Abstract
Context
According to the trophic-rank hypothesis, species may be differentially affected by habitat isolation due to their trophic position in the food chain, i.e. high-order trophic levels may be more negatively affected than low-order levels.
Objectives
The aim of this paper is to study how species richness, abundance and composition of saproxylic beetle communities are affected by patch (=tree) quality and small-scale patch connectivity. Following the trophic-rank hypothesis, we expected predators to be more negatively affected by patch isolation than wood-feeding beetles.
Methods
We studied the beetle community, patch connectivity and patch quality on 28 large oaks. Different connectivity measures were calculated using 50 m-buffers around trees and using distances to the five nearest trees.
Results
Beetle species richness increased with the diameter of oaks, i.e. patch quality. No evidence of the trophic-rank hypothesis was found for species richness patterns. In accordance with the trophic-rank hypothesis, abundance of predatory beetles increased with patch connectivity but lower trophic levels were unaffected or even decreased with patch connectivity.
Conclusions
The structure of invertebrate communities on trees changes with small-scale patch connectivity due to a differential response of low-order and high-order trophic levels. Isolated trees are more exposed to the sun than the more connected trees, which may affect the beetles; however, it was impossible to distinguish the microclimatic from the spatial effects. Although scattered trees generally have a higher conservation value than trees in forests, we conclude that forest trees may be more important for certain trophic levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quality and spatial distribution of habitats are among the basic determinants of species occurrence at different spatial scales and can have a strong impact on community composition and species richness (Saunders et al. 1991; Steffan-Dewenter and Tscharntke 2000; Hunter 2002; Tscharntke and Brandl 2004; Ewers and Didham 2006; Bailey et al. 2010). Habitat fragmentation leads to increasing isolation of the remaining habitat patches, and this has the potential to affect the structure of ecological communities. According to the trophic-rank hypothesis, the sensitivity to habitat loss and isolation increases with trophic level, meaning that the strongest fragmentation effects are on predators, followed by herbivores and then plants (Holt et al. 1999; Davies et al. 2000; Cagnolo et al. 2009; Herrmann et al. 2012). Predators may be more vulnerable to habitat loss and isolation because their area requirements are larger than those of herbivores, e.g. due to declining population size with increasing trophic rank (Holt et al. 1999), and because they suffer from both direct fragmentation effects and reduced prey availability in fragmented habitats (Holt 2002). In accordance with the trophic-rank hypothesis, declines in the abundance of predominantly predatory birds and wasps have been observed with habitat isolation, while herbivorous beetles were unaffected in the same study area (Herrmann et al. 2012). However, a wide range of responses to habitat isolation has been observed, and changes in abiotic conditions with habitat isolation may play an additional role (Ewers and Didham 2006; Brouwers and Newton 2009). Empirical evidence for the trophic-rank hypothesis is still scarce, although trophic interactions may help to better understand complex ecological systems (Gravel et al. 2011).
Thus, more studies on the effects of habitat isolation on different trophic levels are needed, particularly for species-rich groups where many species, such as saproxylic organisms, are negatively affected by habitat fragmentation (Komonen et al. 2000; Ranius et al. 2010; Buse 2012).
Saproxylic (=dead wood-dependent) beetles represent a species-rich and functionally important group of organisms in forest ecosystems. Many saproxylic beetles are specialised in terms of habitat quality (decomposition stage, diameter, fungal colonisation etc.) and in terms of trophic position within the food chain (Palm 1959; Köhler 2000; Möller 2009). The connectivity of suitable habitat affects the incidence of saproxylic beetles and fungi on both a landscape and a within-forest scale (Schiegg 2000; Laaksonen et al. 2008; Ranius et al. 2010). The scale of response of oak-specialist beetles to surrounding habitat has been found to vary from hundreds of metres to several kilometres (Bergman et al. 2012; Ranius et al. 2010). According to the trophic-rank hypothesis we would expect predators within the saproxylic beetle community to be more negatively affected by isolation of oaks than primary consumers.
In Europe, a species-rich fauna and flora is associated with semi-open landscapes with scattered large oaks, which often have a large girth and cavities that provide habitat for a species-rich invertebrate fauna. Landscapes with scattered large trees are of high conservation value, but are globally declining due to intensified agricultural management or abandonment of traditional uses (Ranius and Jansson 2000; Manning et al. 2006; Gibbons et al. 2008; Fischer et al. 2010). Both patch (=tree) quality and connectivity (=tree isolation) might affect the species richness and also the trophic structure of saproxylic beetle communities in scattered tree landscapes. Previous studies have revealed a positive effect of tree diameter and sun exposure on the number of saproxylic insect species and threatened species (Ranius and Jansson 2000; Buse et al. 2007; Horak et al. 2012; Koch Widerberg et al. 2012; Vodka and Cizek 2013).
The aim of this paper is to study how species richness, abundance and composition of saproxylic beetle communities are affected by patch quality and small-scale patch connectivity (where the measure of patch connectivity may also reflect increasing shade by neighbouring trees). This was done by comparing the catches made using flight-interception traps placed in the lower canopies of oak trees. We test the hypothesis that high-order trophic levels (here predators) in the saproxylic beetle community on oaks are more negatively affected by isolation of the oaks than low-order trophic levels (here wood-feeders). In addition to patch quality, connectivity is expected to explain variation in species richness and abundance between oaks. We expected
-
(1)
Species richness and abundance to increase with patch quality and connectivity.
-
(2)
Predatory species to benefit more strongly from patch connectivity than wood-feeding species, in line with the trophic-rank hypothesis.
-
(3)
Threatened species to be more strongly affected by patch quality and small-scale patch connectivity than non-threatened species because they are often specialists and poor dispersers (Kotiaho et al. 2005).
Methods
Study area
The study area is located in the “Gartower Elbmarsch” (53°1′N, 11°27′E) in Germany, a landscape characterised by pastures and meadows interspersed with groups of scattered trees and woodlands (Fig. 1). At least some of the woodlands are occasionally flooded by the nearby River Elbe and are used for non-intensive forestry. We also studied trees in the ancient “Elbholz” woodland, one of the last remaining semi-natural floodplain woodlands in Central Europe. Most of the solitary trees studied were in cattle and sheep pastures. There was a two km distance between the two study plots, but they shared the same tree species composition, same age structure and average connectivity, and were both close to the river Elbe. Our study area is part of the MAB (Man-and-Biosphere) reserve “Elbe River landscape” and part of the Natura 2000 protected area “Elbe lowlands between Schnackenburg and Geesthacht” (227 km2).
Aerial picture of the study area in the MAB (Man-and-Biosphere) reserve “River landscape Elbe” (Germany). Large parts of this area are temporarily grazed by cattle. We used two subplots indicated on the map, and the position of traps in one subplot is marked. The position of the study area within Germany is indicated on the small map on the upper right
Insect sampling and guild classification
Two study plots were situated at locations with a sufficient number of isolated oaks. We assessed tree characteristics and the saproxylic beetle fauna on 28 oaks. Trees were selected to obtain a large variation in tree diameter and small-scale connectivity. We used flight interception traps of the same type as Hyvärinen et al. (2006) to survey the flight-active beetle fauna of the oak trees. The traps were covered to protect them from rain. The perpendicular, crossed windows in the traps measured 50 cm × 30 cm. One trap per tree was placed in the lower canopy, 4-5 metres above ground, from April 27 to August 31, 2007. We used a preservation agent consisting of ethanol, glycerine, water and acidic acid, as used by Renner (1980). We emptied the traps fortnightly and identified all captured beetles to species level. In our analyses we only included species that are saproxylic (Speight 1989; Schmidl and Bussler 2004) and possibly develop in oak wood, as described by Palm (1959) and Köhler (2000) (see Appendix S1). The same literature was used to classify the species into eight trophic levels: mycetophagous = consumers of wood fungi; necrophagous = consumers of dead animals; xylophagous = consumers of woody substrate; zoophagous = predators; xylo- and saprophagous = consumers of wood and dead organic material; saprophagous = consumers of dead organic material; xylomycetophagous = consumers of fungi-infested wood; xylo- and zoophagous = consumers of both wood and animals). To test the trophic-rank hypothesis, we focussed on true predators (zoophagous) and true wood-feeders (xylophagous) which represent one high-order and one low-order trophic level in the food chain. The trophic level of the other groups is uncertain since most groups above the herbivore level are probably omnivores in complex terrestrial food webs (Thompson et al. 2007). The red-list classification was taken from the recent national Red List of Germany (Schmidl and Büche in press). Only species listed as critically endangered, endangered, and vulnerable are considered as threatened.
Environmental variables
To study the impact of patch quality and connectivity on the saproxylic beetle community we measured five tree characteristics representing patch quality and calculated six patch connectivity measures (Table 1). For all oaks selected for the study, we measured diameter at breast height (DBH), amount of dead wood in the canopy, occurrence of tree cavities and bare bark, and colonisation by Cerambyx cerdo (Coleoptera, Cerambycidae), because this longhorn beetle may increase species richness on oaks by creating extensive galleries in the wood (Buse et al. 2008). Patch connectivity was measured in two ways: the nearest neighbour distance, and the amount of habitat in a circle surrounding the habitat patch (=buffer) (Tischendorf and Fahrig 2000). There is evidence that nearest neighbour distances are less powerful than buffer connectivity measures (Moilanen and Nieminen 2002). The first three connectivity measures in our study are different kinds of buffer measures, for which we summed the number of trees in a 50 m buffer zone around each studied oak (Ranius et al. 2010). This rather small buffer zone was used because the trees studied were relatively close to one another, although in other studies larger buffer zones have been found to be more appropriate for saproxylic beetles (Bergman et al. 2012; Ranius et al. 2010). This means that our analyses deal with the characteristics of the pastures or woodlands where the oaks are situated, rather than the characteristics of the wider landscape. Connectivity was measured for three different categories of patches representing different landscape structure qualities: all trees, all oaks, and all large-diameter oaks (Table 1).
Statistical analysis
We used generalised linear models (GLM) to analyse total abundance and species richness of saproxylic beetles in relation to patch quality and connectivity variables. We applied the same procedure for species richness and abundance of single trophic levels in the beetle community. GLMs for species richness were performed with the assumption of a Poisson error distribution. For overall abundance and abundance of trophic levels, we used GLMs with a quasi-Poisson error distribution to account for overdispersion. We then searched for the models which best explained species richness of beetles and of threatened species and beetle abundance by comparing models with all possible combinations of explanatory variables. For every response variable, the best-fit models according to Akaike’s Information Criterion for small sample sizes (AICc) were identified using the ‘dredge’ function in R package MuMIn version 1.7.2 (Barton 2012). We accounted for the two study areas by adding a binary variable to the best-fit models, but deleted the binary parameter if there was no significant impact. Top-down control of wood-feeding beetles by predators was tested using abundance data. We compared the final model for abundance of wood-feeding beetles with an alternative model where abundance of predators was added as a predictor. The final and the alternative model were compared by ANOVA using an F-test. The effects of environmental variables on community structure of saproxylic beetles were analysed with nonmetric multidimensional scaling (NMDS) implemented in the R package ‘vegan’ (Oksanen et al. 2011). NMDS ordination was based on the Bray-Curtis distance. Species occurring with less than 10 individuals in the whole dataset were regarded as accidental catches and omitted from the ordination analysis. All environmental variables were fitted onto the ordination and only significant variables (p < 0.05) are presented. Environmental effects on community structure were tested with a PERMANOVA (999 permutations) implemented in the ‘adonis’-function.
Indicator species analysis was done using the IndVal procedure implemented in the R package ‘labdsv’ to find species that responded to oak connectivity (Dufrene and Legendre 1997). IndVal combines a species-relative abundance with its relative frequency of occurrence in different groups. We divided the samples into two groups based on the median of the mean distance to the nearest five large oaks (DIST_largeoaks) (median = 19.6 m) to identify species that were possibly affected by connectivity of large oaks. Only species with more than 10 individuals were used for the calculation of indicator values (58 species).
All statistical analysis was performed in the software R version 2.13.2 (R Core Development Team 2011).
Results
We caught 241 beetle species and 9,066 individuals on the 28 oaks studied. A total of 171 saproxylic beetle species, represented by 8520 individuals, were classified as inhabitants of oaks (see Appendix S1 in supporting information). We found between 29 and 60 saproxylic beetle species and between 147 and 644 individuals per oak (=trap).
Impact of patch quality and connectivity on overall species richness and abundance
Overall species richness of saproxylic beetles was positively influenced by DBH and to a lesser extent by other patch quality and connectivity variables (see Appendix S2). Model selection (AICc) showed that DBH was the strongest and, after multiple comparison of different models, the only significant predictor for overall species richness (see Appendix S3). No measure of patch connectivity remained in the final model for species richness. Model selection (AICc) for overall abundance patterns showed that three predictors were included in the best model (Appendix S3). Overall abundance was positively influenced by high dead wood amount in the canopy (GLM, z = 5.117, p < 0.001) and was negatively influenced by barkless wood on the trunk (GLM, z = −4.388, p < 0.001). Larger distances to other oaks decreased overall abundance (GLM, z = 2.296, p < 0.031). The best-fit model explained 60 % of the variation.
Impact of patch quality and connectivity on species richness of trophic levels
At eight different trophic levels, predators (mean = 42 %, SE = 1.23 of the species per oak) and wood-feeders (mean = 17 %, SE = 0.97) and fungi-feeders (mean = 17 %, SE = 0.67) were dominant in terms of species richness. However, in terms of abundance, fungi-feeders (total 44 % of all individuals) and predators (total 42 %) were the dominant groups. Species richness patterns of trophic levels did not follow the predictions made by the trophic-rank hypothesis. The species richness of predatory and fungi-feeding beetles was not related to any of the variables tested, while the species richness of wood-feeding beetles was positively related to DBH (GLM, z = 2.965, p = 0.003, see Appendix S2). All connectivity measures, except DIST_medoaks, showed significant negative (buffer measures) or positive (distance measures) relationships with the richness of wood-feeding species in univariate GLMs (Appendix S2). AICc-based model selection revealed that DBH was the strongest predictor for the richness of wood-feeding beetles, explaining 23 % of the deviance (see Appendix S2).
Impact of patch quality and connectivity on abundance of trophic levels
In terms of total abundance, predatory beetles were more strongly influenced by oak connectivity than wood-feeding beetles. Wood-feeder abundance was positively related to DBH and was influenced by connectivity variables, but in a different direction compared to predators (Table 2, see Appendix S3). Increasing numbers of trees in a 50 m buffer around each sampled oak led to decreasing numbers of wood-feeding beetle individuals (Table 2; Fig. 2b). In contrast, the abundance of predatory species increased significantly with the number of oaks in a 50 m radius (Table 2; Fig. 2e), while DBH had no effect (Table 2; Fig. 2e, see also Appendix S3). No evidence for top-down control of wood-feeding beetles was found when abundance of predators (predator effect t = −0.721, p = 0.478) was included as a further predictor.
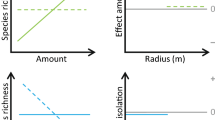
Abundance of wood-feeding (a–c) and predatory (d–f) beetles on 28 oaks and their relation to DBH (a, d) and different patch connectivity measures (b, e = BUF_medoaks; c, f = DIST_tree). Shown are overall species richness (black circles) and richness of threatened beetles (empty circles). Relationships were tested with a GLM (quasi-Poisson errors), and significant relationships are displayed by the regression curve for overall species richness (solid line) and richness of red-listed beetles (dashed line) at the respective trophic level
The alternative model with the predator effect did not perform better than the original final model (F = 0.528, p = 0.475). Model selection revealed that the abundance of beetles feeding on fungi-infested wood was positively related to the number of cavities in the trunk (Table 2, see Appendix S3). Their abundance decreased with increasing number of large oaks in the surrounding area, but also when distances to trees became larger (Table 2). Beetles feeding on wood fungi showed positive relations to the number of dead branches, but no relationship to any of the connectivity variables tested after model selection (Table 2, see Appendix S3).
Community structure
The composition of beetle communities (Fig. 3) correlated significantly with DBH (PERMANOVA, F1,27 = 4.094, p < 0.001), BUF_medoaks (F1,27 = 2.878, p = 0.002) and the number of cavities (F1,27 = 2.131, p = 0.035). Species were mainly segregated by two gradients (Fig. 3). The first gradient represents connectivity of oaks. The second gradient represents an increasing number of hollow oaks with large DBH, and this favoured consumers of dead animals and species of fungi-infested wood (necrophagous and xylomycetophagous). Indicator species analysis showed that seven species had a higher abundance and frequency on oaks with a distance to large oaks of more than 20 m (Table 3). There were two species that had a preference for large oaks located closer than 20 m from other large oaks.
Nonmetrical multidimensional scaling (NMDS) of the saproxylic beetle species based on Bray–Curtis-similarities. Three-dimensional stress was 0.164, but only the first two axes are shown. Species were classified into different feeding groups. Significant variables influencing ordination tested with PERMANOVA are shown as vectors (see Table 1 for explanation of variables)
Threatened species
We identified 31 threatened beetle species listed as vulnerable, endangered or critically endangered in Germany; this represented 18 % of the total number of saproxylic oak-inhabiting species collected. Nine species were listed as near threatened. Overall species richness of threatened beetles was positively influenced by DBH and to a lesser extent also by other patch quality (e.g. number of tree cavities) and connectivity variables (see Appendix S2). There was a positive relationship between species richness of threatened beetles and the number of tree cavities. An increasing number of large oaks in the surroundings decreased richness of threatened beetles. Model selection (AICc) showed that DBH was the strongest and, after multiple comparison of different models, the only significant predictor for richness of threatened species (see Appendix S3). Threatened beetles were more strongly affected by patch quality than non-threatened beetles because the regression coefficient for DBH was larger for threatened (β = 0.520, p < 0.001) than for non-threatened species (β = 0.136, p = 0.071). No measure of patch connectivity remained in the final model for threatened species richness. Model selection (AICc) for abundance of threatened beetles showed that two predictors were included in the best model (Appendix S3), which explained 70 % of the variation. Abundance of threatened beetles was positively influenced by the number of tree hollows and abundance decreased with increasing tree connectivity (BUF_trees).
Discussion
Both patch quality and small-scale connectivity determined abundance, species richness and community structure of oak-inhabiting saproxylic beetles. Overall species richness on the studied large oaks was positively influenced by DBH. Abundance of saproxylic beetles was positively influenced by dead wood in the canopy, vital trunks and by connectivity. Abundance and species richness of wood-feeding species were positively affected by DBH and showed a negative response to connectivity, while predators showed a positive response to patch connectivity and no relation to DBH. We found evidence for the hypothesis that high-order trophic levels such as predators are more negatively affected by isolation of oaks than low-order trophic levels such as wood-feeders (trophic-rank hypothesis). Evidence was found for abundance patterns, but not for species richness patterns.
Strong effects of patch quality on species richness and abundance
In our study, species richness increased with DBH, whereas small-scale connectivity had a significant positive effect on abundance. Our results confirm the well-known positive species richness-DBH relationship of tree-dependent organisms (Ranius and Jansson 2000; Löbel et al. 2006; Ranius et al. 2008; Sverdrup-Thygeson et al. 2010). For species with a colonisation rate limited by the number of dispersal sources in the near vicinity, a connectivity effect would be expected (Johansson et al. 2013). However, in our study, as well as in most other situations, small-scale connectivity of trees was certainly related to habitat openness, which affects microclimate. The latter has been found to affect species richness in many other studies on saproxylic beetles (Müller et al. 2010; Sverdrup-Thygeson et al. 2010; Koch Widerberg et al. 2012) and may also have influenced the outcome of our study. It is thus not possible to distinguish between the impact of connectivity and that of microclimatic conditions on tree-inhabiting beetle communities with our data. Using different experimental spatial arrangements of dead wood pieces might be a possible approach to studying connectivity effects independently of microclimate. Based on our data, we conclude that patch quality for saproxylic beetles on oaks is relevant in terms of DBH, while patch connectivity seems to be of minor importance.
Small-scale connectivity affects trophic structure in beetle communities
We found that in terms of species richness and abundance, wood-feeding beetles responded positively to the isolation of oaks, while abundance of predatory beetles showed the opposite response. Other studies have demonstrated that species richness and abundance of high-order trophic levels decrease at higher fragmentation levels (Davies et al. 2000; Ozanne et al. 2000; Cagnolo et al. 2009), but our study is the first to demonstrate this relationship for the community of saproxylic beetles. Dead-wood dependent beetles have been found to be affected by small-scale habitat fragmentation to different degrees, but this was determined by habitat specificity and not by the trophic level of species (Schiegg 2000). Furthermore, it has been observed that decreasing habitat connectivity may decrease the number of trophic levels in an insect community inhabiting saproxylic fungi (Komonen et al. 2000). According to the trophic-rank hypothesis, low connectivity of habitat patches could lead to the extinction of high-order levels (predators, parasites, parasitoids) probably because of lower population densities in fragmented patches (Holt et al. 1999; Davies et al. 2000; Schuepp et al. 2011; Herrmann et al. 2012). We found no evidence for the extinction of high-order trophic levels. However, predatory saproxylic beetles might be predominantly generalists able to find their prey in different dead wood types; some may also find prey in habitats other than the wood itself, e.g. by visiting flowers. Predators such as checkered beetles (Cleridae) may colonise isolated oaks quickly as they cover large distances of up to several kilometres (Cronin et al. 2000). Low spatial connectivity as studied here does not, therefore, matter for incidence of beetle predators, but population density does decrease with decreasing connectivity.
There are at least two possible explanations for the decrease in abundance and species richness of wood-feeding beetles with higher connectivity. Firstly, predatory species may have a strong regulatory effect on their prey, and thus prey density decreases when predator density increases (top-down control). However, the lack of a significant negative relationship between the abundances of wood-feeders and predators makes this explanation less likely. Secondly, it can be explained by the favourable microclimate for breeding in more sun-exposed conditions. This is consistent with the positive effects of habitat openness which have been found in many other studies on saproxylic beetles (Müller et al. 2010; Sverdrup-Thygeson et al. 2010; Koch Widerberg et al. 2012), including wood-feeding beetles (Vodka et al. 2009).
Conclusions
Landscapes with scattered mature trees are of high conservation value (Ranius and Jansson 2000; Manning et al. 2006; Fischer et al. 2010). However, the number of scattered trees in agricultural landscapes is declining globally and this trend will continue over the coming decades even if management is improved (Gibbons et al. 2008). It is important to know how different ecological groups and threatened species respond to differently structured landscapes (Harrison and Bruna 1999; Dupont and Nielsen 2006; Franc et al. 2007). We showed that predators within the saproxylic beetle community are positively affected by connectivity of oaks, while wood-feeding beetles are negatively affected. For wood-feeding beetles, both species richness and abundance decrease with connectivity, but for predators abundance increases. Moreover, threatened species were more abundant with decreasing connectivity. Whether the observed patterns are caused by different microclimates on isolated trees or by the small-scale connectivity itself remains unclear. However, there is a close relationship between a favourable microclimate for many saproxylic beetles and low small-scale tree connectivity in nature. To conserve saproxylic beetles, we clearly need semi-open habitats with scattered trees; this can be achieved both by continued management of traditional landscape structure and by partial cutting in closed forest environments (Franc and Götmark 2008). A more differentiated strategy may be desirable with respect to the trophic structure in insect communities because different trophic levels may respond in different ways to habitat isolation. We recommend taking multiple trophic levels into consideration for a balanced evaluation of fragmentation effects and for effective management of wood-inhabiting beetles in fragmented landscapes. Future studies should thus bear the potential importance of dense forests for predatory wood-inhabiting beetles in mind, but dense forests might restrict other resources such as flowers.
References
Bailey D, Schmidt-Entling MH, Eberhart P, Herrmann JD, Hofer B, Kormann U, Herzog F (2010) Effects of habitat amount and isolation on biodiversity in fragmented traditional orchards. J Appl Ecol 47:1003–1013
Barton K (2012) MuMIn: multi-model inference. R package version 1.7.2. http://CRAN.R-project.org/package=MuMIn
Bergman K-O, Jansson N, Claesson K, Palmer MW, Milberg P (2012) How much and at what scale? Multiscale analyses as decision support for conservation of saproxylic oak beetles. For Ecol Manage 265:133–141
Brouwers NC, Newton AC (2009) The influence of habitat availability and landscape structure on the distribution of wood cricket (Nemobius sylvestris) on the Isle of Wight, Uk. Landscape Ecol 24(2):199–212
Buse J (2012) “Ghosts of the past”: flightless saproxylic weevils (Coleoptera: Curculionidae) are relict species in ancient woodlands. J Insect Conserv 16(1):93–102
Buse J, Schröder B, Assmann T (2007) Modelling habitat and spatial distribution of an endangered longhorn beetle - A case study for saproxylic insect conservation. Biol Conserv 137(3):372–381
Buse J, Ranius T, Assmann T (2008) An endangered longhorn beetle associated with old oaks and its possible role as an ecosystem engineer. Conserv Biol 22(2):329–337
Cagnolo L, Valladares G, Salvo A, Cabido M, Zak M (2009) Habitat fragmentation and species loss across three interacting trophic levels: effects of life-history and food-web traits. Conserv Biol 23(5):1167–1175
Cronin JT, Reeve JD, Wilkens R, Turchin P (2000) The pattern and range of movement of a checkered beetle predator relative to its bark beetle prey. Oikos 90(1):127–138
Davies KF, Margules CR, Lawrence JF (2000) Which traits of species predict population declines in experimental forest fragments? Ecology 81(5):1450–1461
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67(3):345–366
Dupont YL, Nielsen BO (2006) Species composition, feeding specificity and larval trophic level of flower-visiting insects in fragmented versus continuous heathlands in Denmark. Biol Conserv 131:475–485
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81(1):117–142
Fischer J, Stott J, Law BS (2010) The disproportionate value of scattered trees. Biol Conserv 143(6):1564–1567
Franc N, Götmark F (2008) Openness in management: hands-off vs partial cutting in conservation forests, and the response of beetles. Biol Conserv 141(9):2310–2321
Franc N, Götmark F, Økland B, Nordén B, Paltto H (2007) Factors and scales potentially important for saproxylic beetles in temperate mixed oak forest. Biol Conserv 135:86–98
Gibbons P, Lindenmayer DB, Fischer J, Manning AD, Weinberg A, Seddon J, Ryan P, Barrett G (2008) The future of scattered trees in agricultural landscapes. Conserv Biol 22(5):1309–1319
Gravel D, Massol F, Canard E, Mouillot D, Mouquet N (2011) Trophic theory of island biogeography. Ecol Lett 14(10):1010–1016
Harrison SP, Bruna E (1999) Habitat fragmentation and large-scale conservation: what do we know for sure? Ecography 22:225–232
Herrmann JD, Kormann U, Schuepp C, Stocker Y, Herzog F, Entling MH (2012) Effects of habitat isolation and predation pressure on an arboreal food-web. Community Ecology 13(1):82–87
Holt RD (2002) Food webs in space: on the interplay of dynamic instability and spatial processes. Ecol Res 17:261–273
Holt RD, Lawton JH, Polis GA, Martinez ND (1999) Trophic rank and the species-area relationship. Ecology 80:1495–1504
Horak J, Chumanova E, Hilszczanski J (2012) Saproxylic beetle thrives on the openess in management: a case study on the ecological reuquirements of Cucujus cinnaberinus from Central Europe. Insect Conservation and Diversity 5:403–413
Hunter MD (2002) Landscape structure, habitat fragmentation, and the ecology of insects. Agric For Entomol 4:159–166
Hyvärinen E, Kouki J, Martikainen P (2006) A comparison of three trapping methods used to survey forest-dwelling Coleoptera. Eur J Entomol 103:397–407
Johansson V, Snäll T, Ranius T (2013) Estimates of connectivity reveal non-equilibrium epiphyte occurrence patterns almost 180 years after habitat decline. Oecologia 172:607–615
Koch Widerberg M, Ranius T, Drobyshev I, Nilsson U, Lindbladh M (2012) Increased openness around retained oaks increases species richness of saproxylic beetles. Biodivers Conserv 21(12):3035–3059
Köhler F (2000) Totholzkäfer in Naturwaldzellen des nördlichen Rheinlandes. Landesanstalt für Ökologie, Bodenordnung und Forsten Nordrhein-Westfalen, Recklinghausen
Komonen A, Penttila R, Lindgren M, Hanski I (2000) Forest fragmentation truncates a food chain based on an old-growth forest bracket fungus. Oikos 90(1):119–126
Kotiaho JS, Kaitala V, Komonen A, Paivinen J (2005) Predicting the risk of extinction from shared ecological characteristics. Proc Natl Acad Sci USA 102(6):1963–1967
Laaksonen M, Peuhu E, Varkonyi G, Siitonen J (2008) Effects of habitat quality and landscape structure on saproxylic species dwelling in boreal spruce-swamp forests. Oikos 117(7):1098–1110
Löbel S, Snäll T, Rydin H (2006) Species richness patterns and metapopulation processes - evidence from epiphyte communities in boreo-nemoral forests. Ecography 29:169–182
Manning AD, Fischer J, Lindenmayer DB (2006) Scattered trees are keystone structures - Implications for conservation. Biol Conserv 132:311–321
Moilanen A, Nieminen M (2002) Simple connectivity measures in spatial ecology. Ecology 83(4):1131–1145
Möller G (2009) Struktur- und Substratbindung holzbewohnender Insekten, Schwerpunkt Coleoptera - Käfer. Dissertation, Freie Universität, Berlin
Müller J, Noss RF, Bussler H, Brandl R (2010) Learning from a “benign neglect strategy” in a national park: response of saproxylic beetles to dead wood accumulation. Biol Conserv 143(11):2559–2569
Oksanen J, Blanchet FG, Kindt R et al (2011) vegan: Community Ecology Package. R package version 2.0-0. http://CRAN.R-project.org/package=vegan
Ozanne CMP, Speight MR, Hambler C, Evans HF (2000) Isolated trees and forest patches: patterns in canopy arthropod abundance and diversity in Pinus sylvestris (Scots pine). For Ecol Manage 137:53–63
Palm T (1959) Die Holz- und Rindenkäfer der süd- und mittelschwedischen Laubbäume, Lund
Ranius T, Jansson N (2000) The influence of forest regrowth, original canopy cover and tree size on saproxylic beetles associated with old oaks. Biol Conserv 95:85–94
Ranius T, Johansson P, Berg N, Niklasson M (2008) The influence of tree age and microhabitat quality on the occurrence of crustose lichens associated with old oaks. J Veg Sci 19(5):653–662
Ranius T, Johansson V, Fahrig L (2010) A comparison of patch connectivity measures using data on invertebrates in hollow oaks. Ecography 33(5):971–978
R Core Development Team (2011) R: a language and environment for statistical computing. 2.13.2 edn. R Foundation for Statistical Computing, Vienna
Renner K (1980) Faunistisch-ökologische Untersuchungen der Käferfauna pflanzensoziologischer Biotope im Evesell–Bruch bei Bielefeld-Sennestadt. Berichte des naturwissenschaftlichen Vereins zu Bielefeld Sonderheft 2:145–176
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5(1):18–32
Schiegg K (2000) Are there saproxylic beetle species characteristic of high dead wood connectivity? Ecography 23(5):579–587
Schmidl J, Büche B (in press) Die Rote Liste und Gesamtartenliste der Käfer (Coleoptera, exkl. Lauf- und Wasserkäfer) Deutschlands im Überblick (Stand Sept. 2011). Naturschutz und Biologische Vielfalt 70
Schmidl J, Bussler H (2004) Ökologische Gilden xylobionter Käfer Deutschlands. Naturschutz und Landschaftsplanung 36(7):202–218
Schuepp C, Herrmann JD, Herzog F, Schmidt-Entling MH (2011) Differential effects of habitat isolation and landscape composition on wasps, bees, and their enemies. Oecologia 165(3):713–721
Speight MCD (1989) Saproxylic invertebrates and their conservation. Council of Europe, Strasbourg
Steffan-Dewenter I, Tscharntke T (2000) Butterfly community structure in fragmented habitats. Ecol Lett 3(5):449–456
Sverdrup-Thygeson A, Skarpaas O, Odegaard F (2010) Hollow oaks and beetle conservation: the significance of the surroundings. Biodivers Conserv 19:837–852
Thompson RM, Hemberg M, Starzomski BM, Shurin JB (2007) Trophic levels and trophic tangles: the prevalence omnivory in real food webs. Ecology 88(3):612–617
Tischendorf L, Fahrig L (2000) On the usage and measurement of landscape connectivity. Oikos 90:7–19
Tscharntke T, Brandl R (2004) Plant-insect interactions in fragmented landscapes. Annu Rev Entomol 49:405–430
Vodka S, Cizek L (2013) The effects of edge-interior and understorey-canopy gradients on the distribution of saproxylic beetles in a temperate lowland forest. For Ecol Manag 304:33–41
Vodka S, Konvicka M, Cizek L (2009) Habitat preferences of oak-feeding xylophagous beetles in a temperate woodland: implications for forest history and management. J Insect Conserv 13(5):553–562
Acknowledgments
JB was supported by a DBU (German Federal Environmental Foundation) scholarship during field work in 2007. We thank the administration of the Biosphere Reserve “Niedersächsische Elbtalaue” for permission to do field work. We are also grateful to D. Stengel (Leuphana University Lüneburg) for his assistance in the field. The comments of two anonymous referees improved an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buse, J., Entling, M.H., Ranius, T. et al. Response of saproxylic beetles to small-scale habitat connectivity depends on trophic levels. Landscape Ecol 31, 939–949 (2016). https://doi.org/10.1007/s10980-015-0309-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-015-0309-y