, Ehsani MR, Aminlari M (2018) Production of antioxidant and ACE-inhibitory peptides from Kluyveromyces marxianus protein hydrolysates: purification and molecular docking. J Food Drug Anal 26:696–705
Mitra S, Goyal T, Mehta JL (2011) Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drug Ther 25(5):419–429
Mohanty DP, Mohapatra S, Misra S, Sahu PS (2016) Milk derived bioactive peptides and their impact on human health—a review. Saudi J Biol Sci 23(5):577–583
Mojica L, de Mejia EG, Granados-Silvestre MÁ, Menjivar M (2017) Evaluation of the hypoglycemic potential of a black bean hydrolyzed protein isolate and its pure peptides using in silico, in vitro and in vivo approaches. J Funct Foods 31:274–286
Mora L, Gallego M, Reig M, Toldrá F (2017) Challenges in the quantitation of naturally generated bioactive peptides in processed meats. Trends Food Sci Technol 69:306–314
Mudgil P, Kamal H, Yuen GC, Maqsood S (2018) Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem 259:46–54
Mulvihill EE (2018) Dipeptidyl peptidase inhibitor therapy in type 2 diabetes: control of the incretin axis and regulation of postprandial glucose and lipid metabolism. Peptides 100:158–164
Murray BA, FitzGerald RJ (2007) Angiotensin converting enzyme inhibitory peptides derived from food protein: biochemistry, bioactivity and production. Cur Pharm Des 13:773–791
Nagaoka S, Futamura Y, Miwa K, Awano T, Yamauchi K, Kanamaru Y (2001) Identification of novel hypocholesterolemic peptides derived from bovine milk β-lactoglobulin. Biochem Biophys Res Commun 281:11–17
Nakamura Y, Yamamoto N, Sakai K, Takano T (1995) Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors of angiotensin-I-converting enzyme. J Dairy Sci 78:1253–1257
Nawaz KAA, David SM, Murugesh E, Thandeeswaran M, GopikrishnanKiran K, Mahendran R et al (2017) Identification and in silico characterization of a novel peptide inhibitor of angiotensin converting enzyme from pigeon pea (Cajanuscajan). Phytomedicine 09:013
Nimalaratne C, Bandara N, Wu J (2015) Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem 188:467–472
Nongonierma AB, FitzGerald RJ (2015) Susceptibility of milk protein-derived peptides to dipeptidyl peptidase IV (DPP-IV) hydrolysis. Food Chem 145:845–852
Nongonierma AB, Paolella S, Mudgil P, Maqsood S, FitzGerald RJ (2018) Identification of novel dipeptidylpeptidase IV (DPP-IV) inhibitory peptides in camel milk protein hydrolysates. Food Chem 244:340–348
Nongonierma AB, Cadamuro C, Le Gouic A, Mudgil P, Maqsood S, FitzGerald RJ (2019) Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of a camel whey protein enriched hydrolysate preparation. Food Chem 279:70–79
Ondetti MA, Cushman DW (1984) Angiotensin-converting enzyme inhibitors: biochemical properties and biological actions. CRC Crit Rev Biochem 16:381–411
Oun R, Plumb J, Rowan E, Wheate N (2013) Encapsulation of cisplatin by cucurbituril decreases the neurotoxic and cardiotoxic side effects of cisplatin. Toxicol Lett 221:S92
Pachaiappan R, Tamboli E, Acharya A, Su C, Gopinath SCB, Chen Y, Velusamy P (2018) Separation and identification of bioactive peptides from stem of Tinospora cordifolia (Willd) Miers. PLoS ONE 13(3):e0193717
Palaniswamy M, Angayarkanni J, Nandhini B (2012) Angiotensin converting enzyme inhibitory activity and antioxidant properties of goat milk hydrolysates. Int J Pharm Pharm Sci 4:367–370
Panth N, Paudel KR, Parajuli K (2016) Reactive oxygen species: a key hallmark of cardiovascular disease—a review. Adv Med 2016(9152732):12
Park CJ, Lee JH, Hong SS, Lee HS, Kim SC (1998) High-level expression of the angiotensin-converting enzyme inhibiting peptide, YG-1, as tandem multimers in Escherichia coli. Appl Microbiol Biotechnol 50:71
Paudel KR, Lee UW, Kim DW (2016) Chungtaejeon, a Korean fermented tea, prevents the risk of atherosclerosis in rats fed a high-fat atherogenic diet. J Integr Med 14(2):134–142
Pellegrini A, Dettling C, Thomas U, Hunziker P (2001) Isolation and characterization of four bactericidal domains in the bovine b-lactoglobulin. Biochem Biophys Acta 1526:131–140
Perego S, Cosentino S, Fiorilli A, Tettamanti G, Ferraretto A (2012) Casein phosphopeptides modulate proliferation and apoptosis in HT-29 cell line through their interaction with voltage-operated L-type calcium uptake and apoptosis in Caco2 cells through their interaction with the TRPV6 calcium channel. J Funct Foods 5:847–857
Pihlanto A (2006) Antioxidative peptides derived from milk proteins. Int Dairy J 16:1306–1314
Pina AS, Batalha IL, Roque ACA (2014) Affinity Tags in Protein Purification and Peptide Enrichment: An Overview. Chapter 14, Methods in molecular biology. Humana Press, Clifton. https://doi.org/10.1007/978-1-62703-977-2-14
Piotto SP, Sessa L, Concilio S, Iannelli P (2012) Yadamp: yet another database of antimicrobial peptides. Int J Antimicrob Agents 39:346–351
Polona J, Katja I, Tune W, Margret G, Annette A, Rosa J, Hordur K, Ingrid U (2017) Bioactivity of cod and chicken protein hydrolysates before and after in vitro Gastrointestinal Digestion. Food Technol Biotech 55(3):360–367
Qian ZJ, Jung WK, Kim SK (2008) Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana shaw. Bioresour Technol 99(6):1690–1698
Quirós A, Ramos M, Muguerza B, Delgado MA, Miguel M, Aleixandre A et al (2007) Identification of Novel antihypertensive peptides in milk fermented with Enterococcus faecalis. Int Dairy J 17:33–41
Reddi S, Kumar N, Vij R, Mada SB, Kapila S, Kapila R (2016) Akt drives buffalo casein derived novel peptide mediated osteoblast differentiation. J Nutr Biochem 38:134–144
Roblet C, Akhtar MJ, Mikhaylin S, Pilon G, Gill T, Marette A, Bazinet L (2016) Enhancement of glucose uptake in muscular cell by peptide fractions separated by electrodialysis with filtration membrane from Salmon frame protein hydrolysate. J Funct Foods 22:337–346
Rodriguez-Figueroa JC, Gonzalez-Cordova AF, Torres-Llanez MJ, Garcia HS, Vallejo-Cordoba B (2012) Novel angiotensin-I-enzyme inhibitory peptides produced in fermented milk by specific wild Lactococcus lactis strains. J Dairy Sci 95:5536–5543
Sanchón J, Fernández-Tomé S, Miralles B, Hernández-Ledesma B, Tomé D, Gaudichon C et al (2018) Protein degradation and peptide release from milk proteins in human jejunum. Comparison with in vitro gastrointestinal simulation. Food Chem 239:486–494
Sarmadi BH, Ismail A (2010) Antioxidative peptides from food proteins: a review. Peptides 31:1949–1956
Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ (2005) PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 33:W363–W367
Schrimpf A, Hempel F, Li A, Linne U, Maier UG, Reetz MT, Geyer A (2018) Hinge-type dimerization of proteins by a tetracysteine peptide of high pairing specificity. Biochemistry 57:3658–3665
Shah P, Hsiao FSH, Ho YH, Chen CS (2016) The proteome targets of intracellular targeting antimicrobial peptides. Proteomics 16:1225–1237
Shanmugam VP, Kapila S, Sonfack TK, Kapila R (2015) Antioxidative peptide derived from enzymatic digestion of Buffalo casein. Int Dairy J 42:1–5
Sharma S, Singh R, Rana S (2011) Bioactive peptides: a review. Int J Bioautomation 15:223–250
Shimizu M (2004) Food-derived peptides and intestinal functions. BioFactors 21:43
Silveira ST, Martínez-Maqueda D, Recio I, Hernández-Ledesma B (2013) Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem 141:1072–1077
Siow HL, Choi SB, Gan CY (2016) Structure–activity studies of protease activating, lipase inhibiting, bile acid binding and cholesterol-lowering effects of pre-screened Cumin seed bioactive peptides. J Funct Foods 27:600–611
Song R, Wei RB, Luo HY, Yang ZS (2014) Isolation and identification of an antiproliferative peptide derived from heated products of peptic hydrolysates of half-fin anchovy (Setipinna taty). J Funct Foods 10:104–111
Suetsuna K, Ukeda H, Ochi H (2000) Isolation and characterization of free radical scavenging activities peptides derived from casein. J Nutr Biochem 11(3):128–131
Tang W, Zhang H, Wang L, Qian H, Qi X (2015) Targeted separation of antibacterial peptide from protein hydrolysate of anchovy cooking wastewater by equilibrium dialysis. Food Chem 168:115–123
Taniguchi M, Ochiai A, Kondo H, Fukuda S, Ishiyama Y, Saitoh E, Kato T, Tanaka T (2016) Pyrrhocoricin, a proline-rich antimicrobial peptide derived from insect, inhibits the translation process in the cell-free Escherichia coli protein synthesis system. J Biosci Bioeng 121:591–598
Thong K, Gupta PS, Blann A, Ryder R (2015) The influence of age and metformin treatment status on reported gastrointestinal side effects with liraglutide treatment in type 2 diabetes. Diabetes Res Clin Pract 109:124–129
Thulé PM, Umpierrez G (2014) Sulfonylureas: a new look at old therapy. Curr Diabetes Rep 14:1–8
Tomioka H, Nakagami H, Tenma A, Saito Y, Kaga T, Kanamori T et al (2014) Novel anti-microbial peptide SR-0379 accelerates wound healing via the PI3 Kinase/Akt/mTOR pathway. PLoS ONE 9:e92597
Tulipano G, Sibilia V, Caroli AM, Cocchi D (2011) Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 32:835–838
Uchida M, Ohshiba Y, Mogami O (2011) Novel dipeptidyl peptidase-4- inhibiting peptide derived from β-lactoglobulin. J Pharmacol Sci 117:63–66
Uenishi H, Kabuki T, Seto Y, Serizawa A, Nakajima H (2012) Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-IV)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int Dairy J 22:24–30
Ugwu CP, Abarshi MM, Mada SB, Sanusi B, Nzelibe HC (2019) Camel and horse milk casein hydrolysates exhibit angiotensin converting enzyme inhibitory and antioxidative effects in vitro and in silico. Int J Pept Res Ther 19(4):1573–3139
Umayaparvathi S, Meenakshi S, Vimalraj V, Arumugam M, Sivagami G, Balasubramanian T (2014) Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed Prev Nutr 4:343–353
Van Acker T, Van Malderen SJ, Van Heerden M, McDuffie JE, Cuyckens F, Vanhaecke F (2016) High-resolution laser ablation-inductively coupled plasma-mass spectrometry imaging of cisplatin-induced nephrotoxic side effects. Anal Chim Acta 945:23–30
Vital DAL, de Mejía EG, Dia VP, Loarca-Piña G (2014) Peptides in common bean fractions inhibit human colorectal cancer cells. Food Chem 157:347–355
Waghu FH, Barai RS, Gurung P, Idicula-Thomas S (2016) CAMPR3: a database on sequences, structures antimicrobial peptides. Nucleic Acids Res 44:D1094–D1097
Wang Z, Zhang X (2017) Isolation and identification of anti-proliferative peptides from spirulina platensis using three-step hydrolysis. J Agric Food Sci 97:918–922
Wang JR, Teng D, Tian ZG (2008) Preparation and mechanism of functional antioxidant peptides. Nat Prod Res Dev 20:371–375
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44:D1087–D1093
Wang X, Chen H, Fu X, Li S, Wei J (2017) A novel antioxidant and ace inhibitory peptide from rice bran protein: biochemical characterization and molecular docking study. LWT-Food Sci Technol 75:93–99
Wang J, Liao W, Nimalaratne C, Chakrabarti S, Wu J (2018) Purification and characterization of antioxidant peptides from cooked eggs using a dynamic in vitro gastrointestinal model in vascular smooth muscle A7r5 cells. Nat Partn J Sci Food 2:7
Wu Q, Jia J, Yan H, Du J, Gui Z (2015) A novel angiotensin-capital I, Ukrainian converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: biochemical characterization and molecular docking study. Peptides 68:17–24
Xu X, Gao Y (2015) Purification and identification of angiotensin I-converting enzyme-inhibitory peptides from apalbumin 2 during simulated gastrointestinal digestion. J Agric Food Sci 95:906–914
Xue Z, Wen H, Zhai L, Yu Y, Li Y, Yu W, Cheng A, Wang C, Kou X (2015) Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.). Food Res Int 77:75–81
Yahya MA, Alhaj OA, Al-Khalifa AS (2017) Antihypertensive effect of fermented skim camel (Camelus dromedaries) milk on spontaneously hypertensive rats. Nutr Hosp 34(2):416–421
Yamauchi R, Ohinata K, Yoshikawa M (2003) β-Lactotensin and neurotensin rapidly reduce serum cholesterol via NT2 receptor. Peptides 24:1955–1961
Yigzaw Y, Hinckley P, Hewig A, Vedantham G (2009) Ion exchange chromatography of proteins and clearance of aggregates. Curr Pharm Biotechnol 10:421–426
Zhang H, Yokoyama WH, Zhang H (2012) Concentration-dependent displacement of cholesterol in micelles by hydrophobic rice bran protein hydrolysates. J Agric Food Sci 92:1395–1401
Zhang Y, Chen R, Ma H, Chen S (2015) Isolation and identification of dipeptidyl peptidase IV-inhibitory peptides from trypsin/chymotrypsin-treated goat milk casein hydrolysates by 2D-TLC and LC-MS/MS. J Agric Food Chem 63:8819–8828
Zhang Y, Chen R, Chen X, Zeng Z, Ma H, Chen S (2016) Dipeptidyl peptidase IV-inhibitory peptides derived from Silver carp (Hypophthalmichthys molitrix val.) proteins. J Agric Food Chem 64:831–839
Zhang F, Cui X, Fu Y, Zhang J, Zhou Y, Sun Y, Wang X, Li Y, Liu Q, Chen T (2017) Antimicrobial activity and mechanism of human milk-sourced peptide casein. Biochem Biophys Res Commun 485:698–704
Zhang G, Zheng S, Feng Y, Shen G, Xiong S, Du H (2018) Changes in nutrient profile and protein and antioxidant activities of different fish soups, before and after simulated gastrointestinal digestion. Molecules 23:1965
Zheng Q, Qiu D, Liu X, Zhang L, Cai S, Zhang X (2015) Antiproliferative effect of dendrobium catenatum lindley polypeptides against human liver, gastric and breast cancer cell lines. Food Funct 6:1489–1495
Zhu YH, Liu R, Wu H, Wang LC (2012) Progress of structure-activity relationship of bioactive peptides. China J Trad Chin Med Pharm 27:2625–2628
Zou T, He T, Li H, Tang H, Xia E (2016) The structure–activity relationship of the of the antioxidant peptides from natural proteins. Molecules 21:72
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mada, S.B., Ugwu, C.P. & Abarshi, M.M. Health Promoting Effects of Food-Derived Bioactive Peptides: A Review. Int J Pept Res Ther 26, 831–848 (2020). https://doi.org/10.1007/s10989-019-09890-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09890-8