Abstract
The information emerging from the studies demonstrates adrenergic system and nociceptin/orphanin FQ (N/OFQ) play a crucial role on appetite regulation but there is no information for their interaction. The purpose of this study was to examine the effects of intracerebroventricular (ICV) injection of prazosin (α1 receptor antagonist), yohimbine (α2 receptor antagonist), metoprolol (β1 adrenergic receptor antagonist), ICI 118,551 (β2 adrenergic receptor antagonist) and SR59230R (β3 adrenergic receptor antagonist) on N/OFQ-induced hyperphagia by 3-h food-deprived neonatal broiler chicken. In experiment 1, chicken injected with saline, prazosin (10 nmol), N/OFQ (16 nmol) and co-injection of prazosin + N/OFQ. In experiment 2, ICV injection of saline, yohimbine (13 nmol), N/OFQ (16 nmol) and yohimbine + N/OFQ applied to the birds. In experiment 3, injections were saline, metoprolol (24 nmol), N/OFQ (16 nmol) and metoprolol + N/OFQ. In experiment 4, the birds received ICV injection of saline, ICI 118,551 (5 nmol), (C) N/OFQ (16 nmol) and co-administration of ICI 118,551 + N/OFQ. In experiment 5, chicken injected with saline, SR59230R (20 nmol), N/OFQ (16 nmol) and SR59230R + N/OFQ. Then, cumulative food intake was recorded until 120 min after injection. According to the results, ICV injection of N/OFQ significantly increased food intake (P < 0.001). The effect of N/OFQ significantly amplified by co-injection of N/OFQ + β2 adrenergic receptor antagonist (P < 0.001). Also, administration of β1 or β3 adrenergic receptor antagonist had no effect on N/OFQ-induced hyperphagia (P > 0.05). These results suggest that the effect of N/OFQ on cumulative food intake is mediated via β2 adrenergic receptors in neonatal chicken.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For many years a dual-center hypothesis served to explain the neural regulation of food intake in animals (Denbow 1994). Several studies suggest many aspects of food intake control in chickens appear similar to that in mammals but there are some responses that are anomalous in chicks. In particular, domestic fowl differ from mammals in the stimulatory mechanisms for feeding (Bungo et al. 2005). Feeding behavior is a complex physiologic phenomenon which interacts via several neurotransmitters by a wide distributed neurological network on food intake regulation (D’Addario et al. 2014).
Norepinephrine (NE) is a well-known catecholamine which acts as a neurotransmitter in the central nervous system (CNS) (Tachibana et al. 2009). NE usually interacts with α and β adrenergic receptors. They are divided into two major types of α adrenergic receptors: α1 and α2 and three types of β adrenergic receptors, β1, β2 and β3 (Lei 2014). There is evidence that the brain adrenergic system plays an important role in the regulation of food intake in mammals and avian (Bungo et al. 1999). In the domestic fowl, ICV injection of NE into the paraventricular nucleus of the hypothalamus (PVN) stimulated food intake in chickens (Denbow and Sheppard 1993). This response is mediated by α2 receptors where the central administration of NE or clonidine (α2 receptor agonist) induced feeding and this effect was suppressed by yohimbine (α2 receptor antagonist) not by prazosin (α1 receptor antagonist) (Wellman et al. 1993). Controversial reports exist on role of α-adrenergic receptors on feeding behavior in avian. ICV injection of clonidine stimulated food intake in meat-type chicks (broiler) (Bungo et al. 1999). Also, Denbow et al. (1981) reported ICV administration of NE had no effect on food consumption in broiler or layer-type chicken (layers). Effects of β adrenergic receptors on body metabolism have been detected, and a mediatory effect of β adrenergic on appetite is possible. ICV injection of isoproterenol (nonselective β adrenergic receptor agonist) reduces food intake in rats (Wellman 1992) where injection of β3 adrenergic receptor agonist showed an anorexigenic effect (Tsujii and Bray 1998). ICV injection of salbutamol (β2 adrenergic receptor agonist) decreases food intake in rats (Kanzler et al. 2011). Avian reports revealed ICV injection of isoproterenol (β1 and β2 adrenergic receptor agonist) decreased food and water intake in broilers, respectively (Baghbanzadeh et al. 2010).
Nociceptin/orphanin FQ (N/OFQ) is a 17 amino acid neuropeptide that binds to NOP receptor, the fourth member of the opioid receptor family (Alt et al. 2012). The endogenous ligand N/OFQ binds to the opioid receptors (μ, δ, and к) with a very low affinity (Alt et al. 2012). The N/OFQ implicated in a variety of physiological functions, including stress vulnerability, anxiety, locomotion and memory (Roozendaal et al. 2007). N/OFQ has an important role in appetite regulation where intracerebroventricular (ICV) administration of N/OFQ increases food intake in rats (Olszewski et al. 2010) and birds (Zendehdel et al. 2013, 2015). This effect reported by injection of N/OFQ into the nucleus accumbens (NAcc), ventromedial hypothalamus, paraventricular nucleus, arcuate nucleus (ARC) and the nucleus of the solitary tract (Bungo et al. 2009).
Limited information exists for interaction of N/OFQ and adrenergic system. It is reported N/OFQ inhibits noradrenaline release in the mouse cortex via NOP receptors (Werthwein et al. 1999). Presynaptic N/OFQ receptors modulate noradrenaline release in the rat neocortex (Marti et al. 2003). The N/OFQ is able to inhibit the noradrenaline release from the dorsal raphe and locus coeruleus (Gavioli and Calo 2013). The information emerging from the biochemical, biophysical and pharmacological studies demonstrated adrenergic system and N/OFQ play key role in feeding behavior but there is no information for their interaction. Therefore, these mechanisms are important for newly hatched chicks, which must recognize and ingest food voluntarily to survive (Alizadeh et al. 2015). There is no report on the interaction of adrenergic system on N/OFQ-induced feeding behavior. So, the aim of this present series of experiments was to establish the role of adrenergic α1, α2, β1, β2 and β3 receptors on N/OFQ-induced food intake in 3 hour food deprived (FD3) broiler chicken.
Materials and Methods
Animals
A total of 240 one-day-old broiler chicken (Ross 308) purchased from a local hatchery (Eshragh Co. Iran). At 2 days of age birds were randomly transferred to individual cages and kept at a temperature of 30 ± 1 °C with 50 ± 2 % humidity (Olanrewaju et al. 2006). Birds housed according to a completely randomized design. During the study birds had ad libitum access to fresh water and a starter diet containing 21 % crude protein and 2850 kcal/kg of metabolizable energy (Animal Science Research Institute Co., Iran). Three hours prior to the injections, birds were food deprived (FD3) but had free access to water. Animal handling and experimental procedures performed according to the Guide for the Care and Use of Laboratory animals by the National Institutes of Health (USA) and the current laws of the Iranian government for animal care.
Experimental Drugs
The N/OFQ, prazosin (α1 receptors antagonist), yohimbine (α2 receptors antagonist), metoprolol (β1 adrenergic receptors antagonist), ICI 118,551 (β2 adrenergic receptor antagonist), SR 59230R (β3 adrenergic receptor antagonist) and Evans blue purchased from Sigma Co. (Sigma, USA). Drugs at first dissolved in absolute dimethyl sulfoxide (DMSO) then diluted with 0.85 % saline containing Evans blue at a ratio of 1/250. DMSO with this ratio does not have a cytotoxic effect (Blevins et al. 2002; Qi et al. 2008).
Intracerebroventricular Injection Procedures
Intracerebroventricular (ICV) injections conducted at five days of age. A total of five experiments designed to investigate the interconnection of adrenergic and N/OFQ systems on food intake in neonatal birds. Each experiment included four treatment groups with 12 replicates per group (n = 48 chickens per experiment). In each experiment, chicks weighed and allocated into experimental groups based on their body weight so that the average weight (45 ± 5 g) between treatment groups was as uniform as possible. Each chicken received ICV injected once in each experiment. Injections were done using a microsyringe (Hamilton, Switzerland) without anesthesia based on the method described by Davis et al. (1979) and Furuse et al. (1997). In this technique, the head of the chick was held with an acrylic device in which the bill holder was at 45° and the calvarium was parallel to the surface of table (Van Tienhoven and Juhasz 1962). An orifice was made in a plate that was located over the skull immediately over the right lateral ventricle. The microsyringe was inserted into the ventricle through the orifice in the plate. The tip of the needle perforated only 4 mm below the skin of the skull (Jonaidi and Noori 2012). There is no physiological stress using this technique in neonatal chicken (Saito et al. 2005). Injections were done in a volume of 10 μL (Furuse et al. 1999). The control group received control solution as 10 μL of saline containing Evan’s blue (Furuse et al. 1999). At the end of the experiments, to recognize the accuracy of injection, chicken were killed by decapitation. Accuracy of placement of the injection in the ventricle was verified by the presence of Evans blue followed by slicing the frozen brain tissue. Only data from individuals in which dye was present in their lateral ventricle were used for analysis. All experimental procedures were done from 8:00 a.m. until 3:30 p.m. Also, the time course of food consumption was selected by previous studies (Jonaidi and Noori 2012; Zendehdel and Hassanpour 2014; Hassanpour et al. 2015; Zendehdel et al. 2016).
Food Intake Measurement Procedure
In this study, five experiments designed, each with four treatment groups (A–D) (n = 48). In experiment 1, FD3 chickens were ICV injected with (A) saline, (B) prazosin (10 nmol), (C) N/OFQ (16 nmol) and (D) co-administration of prazosin + N/OFQ. In experiment 2, ICV injection of (A) saline, (B) yohimbine (13 nmol), (C) N/OFQ (16 nmol) and (D) co-injection of yohimbine + N/OFQ were applied to the birds. In experiment 3, ICV injections were (A) saline, (B) metoprolol (24 nmol), (C) N/OFQ (16 nmol) and (D) metoprolol + N/OFQ. In experiment 4, the birds received an ICV injection of (A) saline, (B) ICI 118,551 (5 nmol), (C) N/OFQ (16 nmol) and (D) co-administration of ICI 118,551 + N/OFQ. In experiment 5, chickens were ICV injected with (A) saline, (B) SR 59230R (20 nmol), (C) N/OFQ (16 nmol) and (D) SR 59230R + N/OFQ. To find the possible relationship between these two systems, effective and sub-effective doses of pharmacologic agents administered to confront nullifying effects of the agents. In other words, when an effective dose of a system administered, the sub-effective dose of the other system considered. Immediately after injection, chickens were returned to their individual cages and provided with access to pre-weighed food ad libitum and water. Cumulative food intake (g) was measured at 30, 60 and 120 min after the injection. Food consumption was calculated as a percentage of body weight (%BW) to minimize impact of body weight on the amount of food intake. Each bird just used once in each experimental group. These doses of antagonists and N/OFQ were calculated based on previous and pilot studies (Bungo et al. 1999; Abbasnejad et al. 2005; Tachibana et al. 2009; Zendehdel and Hassanpour 2014; Zendehdel et al. 2013, 2015).
Statistical Analysis
Data is presented as mean ± standard error of the mean (SEM). Cumulative food intake (as percent of body weight) analyzed by repeated measure two-way analysis of variance (ANOVA) using SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA). For treatment showing a main effect by ANOVA, means compared by Tukey–Kramer test. P < 0.05 was considered as significant differences between treatments.
Results
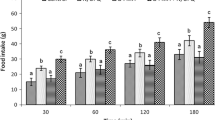
Effects of central adrenergic system on N/OFQ-induced hyperphagia in FD3 neonatal broiler chicks presented in Figs. 1, 2, 3, 4 and 5. In experiment 1, ICV injection of sub effective dose of prazosin (10 nmol) had no significant effect on food intake compared to control group in FD3 neonatal broiler (P > 0.05). The ICV injection of an effective dose of N/OFQ (16 nmol) significantly increased food consumption at 30, 60 and 120 min post-injection (P < 0.001). Co-injection of prazosin (10 nmol) and N/OFQ (16 nmol) had no effect on N/OFQ-induced hyperphagia at 30, 60 and 120 min post-injection (P > 0.05).
Effect of ICV injection of prazosin (10 nmol), N/OFQ (16 nmol) and their combination on percent of body weight cumulative food intake in neonatal layer type chicken (n = 48). prazosin: α1 receptor antagonist, N/OFQ: nociceptin/orphanin FQ. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,18) = 41.29, P < 0.001; prazosin, F(l,64) = 0.01, P > 0.05; N/OFQ, F(l,64) = 71.25, P < 0.001; prazosin × N/OFQ interaction, F(l,64) = 0.42, P > 0.05. Asterisk indicates significant difference with saline group (P < 0.001)
Effect of ICV injection of yohimbine (13 nmol), N/OFQ (16 nmol) and their combination on percent of body weight cumulative food intake in neonatal layer type chicken (n = 48). yohimbine: α2 receptor antagonist, N/OFQ: nociceptin/orphanin FQ. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,94) = 60.18, P < 0.001; yohimbine, F(l,43) = 0.08, P > 0.05; N/OFQ, F(l,43) = 53.71, P < 0.001; yohimbine × N/OFQ interaction, F(l,43) = 1.06, P > 0.05. Asterisk indicates significant difference with saline group (P < 0.001)
Effect of ICV injection of metoprolol (24 nmol), N/OFQ (16 nmol) and their combination on percent of body weight cumulative food intake in neonatal layer type chicken (n = 48). metoprolol: β1 adrenergic receptor antagonist, N/OFQ: nociceptin/orphanin FQ. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,13) = 114.37, P < 0.001; metoprolol, F(l,46) = 1.34, P > 0.05; N/OFQ, F(l,46) = 113.01, P < 0.001; metoprolol × N/OFQ interaction, F(l,46) = 0.17, P > 0.05. Asterisk indicates significant difference with saline group (P < 0.001)
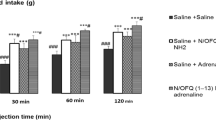
Effect of ICV injection of ICI 118,551 (5 nmol), N/OFQ (16 nmol) and their combination on percent of body weight cumulative food intake in neonatal layer type chicken (n = 48). ICI 118,551: β2 adrenergic receptor antagonist, N/OFQ: nociceptin/orphanin FQ. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,55) = 154.39, P < 0.001; ICI 118,551, F(l,75) = 0.05, P > 0.05; N/OFQ, F(l,75) = 51.97, P < 0.001; ICI 118,551 × N/OFQ interaction, F(l,75) = 40.16, P < 0.001. Asterisk indicates significant difference with saline group (P < 0.001). Double asterisk indicates significant difference with N/OFQ group (P < 0.001)
Effect of ICV injection of SR 59230R (20 nmol), N/OFQ (16 nmol) and their combination on percent of body weight cumulative food intake in neonatal layer type chicken (n = 48). SR 59230R: β3 adrenergic receptor antagonist, N/OFQ: nociceptin/orphanin FQ. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,35) = 127.61, P < 0.001; SR 59230R, F(l,42) = 1.37, P > 0.05; N/OFQ, F(l,42) = 86.11, P < 0.001; SR 59230R × N/OFQ interaction, F(l,42) = 0.19, P > 0.05. Asterisk indicates significant difference with saline group (P < 0.001)
In experiment 2, ICV administration of sub effective dose of yohimbine (α2 receptor antagonist, 13 nmol) had no effect on cumulative food consumption in FD3 neonatal broiler (P > 0.05). ICV injection of 16 nmol of N/OFQ had a hyperphagic effect at 30, 60 and 120 min post-injection (P < 0.001). However, there was no significant effect on N/OFQ-induced feeding behavior by combine injection of yohimbine + N/OFQ (P > 0.05).
In experiment 3, ICV injection of sub effective dose of β1 adrenergic receptor antagonist, metoprolol (24 nmol) had no effect on food consumption in comparison to control group (P > 0.05). ICV injection of 16 nmol N/OFQ induced hyperphagia (P < 0.001); and this effect was not altered by co-injection of metoprolol (24 nmol) and N/OFQ (16 nmol) at 30, 60 and 120 min post-injection (P > 0.05).
In experiment 4, ICV injection of ICI 118,551 (β2 adrenergic receptor antagonist, 5 nmol) had no significant effect on feeding behavior in FD3 neonatal broiler chicks compared to control group (P > 0.05). The ICV injection of N/OFQ (16 nmol) significantly increased food consumption in chickens (P < 0.001). Also, the hyperphagic effect of N/OFQ significantly amplified by co-injection of ICI 118,551 + N/OFQ at 30, 60 and 120 min post-injection (P < 0.001).
In experiment 5, ICV injection of β3 adrenergic receptor antagonist (SR 59230R) at dose of 20 nmol, had no effect on food intake in FD3 neonatal broiler compared to control group (P > 0.05). ICV injection of 16 nmol N/OFQ significantly increased food intake in neonatal chickens (P < 0.001). Co-injection of SR 59230R and N/OFQ was not able to fluctuate N/OFQ-induced hyperphagia at 30, 60 and 120 min post-injection (P > 0.05).
Discussion
Based on the literature this paper is the first report on relationship between N/OFQ and adrenergic system on food intake in FD3 neonatal broiler chickens. According to the data N/OFQ had a hyperphagic effect (Figs. 1, 2, 3, 4, 5) which was in agreement with previous reports in rats (Economidou et al. 2006; Olszewski et al. 2010) and meat-type chickens (Abbasnejad et al. 2005; Tajalli et al. 2006; Bungo et al. 2009). The distribution of adrenergic receptors differs in different parts of the avian brain. It is known that α2 adrenergic receptors increase food intake by poultry (Baghbanzadeh et al. 2010) while β adrenergic receptor agonists decreased food intake by rats (Wellman et al. 1993; Tsujii and Bray 1998) and poultry (Zendehdel and Hassanpour 2014).
In this study, there was no relationship between α adrenergic receptors and N/OFQ on food intake regulation. In poultry, injection of NE into the ventromedial nucleus, paraventricular nucleus, and medial septal sites stimulated food intake while inhibited when injected near the lateral septal organ and the anterior portion of both the nucleus reticularis superior and pars dorsalis (Denbow 1999). The ICV injection of clonidine (α2 receptor agonist) simulated ingestion by post-synaptic α2 receptor in the layer-type chicken (Tachibana et al. 2009). The arcuate nucleus (ARC) is accessible to circulating signals of energy balance via the underlying median eminence, as this is not protected by the blood–brain-barrier (Chen et al. 2006). There are two primary neurons within the ARC which integrate signals of nutritional status and influence energy homeostasis: pro-opiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART) which inhibits food intake while neuropeptide Y (NPY) and agouti related protein (AgRP) stimulates food consumption (D’Addario et al. 2014). The NE excites approximately half of the neurons in the ARC, probably because of a direct postsynaptic response via α1 or β receptors. Peripherally administered ghrelin may activate NPY/AgRP neurons in the ARC through the noradrenalin system (Date et al. 2006). It is revealed orexigenic effect of α adrenergic receptors mediates via NPY in chicks (Tachibana et al. 2009). In mammals, orexigenic effects of NPY at least partially mediated by brain α adrenergic receptors. It is therefore likely there are similar orexigenic mechanisms related to NPY and α adrenergic receptors in chicks and mammals (Tachibana et al. 2009). In this study, we used sub-effective dose of α adrenergic receptors antagonists, which blocks receptor without effect on food intake to assay possible interaction(s) with N/OFQ. However, no interconnection detected for α1 and α2 adrenergic receptors with N/OFQ.
As seen in this study, blockade of β2 adrenergic receptors with sub effective dose of ICI 118,551, amplified N/OFQ-induced hyperphagia in FD3 neonatal broiler. In the chicken, β1 and β3 receptor antagonists had no effect on food intake (Zendehdel and Hassanpour 2014). It is reported, ICV injection of propranolol (a nonselective β receptor antagonist) alone in the third ventricle increase in food intake by a tonic inhibitory influence of the β1 or β2 adrenergic receptors in rat (Tsujii and Bray 1998). In all rodent species studied, N/OFQ inhibits release of NE. it is revealed the NOP1 receptors involved in preventing of NE (Siniscalchi et al. 2002). There is evidence that food intake regulation mechanisms are different among mammals and birds. For example, ghrelin known as an orexigenic neurotransmitter in mammals but has anorexigenic effect in avian (Zendehdel and Hassanpour 2014). So far, numerous researches done to the determine effect of neurotransmitters on feeding behavior in mammals, but aspects of food intake regulation in avian still unclear (Hassanpour et al. 2015). Given the estimated 300 million years of evolutionary distance between mammals and avian, it is not surprising that significant differences have been found in the activities of a number of components involved in the regulation of energy homeostasis between mammals and birds(Alizadeh et al. 2015).
Based on the literature, the ARC is the site where N/OFQ exerts most potent orexigenic effect (Abbasnejad et al. 2005). N/OFQ elicits hyperphagic role in the ARC neurons by activation of inward K+ currents. N/OFQ may therefore influence CART’s effect on food intake by attenuating its release from ARC neurons via the NOP receptor (Barnes et al. 2006). The CART and AgRP neurons in the ARC express NOP receptors (Bewick et al. 2005). N/OFQ via NOP receptors decreased and increased the release of CART and AgRP, respectively. So, the ARC is the main site of action for the hyperphagic effect of N/OFQ (Polidori et al. 2000). It is reported interconnection exist between the α2 and NOP at the level of the receptors themselves or at a step behind the receptors via G-protein-coupled receptors (GPCRs) or ion channels (Werthwein et al. 1999). However, no relationship found between α adrenergic receptors and N/OFQ on food intake regulation, but blockade of β2 adrenergic receptors with sub effective dose amplified N/OFQ-induced hyperphagia in FD3 neonatal broiler chickens. In a sole study, Roozendaal et al. (2007) reported β1 adrenergic receptors antagonist atenolol (2.0 nmol) administered concurrently into the basolateral complex of the amygdala potentiated the dose–response effects of OFQ/N, but, there is no report for β2 adrenergic receptors (Roozendaal et al. 2007). We think this can be one of the differences between avian and mammalian for central neurotransmitters activity.
Recently a new mechanism reported corticotropin-releasing factor (CRF) facilitates the memory-modulatory effects of noradrenergic via β adrenergic receptors (Roozendaal et al. 2008). The NE effects on memory consolidation involve activation of both postsynaptic α1 and β adrenergic receptors. The β adrenergic receptors coupled to adenylate cyclase to stimulate the cAMP signaling pathway while α1 receptors activation indirectly modulates the β receptors (Roozendaal et al. 2008). CRF and its receptors, play crucial effects on energy intake, body weight regulation and known as a hypophagic neurotransmitter (Zendehdel et al. 2016). N/OFQ inhibited stress-induced anorexia by blockade of hypothalamic CRF2 receptors (Ciccocioppo et al. 2004). The CRF, β adrenergic receptors and N/OFQ belong to GPCRs. Because CRF receptors like the β adrenergic receptors influence adenylate cyclase activity and cAMP accumulation, presumably activation of β adrenergic receptors increases CRF levels in the brain (Roozendaal et al. 2008). On the other hand, microinjection of N/OFQ into the ventromedial hypothalamus increase food intake by blockade of CRF. The mechanisms underlying how N/OFQ interacts with CRF is still unknown (Ciccocioppo et al. 2004). Based on new findings of the current study, a relationship exists between N/OFQ and β adrenergic receptors via CRF on central food intake regulation in broiler chicken. However, because of limitation of the current study, we were not able to determine CRF level following co-injection of N/OFQ and β1 or β2 adrenergic receptor antagonists. Based on our knowledge, there was no report on molecular and cellular interaction between β adrenergic receptors and N/OFQ. So, we think further researches needed to determine molecular pathway(s) for the relationship. Importantly, very little information is present on this topic even in mammals. Presumably, obtained results can use for the first time as base information and further studies are necessary to investigate the possible involvement of N/OFQ and adrenergic system with other neurotransmitters on feeding regulation.
References
Abbasnejad M, Jonaidi H, Denbow DM, Pour Rahimi AM (2005) Feeding and locomotion responses to centrally injected nociceptin/orphanin FQ in chicks. Physiol Behav 85:383–386
Alizadeh A, Zendehdel M, Babapour V, Charkhkar S, Hassanpour S (2015) Role of cannabinoidergic system on food intake in neonatal layer-type chicken. Vet Res Commun 39:151–157
Alt C, Lam JS, Harrison MT, Kershaw KM, Samuelsson S, Toll L, D’Andrea A (2012) Nociceptin/orphanin FQ inhibition with SB612111 ameliorates dextran sodium sulfate-induced colitis. Eur J Pharmacol 683:285–293
Baghbanzadeh A, Hajinezhad MR, Shohreh B, Maleklou R (2010) Intralateral hypothalamic area injection of isoproterenol and propranolol affects food and water intake in broilers. J Comp Physiol A 196:221–226
Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA (2006) Increased expression of mu opioid receptors in animals susceptible to dietinduced obesity. Peptides 27:3292–3298
Bewick GA, Dhillo WS, Darch SJ, Murphy KG, Gardiner JV, Jethwa PH et al (2005) Hypothalamic cocaine- and amphetamine-regulated transcript (CART) and agouti-related protein (AgRP) neurons coexpress the NOP1 receptor and nociceptin alters CART and AgRP release. Endocrinology 146(8):3526–3534
Blevins JE, Stanley BG, Reidelberger RD (2002) DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav 71:277–282
Bungo T, Dodo K, Kawamura K, Izumi T, Ueda H (2005) Effects of various µ- and δ-opioid ligands on food intake in the meat-type chick. Physiol Behav 85:519–523
Bungo T, Shimojo M, Masuda Y, Choi YH, Denbow DM, Furuse M (1999) Induction of food intake by a noradrenergic system using clonidine and fusaric acid in the neonatal chick. Brain Res 826:313–316
Bungo T, Shiraishi J, Yanagita K, Ohta Y, Fujita M (2009) Effect of nociceptin/orphanin FQ on feeding behavior and hypothalamic neuropeptide expression in layer-type chicks. Gen Comp Endocrinol 163:47–51
Chen RZ, Frassetto A, Fong TM (2006) Effects of the CB1 cannabinoid receptor inverse agonist AM251 on food intake and body weight in mice lacking l-opioid receptors. Brain Res 1108:176–178
Ciccocioppo R, Cippitelli A, Economidou D, Fedeli A, Massi M (2004) Nociceptin/orphanin FQ acts as a functional antagonist of corticotropin-releasing factor to inhibit its anorectic effect. Physiol Behav 82:63–68
D’Addario C, Micioni Di Bonaventura MV, Puccia M, Romano A, Gaetani S, Ciccocioppo R, Cifani C, Maccarrone M (2014) Endocannabinoid signaling and food addiction. Neurosci Biobehav Rev 47:203–224
Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M (2006) Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab 4:323–331
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of l-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Denbow DM (1994) Peripheral regulation of food intake in poultry. J Nutr 124:1349S–1354S
Denbow DM (1999) Food intake regulation in birds. J Exp Zool 283:333–338
Denbow DM, Cherry JA, Siegel PB, Van Kery HP (1981) Eating, drinking and temperature response of chicks to brain catecholamine injections. Physiol Behav 27:265–269
Denbow DM, Sheppard BJ (1993) Food and water intake responses of the domestic fowl to norepinephrine infusion at circumscribed neural sites. Brain Res Bull 31:121–128
Economidou D, Policani F, Angellotti T, Massi M, Terada T, Ciccocioppo R (2006) Effect of novel NOP receptor ligands on food intake in rats. Peptides 27:775–783
Furuse M, Ando R, Bungo T, Ao R, ShimoJO M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 40:698–700
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Gavioli EC, Calo G (2013) Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharmacol Ther 140(1):10–25
Hassanpour S, Zendehdel M, Babapour V, Charkhkar S (2015) Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. Br Poult Sci 56(4):443–451
Jonaidi H, Noori Z (2012) Neuropeptide Y-induced feeding is dependent on GABAA receptors in neonatal chicks. J Comp Physiol A 198:827–832
Kanzler SA, Januario AC, Paschoalini MA (2011) Involvement of β3 -adrenergic receptors in the control of food intake in rats. Braz J Med Biol Res 44(11):1141–1147
Lei S (2014) Cross interaction of dopaminergic and adrenergicsystems in neural modulation. Int J Physiol Pathophysiol Pharmacol 6(3):137–142
Marti M, Stocchi S, Paganini F, Mela F, Risi CD, Calo G, Guerrini R, BArnes TA, LAmbert DG, Beani L, Bianchi C, Morari M (2003) Pharmacological profiles of presynaptic nociceptin/orphanin FQ receptors modulating 5-hydroxytryptamine and noradrenaline release in the rat neocortex. Br J Pharmacol 138:91–98
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J Poult Sci 5(4):301–308
Olszewski PK, Grace MK, Fard SS, Grevès ML, Klockars A, Massi M, Schiöth HB, Levine AS (2010) Central nociceptin/orphanin FQ system elevates food consumption by both increasing energy intake and reducing aversive responsiveness. Am J Physiol Regul Integr Comp Physiol 299:R655–R663
Polidori C, de Caro G, Massi M (2000) The hyperphagic effect of nociceptin/orphanin FQ in rats. Peptides 21:1051–1062
Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res 236:52–60. doi:10.1016/j.heares.2007.12.002
Roozendaal B, Lengvilas R, McGaugh JL, Civelli O, Reinscheid RK (2007) Orphanin FQ/nociceptin interacts with the basolateral amygdala noradrenergic system in memory consolidation. Learn Mem 14:29–35
Roozendaal B, Schelling G, McGaugh JL (2008) Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the β-adrenoceptor–cAMP pathway: dependence on glucocorticoid receptor activation. J Neurosci 28(26):6642–6651
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Siniscalchi A, Rodi D, Morari M, Marti M, Cavallini S, Marino S, Beani L, Bianchi C (2002) Direct and indirect inhibition by nociceptin/orphanin FQ on noradrenaline release from rodent cerebral cortex in vitro. Br J Pharmacol 136:1178–1184
Tachibana T, Sugahara K, Ueda H, Cline MA (2009) Role of adrenergic alpha-2-receptors on feeding behavior in layer-type chicks. Gen Comp Endocrinol 161:407–411
Tajalli S, Jonaidi H, Abbasnejad M, Denbow DM (2006) Interaction between nociceptin/orphanin FQ (N/OFQ) and GABA in response to feeding. Physiol Behav 89:410–413
Tsujii S, Bray GA (1998) A beta-3 adrenergic agonist (BRL-37,344) decreases food intake. Physiol Behav 63:723–728
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Wellman PJ (1992) Overview of adrenergic anorectic agents. Am J Clin Nutr 55(1):193S–198S
Wellman PJ, Davis BT, Morien A, McMahon L (1993) Modulation of feeding by hypothalamic paraventricular nucleus α1- and α2-adrenergic receptors. Life Sci 53:669–679
Werthwein S, Bauer U, Nakazi M, Kathmann M, Schlicker E (1999) Further characterization of the ORL1 receptor-mediated inhibition of noradrenaline release in the mouse brain in vitro. Br J Pharmacol 127:300–308
Zendehdel M, Ghashghayi E, Hassanpour S, Baghbanzadeh A, Jonaidi H (2016) Interaction between opioidergic and dopaminergic systems on food intake in neonatal layer type chicken. Int J Pept Res Ther 22:83–92
Zendehdel M, Hamidi F, Hassanpour S (2015) The effect of histaminergic system on nociceptin/orphanin FQ induced food intake in chicken. Int J Pept Res Ther 21:179–186
Zendehdel M, Hassanpour S (2014) Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci 64:383–391
Zendehdel M, Mokhtarpouriani K, Babapour V, Baghbanzadeh A, Pourrahimi M, Hassanpour S (2013) The effect of serotonergic system on nociceptin/orphanin FQ induced food intake in chicken. J Physiol Sci 63:271–277
Acknowledgments
This research was supported by a grant from the Research Council of the Faculty of Veterinary Medicine, University of Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Morteza Zendehdel, Zahra Parvizi, Shahin Hassanpour, Ali Baghbanzadeh and Farshid Hamidi declare that they have no conflict of interest.
Informed Consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Human and Animal Rights
All experiments executed according to the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Ethics Committee.
Rights and permissions
About this article
Cite this article
Zendehdel, M., Parvizi, Z., Hassanpour, S. et al. Interaction Between Nociceptin/Orphanin FQ and Adrenergic System on Food Intake in Neonatal Chicken. Int J Pept Res Ther 23, 155–161 (2017). https://doi.org/10.1007/s10989-016-9548-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-016-9548-2