Abstract
While spatial heterogeneity is one the most studied ecological concepts, few or no studies have dealt with the subject of ambient sound heterogeneity from an ecological perspective. Similarly to ambient light conditions, which have been shown to play a significant role in ecological speciation, we investigated the existence of ambient sound heterogeneity and its possible relation to habitat structure and specifically to habitat types (as syntaxonomically defined ecological units). Considering that the structure and composition of animal communities are habitat type specific and that acoustic signals produced by animals may be shaped by the habitat’s vegetation structure, natural soundscapes are likely to be habitat specific. We recorded ambient sound in four forest and two grassland habitat types in Northern Greece. Using digital signal techniques and machine learning algorithms (self organizing maps, random forests), we concluded that ambient sound is not only spatially heterogeneous, but is also directly related to habitat type structure, pointing towards the existence of habitat type specific acoustic signatures. We provide evidence of the importance of soundscape heterogeneity and ambient sound signatures and a possible solution to the social cues versus vegetation characteristics debate in habitat selection theory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatial heterogeneity is one of the most important determinants of ecological processes as it “forms the fundamental basis of the structure and functioning of landscapes” (Wu 2004, p. 125). For landscape ecology in particular, heterogeneity has been and continues to be a foundational concept (Urban et al. 1987; Turner 2005; Wiens 2008). Heterogeneity is still actively researched in landscape ecology (Fahrig et al. 2011; Wang et al. 2012), although particular forms of spatial heterogeneity, instrumental for the structure and function of landscapes, are rarely studied in an ecological context. Examples of rarely studied heterogeneity include the role of ambient light gradients in cichlid speciation (Seehausen et al. 2008) or the influence of habitat heterogeneity on the evolution of animal communication signals (Morton 1975). Nonetheless, as (i) acoustic communication is potentially constrained by certain habitat characteristics (Endler 1993b) and (ii) communication among animals has effects on ecological processes (Wagner and Danchin 2010), studies on ambient sound heterogeneity could provide new insights on dispersal (Clobert et al. 2009), habitat choice (Vermeij et al. 2010) or even evolution (Maan and Seehausen 2011).
More recently, landscape ecological studies investigated ambient sound variability in both temporal and spatial dimensions (Matsinos et al. 2008; Farina et al. 2011a, b; Krause et al. 2011). More importantly, soundscape ecology, an “emerging new science”, has been recording and measuring soundscapes, assessing their dynamics and developing tools for their study (Pijanowski et al. 2011a, b).Covering a broad range of sound frequencies and exhibiting temporal and spatial variability, terrestrial soundscapes include animal signals, mostly emitted by bird, insect and amphibian species (biophony), potential anthropogenic noise (anthrophony), and abiotic sources such as wind, rain, and running water (geophony) (Pijanowski et al. 2011a, b). A large part of soundscape ecology, partly owing to its roots in acoustic ecology (Truax and Barrett 2011), focuses on investigating the dynamics and interactions of these three components of the soundscape (Matsinos et al. 2008; Mazaris et al. 2009; Barber et al. 2011; Krause et al. 2011; Pijanowski et al. 2011a, b).
While the assessment of the spatiotemporal dynamics of soundscapes, as indicated above, has been well under way, explicit soundscape–landscape connections have been little studied. Habitat types, as an ecologically relevant unit (Drakou et al. 2011), which has already been used to study ambient sound in behavioural ecology (Waser and Brown 1986; Slabbekoorn 2004a, b; Nicholls and Goldizen 2006), could offer a way to explore this relationship. Considering that acoustic animal communities are habitat type specific (Slabbekoorn 2004a) and that acoustic signals produced by animals may be shaped by the vegetation structure of the habitat (Morton 1975; Ey and Fischer 2009), the acoustic features of ambient sound are likely to be habitat specific. Research in aquatic ecosystems indicates that a relationship exists (Radford et al. 2011; Simpson et al. 2011; Stanley et al. 2012), but the only relevant study in terrestrial ecosystems did not explicitly investigate the habitat type-ambient sound relationship (Farina et al. 2011a).
A potential habitat-specific acoustic signature could not only be utilised for identification and monitoring techniques (Sueur et al. 2008b; Farina et al. 2011a), but more importantly it could also be viewed as a source of environmental heterogeneity that is also connected to public information sensu Wagner and Danchin (2010), especially as interpreted recently by Farina et al. (2011a). Farina et al. (2011a), borrowing from biosemiotics, interpret the soundscape as an “organized structure” that allows organisms to locate resources in space and time. They introduce a new concept, the “soundtope”, i.e. an area of similar acoustic conditions that allows for the presence of interacting species. Radford et al. (2010) report distinct soundtopes or acoustic signatures in coastal areas of aquatic ecosystems and it is possible that similar phenomena could also take place in terrestrial landscapes.
However, most studies that have actually investigated ambient sound differences among terrestrial habitat types are mainly focused on behavioural rather than ecological processes (Ryan 1988; Slabbekoorn 2004a; Kirschel et al. 2009) or study heterogeneity in the anthro-, bio-, or geo- phonies of the soundscape (Matsinos et al. 2008; Mazaris et al. 2009). Additionally, behavioural studies, despite successfully reporting acoustic differences among habitat types, were constrained by several issues: (i) habitat types studied were drastically different (Tobias et al. 2010) or very similar (Trefry and Hik 2010) (ii) only a small number of habitat types were considered in most of cases (mostly just two habitat types (Slabbekoorn 2004a; Kirschel et al. 2009; Trefry and Hik 2010)), and (iii) in the cases where more than two different habitat types were taken into account, urban noise, wind turbulence or running water sounds were also considered (Slabbekoorn 2004b). On the other hand, soundscape studies, while studying ambient sound, either focus on the soundscape through a human-observer perspective (e.g. Matsinos et al. 2008; Mazaris et al. 2009) or are interested in just one soundscape component (Barber et al. 2011; Krause et al. 2011; Francis et al. 2011).
Furthermore, to our knowledge, none of the studies on ambient sound tried to connect differences in ambient sound to the vegetation structure versus social cues debate in habitat selection (Goodale et al. 2010), a debate about the relative importance of vegetation characteristics versus inter- and intra-specific cues for habitat choice. From an evolutionary perspective, it has been shown that heterogeneity in ambient light conditions can cause divergent selection visual signals associated with communication (Seehausen et al. 2008). Similarly to ambient light conditions (Endler 1993a), ambient sound heterogeneity among habitats could be interpreted as a source of environmental heterogeneity and could be of evolutionary importance. Finally, since environmental heterogeneity is an important variable in habitat selection and movement of mobile organisms (Wiens et al. 1993), attention should be paid on the possible influence of spatially heterogeneous ambient sound as used by animals in informed dispersal decisions (Clobert et al. 2009).
As indicated above, observed heterogeneity in ambient sound among habitats, plus an explicit correspondence of the “soundtope” to habitat type, i.e. an “acoustic signature”, could have important implications for the ecology and evolution of animals that utilise sound for communication or resource localisation. Thus, the explicit aim of our research was to determine whether a relationship exists between habitat type and the soundscape. This is the first study to use acoustics and robust learning techniques to tease apart the connection between habitat structure, ambient sound and habitat choice. More specifically, we recorded and analysed digital recordings of seven habitat types and successfully subjected them to ordination and classification analysis.
Materials and methods
Our study rests on the hypothesis that vegetation structure and animal community differences can produce habitat specific ambient sound. We used self organizing maps (SOM), an ordination technique, to reveal habitat similarities based on their sound profiles. We applied random forests (RF), a classification method, to assess whether habitat types can be effectively and accurately discriminated using ambient sound characteristics, similarly to Yovel et al. (2008) who worked on ultrasound-based classification of vegetation types.
Study area
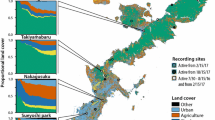
We collected ambient sound samples in Greece in September 2010 at 32 sites within the area of the Kerkini reservoir in Greece (see Fig. 1). The landscape of Kerkini and the surrounding mountains Krousia and Belles (23°05′N, 41°15′E) is composed of wetland and mountain ecosystems of national and European importance. The area is protected under national (National Park), European (Natura 2000 site) and international (Ramsar site) laws and treaties. We use habitat type as a coherent unit that were syntaxonomically defined, described and delineated through fieldwork and sample collection which has been carried out as part of a project entitled ‘Identification and Description of Habitat Types in Areas of Interest for the Conservation of Nature’, funded by the Greek Ministry of the Environment. Since our interest was in discriminating habitat types through their acoustic features, we were interested in habitat types that were:
Land cover map of the Lake Kerkini area. The points represent the recording sites. Oak forest and oak forest edge sites would not be undistinguishable in print due to scale of the map, as due to the study design they are spatially proximate (Source Greek Ministry of the Environment, Energy and Climate Change, www.ypeka.gr). (Color figure online)
-
I.
Structurally dissimilar but spatially close: (a) deciduous (beech) and coniferous (pine) forests that are spatially close (see Fig. 1) and (b) undisturbed oak forest and disturbed oak forest edge. These habitat types differ in leaf morphology, for instance pine (needles) versus beech (rounded), leaf litter, tree density and animal community.
-
II.
Structurally similar to explore variance within groups of different forests and different grasslands. For example, the vegetation of grassland habitats mainly consisted of low grass and very few scattered trees in the landscape, in contrast to the forested landscape of the other habitat types.
Additionally, the acoustic animal community of the grasslands was composed of both insect stridulations and bird vocalisations, while in the deciduous forests it consisted mostly of birds (DB, personal observation). We conducted recordings in seven habitats types (Table 1). Samples were collected at sites that could be assigned unambiguously to one of the seven habitats. Habitat types were identified by DB with the help of the Habitat’s Directive interpretation manual and the habitat type map of the Kerkini area. Vegetation data like leaf morphology, tree density and leaf litter are readily available from relevant sources, are straightforward characteristic that can be assessed by direct observation and are widely used among ecologists.
Recording procedure and feature extraction
We used an omnidirectional Telinga Stereo DAT-Mic microphone and a Marantz PMD-660 solid state recorder to record mono uncompressed digital files, at a 16 bits/44.1 kHz sampling rate. We pointed the microphone towards the North at 1.5 m above the ground. We carried out all the recordings using the same procedure and instrument settings. To investigate possible effects on ambient sound from road caused fragmentation (Šálek et al. 2010) we carried out half of the recordings made in the oak forest 8 m from a forest road. We called this the oak forest edge habitat type. For consistency, we carried out all between 13:00 and 16:00 in late September 2010, on sunny days with a temperature at 25.3 ± 1.3 °C (n = 5). As we were mostly interested in ambient sound produced by the animal communities, we avoided early morning recordings (dawn chorus is dominated by birds) or nights (wetland habitats dominated by anurans). Similarly, September was chosen as, considering the diversity of habitat types investigated, it could offer us a more diverse animal communities (e.g. in spring the soundscape would be dominated by birds, in summer by insects).
To avoid contamination, we did not capture any human made sound such as road traffic, airplane traffic or woodcutting machinery during the recording sessions. We made three 2 min recordings made at each site (see Table 1). This sampling led to a total of 96 recordings of 2 min duration. Eight segments of 10 s in duration were randomly extracted from each 2 min audio file leading to a total of 768 audio segments.
A set of eight features that rely more on statistical features than on spectral analysis was chosen. These acoustic parameters included typical parameters used in audio and soundscape classification (Mitrovic et al. 2010) and entropy indices potentially correlated with species richness (Sueur et al. 2008b). We also developed a new parameter based on the correlation of the signal with 1/f noise. 1/f noise is a feature of complex phenomena discovered in geophysical and economic time series, music, protein dynamics, DNA-base sequences, ecological time series (Halley and Kunin 1999) and is also reported to occur in natural soundscapes (De Coensel et al. 2003). We generated 10 s of 1/f noise and stored it as a digital audio file in Matlab R2009a (The Mathworks Inc 2009). This reference file was then correlated with each 10 s segment using a non-parametric Spearman correlation test. The final set of features extracted is shown in Table 2. We computed the parameters for each 10 s segment and averaged per 2 min recording session. Thus we created a 96 × 8 matrix for further analyses. All signal manipulation and analyses were performed using the R package seewave (Sueur et al. 2008a; R Development Core Team 2009).
Machine Learning
Machine Learning is a growing area within eco-informatics, which can help in revealing structure in complex data (Olden et al. 2008). Among the most often used algorithms in unsupervised machine learning are the SOM (Kohonen 2001) with many applications in ecology and the environmental sciences (Chon 2011). For supervised machine learning, RF (Breiman 2001) have been recently applied to the ecological sciences and are considered as a promising tool for ecological classification (Cutler et al. 2007). Both algorithms, in our case, offer robustness and easy interpretation of the results while the RF has the additional advantage of an error estimation method that eliminates the need for validation tests (Chapman et al. 2010).
SOM is an unsupervised learning algorithm especially suited to exploratory data analysis, visualization, ordination and clustering (Kohonen 2001). SOM is a particular case of artificial neural networks based on competitive learning, i.e. the output neurons of the network compete among themselves to be activated. SOM consists of an input layer of nodes (input data), with a size n × m (in our case the 96 × 8 matrix), where n is the number of training samples and m is the number of features (variables) used for the learning; the output layer is a grid of j neurons, where j is the size of the feature map. The algorithm starts by initialising the iterations i: i = 0; next it initialises the weight vectors W ij of the each neuron and proceeds by presenting the training samples as m-dimensional vectors x(i) = [x 1(i),…, x m (i)] to the output neurons. The algorithm computes the distances d i between the input vector x, and all the output neuron vectors j as: \( d_{j} \left( i \right) = \sum\nolimits_{n - 1}^{m} {[x_{n} \left( i \right) - W_{nj} \left( i \right)]}^{2} \), where x n (t) is the nth observation vector, and W nj (i) is the weight vector of each output neuron at iteration i. The weight vector of the neuron that has the least d j (i) to the input vector x (called the winning neuron) is updated with a learning rate r, along with its neighbouring neurons in a h radius. Both r and h are decreasing functions of i. This feature of the algorithm allows for neurons that lie closely in the feature map, to represent areas lying close in the input vector space i.e. the topography presentation properties of the SOM. The algorithm stops when i = i max.
Due to different units among variables, we standardised the input matrix by mean subtraction and standard-deviation division (Kohonen 2001). Additionally, we determined the size of the SOM heuristically using quality measures; based on the quantization and topographic error measures we set the map size to 48 neurons (6 × 8). Finally, we initialised the SOM using random sampling without replacement from the input data and a used linearly declining learning rate r from 0.05 to 0.01. We used the Kohonen (Wehrens and Buydens 2007) package of the R environment for the SOM generation.
As finding clusters in the SOM is not a straightforward procedure (Kohonen 2001), a fuzzy clustering algorithm on the distances between SOM neurons was used with the cluster package of R.
RF is an ensemble learning classifier; many weak classifiers are combined into a more powerful one. In particular, RF works by a procedure called bagging, where many classification and regression trees (Breiman et al. 1984), called a committee, are constructed independently using only a random sample of the entire dataset and a random subset of the explanatory variables. The output classes are predicted using a majority vote of the committee of the trees grown (Breiman 2001). Two important features of the RF algorithm are the out of bag (OOB) error estimates and the variable importance measures. OOB is an error estimate based on the cases not included in the sample data at each iteration, making the use of a test set for validation unnecessary. The variable importance measure allows for the quantification of the importance of each feature in the classification and is called the Gini index. When each tree splits, the importance of the splitting feature is measured as the improvement in the split criterion and is accumulated over all the trees in the forest separately for each feature. Larger Gini index for a feature is an indication of higher importance. The split criterion is based on the total decrease in node impurities from splitting on each feature (Friedman et al. 2001).
We ran two different classifications in a hierarchical classification approach (Chapman et al. 2010). The first classification was a run including the six broad habitat classes. The second classification was a run that was implemented to discriminate between the core and edge oak forest types.
The RF algorithm is implemented in the R package randomForest (Liaw and Wiener 2002). We used a tuning algorithm provided by the RF package to select the number of variables at each split using and conducted sampling without replacement. We determined the number of iterations heuristically, based on the convergence of the OOB error rate and assessed the accuracy of the classification using the OOB error estimates, a confusion matrix and the Kappa statistic. Finally, the important variables for both classifications were assessed using the Gini index.
Results
Ordination and clustering using SOM
Resulting from the use of SOM and fuzzy clustering, a feature map was produced that displayed the mapping and clustering of the habitat types and the distribution of the relative feature weights (Fig. 2).
Self organizing map (SOM) analysis: the points in the map represent the positioning of the vegetation types in a single neuron. The positions of vegetation types within the neurons are random and do not represent actual relationships. The shading of the neurons represents the clustering of the SOM neurons into four clusters from the fuzzy clustering algorithm
As our hypothesis predicted, vegetation and animal community differences produced habitat specific ambient sound patterns. This is evident in the SOM results (Fig. 2) as the grassland habitats (AG and WE) types were clearly distinct from forest habitats (particularly deciduous), and the forest habitat formed discrete clusters.
However, not only were grasslands and forests distinct, but there was variation among the forests too. Forest types with similar vegetation characteristics, namely leaf morphology, tree density and leaf substrate were clustered together. More specifically, deciduous forest habitat types (FAP, FB, FO and FOE) formed two distinct clusters.
Classification using RF
The first RF algorithm model on the vegetation type classification yielded an OOB classification error of 8.33 % (Table 3).
The AG and PF classes had zero error rate and the class with the biggest error was the class with the less representatives, FAP. The second RF model, between FO and FOE had an error rate of 6 %, with only one misclassification per class. CENT and SFM features were the most important features for the six class model. For the classification between the oak forest and its edge the most important feature was SKEW. Our 1/f noise based feature was the feature with the least decrease in Gini, for (Fig. 3).
Important features for the random forest classifications based on the Gini index. The same eight features were used for both classifications: the one with six classes (top) and the one with only the oak forest and its edge (bottom). Variable importance is measured as mean decrease in Gini: high mean decrease in Gini means high variable importance
Discussion
Both the SOM ordination and the RF classification results (Fig. 2; Table 3) revealed significant ambient sound heterogeneity in the study area. Considering that environmental acoustic conditions have been interpreted as a constraint on animal acoustic communication (Morton 1975), the latter is potentially influenced by the habitat as sensory environment i.e. “the medium through which signals are propagated and the background in which they are perceived” (Maan and Seehausen 2011, p. 597). Divergence due to habitat characteristics that affect communication signals has been discovered in several species, such as green hylia Hylia prasina (Kirschel et al. 2009), satin bowerbirds Ptilonorhynchus violaceus (Nicholls and Goldizen 2006), felids (Peters and Peters 2010) and primates (Waser and Brown 1986). The likely occurrence of ambient sound heterogeneity suggests that ambient sound should act as a spatially diffuse selective pressure on (i) the evolution of signals and signaller and receiver behavioural properties (Endler 1993a) and (ii) ecological processes that influence the spatial distribution of animals in the landscape (Francis et al. 2011).
The SOM revealed several ambient sound similarities among the habitat types. FB and FAP share some structural features: trees have all large rounded leaves, tree density is similar and leaf litter is deep, while the FO and FOE (which are spatially and structurally close) are denser, with less leaf litter. The FP, where lower tree density, fewer dead leaves, and different leaf morphology (needles) are the key characteristics, is different from the other forests and is clustered with some the wetland recordings. Finally, the biotic soundscape of the deciduous forest habitats was shaped by similar animal communities that were mainly composed of birds but no insects, while in the pine forest, insect stridulations were present (DB, personal observation).
The most important features for the first classification were the CENT and SFM measures (Fig. 3).
The importance of these parameters, which estimate the mean frequency and the flatness of the spectrum, respectively, is probably due to differences in vegetation structure (Waser and Brown 1986; Peters and Peters 2010) and vocal animal community composition of the habitat types (Sueur et al. 2008b). The second classification discriminated between OF and FOE habitat types. The SKEW measure was the most important variable indicating differences due to vegetation structure and vocal animal community composition.
The classification procedure was based on a set of features including an index based on the correlation between the ambient sound and 1/f noise spectral profiles. Based on the Gini decrease in accuracy, the weight of this index appeared low for all classifications. Despite the fact that 1/f noise has been reported in natural soundscapes (De Coensel et al. 2003) the variance of the feature was not enough for habitat type discrimination. Still, this index could be relevant when comparing natural and human soundscapes, as anthropogenic soundscapes display a higher percentage of low frequency noise.
Acoustic signatures
However, the most important contribution of our research was evidence for the existence of habitat type acoustic signatures. The high accuracy of the RF classification (Table 3) supposes significant differences in the ambient sound of distinct habitat types. Even closely related habitat types such as FO and FOE (as confirmed by the SOM, Fig. 2) can be effectively discriminated using ambient sound features and the RF algorithm (Table 3). Additionally, although differences among habitats have been previously studied (e.g. Slabbekoorn 2004a), this is the first study that uses machine learning techniques to actually show the existence of habitat specific acoustic signatures.
Studies on aquatic ecosystems have also provided evidence of distinct coastal habitat ambient sound (Radford et al. 2010). Recent results on reef ecosystems have further shown that several organisms use ambient sound for habitat selection: coral larvae (Vermeij et al. 2010); fish (Radford et al. 2011); and crustaceans (Simpson et al. 2011). Similar to reef ecosystems, ambient sound could be an information source for terrestrial organisms for habitat selection and as Francis et al. (2011) suggest, ‘soundscape orientation’ may even “guide movements at great distances” (ibid, p. 1278).
Moreover, while previous theory suggested that birds select suitable habitat mostly based on structural cues i.e. vegetation characteristics (MacArthur et al. 1962), recent studies have shown that birds may be more attracted to suitable habitat by acoustic cues produced by con- or even hetero-specifics (Mönkkönen et al. 1990; Hahn and Silverman 2006; Betts et al. 2008). The biotic and abiotic sources of each habitat-specific acoustic environment could be indices for a suitable habitat. However, if the hypothesis (acoustic adaptation hypothesis) that animal songs are adapted to specific habitats (Morton 1975; Nicholls and Goldizen 2006; Boncoraglio and Saino 2007; Peters and Peters 2010) has any merit and the habitat can indeed affect the evolutionary fitness of organisms (Maan and Seehausen 2011), social cues may not be the only extra-vegetation criterion for habitat selection. Signal attenuation characteristics and ambient noise heterogeneity could be determinants of habitat choice as well.
Our discovery of terrestrial acoustic signatures impacts on the social cues versus vegetation characteristics debate in habitat selection theory (Melles et al. 2009). It suggests that the two theories may not be incompatible, providing they additionally consider ambient sound signatures and the possibility of habitat dependant ambient sound. It is possible that a complex function incorporating vegetation characteristics, social cues and ambient sound determines habitat choice. As vegetation structure has an effect on animal communities and animal communities have an effect on ambient sound, habitat choice is probably a more complex than the simple vegetation versus social cues dichotomy. Thus, on broad spatial scales soundscape orientation may be more important than other cues like con-specific attraction or visual identification of vegetation (Francis et al. 2011). For example, Huijbers et al. (2012) recently provided empirical support for multiple (visual, olfactory and acoustic) cue response in early juvenile Haemulon flavolineatum and they propose a cue-use model that hinges on distance-from-habitat: large distance—acoustic cues, medium distance—olfactory cues and small distance—visual cues.
Tied to the above, is the recent theoretical proposal by Farina et al. (2011a, b) to consider the soundtope as a distinct ecological unit in landscapes. While Farina et al. (2011a, b) propose the soundtope, they do not try to investigate its possible relationship to habitat or other landscape characteristics but instead interpolate soundscape metrics across the landscape, similar to Matsinos et al. (2008), Mazaris et al. (2009) and Pijanowski et al. (2011b). Our results reveal that the soundtope, defined as a distinct soundscape, is directly related to the habitats present in a landscape and that a distance based interpolation (e.g. Matsinos et al. (2008); Mazaris et al. (2009)), that does not take into account the spatial structure of the landscape could possibly be lacking accuracy. Our work echoes the work of Krause et al. (2011), who similarly to the above, try to extrapolate sound recording features across the landscape using vegetation data. However, while they presuppose the existence of acoustic signatures and try to locate them in the landscape using remote sensing tools, we actually bring forth evidence of their actual existence. Ambient sound thus displays clumped heterogeneity, contingent on the habitat type level of organization (Kotliar and Wiens 1990; Allen 1998) and clumping can be important in cue-use success. And while within habitat and above habitat heterogeneity is certain to play a role in determining ecological processes, ambient sound patchiness (sensu Kotliar and Wiens (1990)) at the habitat type level has been shown to influence habitat choice (Huijbers et al. 2012) and signal evolution (Waser and Brown 1986; Nicholls and Goldizen 2006; Peters and Peters 2010).
In conservation related terms, even though automated recognition of species or estimation of species richness through acoustic methods has recently received great interest (Depraetere et al. 2012), to our knowledge, no study has dealt with habitat type recognition through sound features. Although taxonomy and phytosociology are indispensable tools for conservation, the method developed here, relies on a simple analysis process that does not require particular knowledge of taxonomy or acoustics and could be of use in cases of limited funds, time or specialised personnel. For example, the detection of changes in the community structure of a forest edge created by forest roads traditionally requires species census (Šálek et al. 2010). Our method could track such changes with accuracy above 90 % with minimal sampling (30 sites × 2 min), as Kennedy et al. (2010) already succeeded using 2-min identification systems. Acoustic classification appears then as useful tool that could be used in different research and conservation contexts (Farina et al. 2011a, b).
In conclusion, we found that ambient sound is spatially heterogeneous and that the “spatial pattern of the soundscape” (Farina et al. 2011a), is inextricably related to the spatial pattern of vegetation. This finding gives new insights to the relationship between soundscapes and organisms that use sound to communicate. Moreover, it should be stressed that existence of acoustic signatures or soundtopes (Farina et al. 2011a, b) in direct relation to habitat types is a novel finding that as shown above, could have significant applications in habitat selection and ecological evolution theorising and on-the-ground research. Finally, our results suggest that soundscapes are important for the maintenance of biodiversity in different habitat types. Further applications and refinement of habitat acoustics could also not only reveal more nuanced similarities and dissimilarities among habitat types, but also provide insights into the ecological and evolutionary processes that cause acoustic signatures to emerge (Pijanowski et al. 2011a, b).
References
Allen TFH (1998) The landscape “level” is dead: persuading the family to take it off the respirator. In: Peterson DL, Parker VT (eds) Ecological scale. Columbia University Press, New York, pp 35–54
Barber JR, Burdett CL, Reed SE, Warner KA, Formichella C, Crooks KR, Theobald DM, Fristrup KM (2011) Anthropogenic noise exposure in protected natural areas: estimating the scale of ecological consequences. Landscape Ecol 26:1281–1295
Betts GB, Hadley AS, Rodenhouse N, Nocera JJ (2008) Social information trumps vegetation structure in breeding-site selection by a migrant songbird. Proc Biol Sci 275:2257–2263
Boncoraglio G, Saino N (2007) Habitat structure and the evolution of bird song: a meta-analysis of the evidence for the acoustic adaptation hypothesis. Funct Ecol 21:134–142
Breiman L (2001) Random forests. Mach Learn 45:5–32
Breiman L, Friedman JH, Olshen RA, Stone CG (1984) Classification and regression trees. Wadsworth International Group, Belmont, California, USA
Chapman DS, Bonn A, Kunin WE, Cornell SJ (2010) Random forest characterization of upland vegetation and management burning from aerial imagery. J Biogeogr 37:37–46
Chon TS (2011) Self-organizing maps applied to ecological sciences. Ecol Inf 6:50–61
Clobert J, Le Galliard JF, Cote J, Meylan S, Massot M (2009) Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett 12:197–209
Cutler DR, Edwards TC Jr, Beard KH, Cutler A, Hess KT, Gibson J, Lawler JJ (2007) Random forests for classification in ecology. Ecology 88:2783–2792
De Coensel B, Botteldooren D, De Muer T (2003) 1/f noise in rural and urban soundscapes. Acta Acust United Ac 89:287–295
Depraetere M, Pavoine S, Jiguet F, Gasc A, Duvail S, Sueur J (2012) Monitoring animal diversity using acoustic indices: implementation in a temperate woodland. Ecol Indic 13:46–54
Drakou EG, Kallimanis AS, Mazaris AD, Apostolopoulou E, Pantis JD (2011) Habitat type richness associations with environmental variables: a case study in the Greek Natura 2000 aquatic ecosystems. Biodivers Conserv 20:929–943
Endler JA (1993a) The color of light in forests and its implications. Ecol Monogr 63:2–27
Endler JA (1993b) Some general comments on the evolution and design of animal communication systems. Philos Trans R Soc Lond B Biol Sci 340:215–225
Ey E, Fischer J (2009) The “acoustic adaptation hypothesis”: a review of the evidence from birds, anurans and mammals. Bioacoustics 19:21–48
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
Farina A, Lattanzi E, Malavasi R, Pieretti N, Piccioli L (2011a) Avian soundscapes and cognitive landscapes: theory, application and ecological perspectives. Landscape Ecol 26:1257–1267
Farina A, Pieretti N, Piccioli L (2011b) The soundscape methodology for long-term bird monitoring: a Mediterranean Europe case-study. Ecol Inf 6:354–363
Francis CD, Paritsis J, Ortega CP, Cruz A (2011) Landscape patterns of avian habitat use and nest success are affected by chronic gas well compressor noise. Landscape Ecol 26:1269–1280
Friedman J, Hastie T, Tibshirani R (2001) The elements of statistical learning. Springer series in statistics. http://www-stat.stanford.edu/~tibs/ElemStatLearn. Accessed 11 Dec 2012
Goodale E, Beauchamp G, Magrath RD, Nieh JC, Ruxton GD (2010) Interspecific information transfer influences animal community structure. Tree 25:354–361
Hahn BA, Silverman ED (2006) Social cues facilitate habitat selection: American redstarts establish breeding territories in response to song. Biol Lett 2:337–340
Halley JM, Kunin WE (1999) Extinction risk and the 1/f family of noise models. Theor Popul Biol 56:215–230
Huijbers CM, Nagelkerken I, Lössbroek PAC, Schulten IE, Siegenthaler A, Holderied MW, Simpson SD (2012) A test of the senses: fish select novel habitats by responding to multiple cues. Ecology 93:46–55
Kennedy EV, Holderied MW, Mair JM, Guzman HM, Simpson SD (2010) Spatial patterns in reef-generated noise relate to habitats and communities: evidence from a Panamanian case study. J Exp Mar Biol Ecol 395:85–92
Kirschel ANG, Blumstein DT, Cohen RE, Buermann W, Smith TB, Slabbekoorn H (2009) Birdsong tuned to the environment: green hylia song varies with elevation, tree cover, and noise. Behav Ecol 20:1089–1095
Kohonen T (2001) Self-organizing maps. Springer, Berlin, Heidelberg
Kotliar NB, Wiens JA (1990) Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos 59:253–260
Krause B, Gage SH, Joo W (2011) Measuring and interpreting the temporal variability in the soundscape at four places in Sequoia National Park. Landscape Ecol 26:1247–1256
Liaw A, Wiener M (2002) Classification and regression by random forest. R News 2:18–22
Maan ME, Seehausen O (2011) Ecology, sexual selection and speciation. Ecol Lett 14:591–602
MacArthur RH, MacArthur JW, Preer J (1962) On bird species diversity. II. Prediction of bird census from habitat measurements. Am Nat 96:167–174
Matsinos YG, Mazaris AD, Papadimitriou KD, Mniestris A, Hatzigiannidis G, Maioglou D, Pantis JD (2008) Spatiotemporal variability in human and natural sounds in a rural landscape. Landscape Ecol 23:945–959
Mazaris AD, Kallimanis AS, Chatzigianidis G, Papadimitriou K, Pantis JD (2009) Spatiotemporal analysis of an acoustic environment: interactions between landscape features and sounds. Landscape Ecol 24:817–831
Melles SJ, Badzinski D, Fortin MJ, Csillag F, Lindsay K (2009) Disentangling habitat and social drivers of nesting patterns in songbirds. Landscape Ecol 24:519–531
Mitrovic D, Zeppelzauer M, Breiteneder C (2010) Features for content-based audio retrieval. Adv Comput 78:71–150
Mönkkönen M, Helle P, Soppela K (1990) Numerical and behavioural responses of migrant passerines of resident tits (Parus spp.): heterospecific attraction in northern breeding bird communities. Oecologia 85:218–225
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Nicholls JA, Goldizen AW (2006) Habitat type and density influence vocal signal design in satin bowerbirds. J Anim Ecol 75:549–558
Olden JD, Lawler JJ, Poff NLR (2008) Machine learning methods without tears: a primer for ecologists. Q Rev Biol 83:171–193
Peters G, Peters MK (2010) Long-distance call evolution in the Felidae: effects of body weight, habitat, and phylogeny. Biol J Linn Soc 101:487–500
Pijanowski BC, Farina A, Gage SH, Dumyahn SL, Krause BL (2011a) What is soundscape ecology? An introduction and overview of an emerging new science. Landscape Ecol 26:1213–1232
Pijanowski BC, Villanueva-Rivera LJ, Dumyahn SL, Farina A, Krause BL, Napoletano BM, Gage SH, Pieretti N (2011b) Soundscape ecology: the science of sound in the landscape. BioScience 61:203–216
Radford CA, Stanley JA, Tindle CT, Montgomery JC, Jeffs AG (2010) Localised coastal habitats have distinct underwater sound signatures. Mar Ecol Prog Ser 401:21–29
Radford CA, Stanley JA, Simpson SD, Jeffs AG (2011) Juvenile coral reef fish use sound to locate habitats. Coral Reefs 30:295–305
R Development Core Team (2009) R: a language and environment for statistical computing. Version 2.10.1. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org
Ryan MJ (1988) Constraints and patterns in the evolution of anuran acoustic communication. In: Fritzch B, Ryan MJ, Wilczynski W, Hetherington T, Walkowiad W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 637–677
Šálek M, Svobodová J, Zasadil P (2010) Edge effect of low-traffic forest roads on bird communities in secondary production forests in central Europe. Landscape Ecol 25:1113–1124
Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HDJ, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, Imai H, Okada N (2008) Speciation through sensory drive in cichlid fish. Nature 455:620–626
Simpson SD, Radford AN, Tickle EJ, Meekan MG, Jeffs AG (2011) Adaptive avoidance of reef noise. PLoS One 6:e16625
Slabbekoorn H (2004a) Habitat-dependent ambient noise: consistent spectral profiles in two African forest types. J Acoust Soc Am 116:3727–3733
Slabbekoorn H (2004b) Singing in the wild: the ecology of birdsong. In: Marler P, Slabbekoorn H (eds) Nature’s music: the science of birdsong. Elsevier Academic Press, London, pp 178–205
Stanley JA, Radford CA, Jeffs AG (2012) Location, location, location: finding a suitable home among the noise. Proc Biol Sci 279:3622–3631
Sueur J, Aubin T, Simonis C (2008a) Equipment review Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics 18:213–226
Sueur J, Pavoine S, Hamerlynck O, Duvail S (2008b) Rapid acoustic survey for biodiversity appraisal. PLoS One 3:e4065
The Mathworks Inc (2009) MATLAB v. R2009b Documentation. The Mathworks Inc
Tobias JA, Aben J, Brumfield RT, Derryberry EP, Halfwerk W, Slabbekoorn H, Seddon N (2010) Song divergence by sensory drive in Amazonian birds. Evolution 64:2820–2839
Trefry SA, Hik DS (2010) Variation in pika (Ochotona collaris, O. princeps) vocalizations within and between populations. Ecography 33:784–795
Truax B, Barrett GW (2011) Soundscape in a context of landscape and acoustic ecology. Landscape Ecol 26:1201–1207
Turner MG (2005) Landscape ecology: what is the state of the science? Annu Rev Ecol Evol Syst 36:319–344
Urban DL, O’Neil RV, Shugart HH Jr (1987) Landscape ecology: a hierarchical perspective can help scientists understand spatial patterns. BioScience 37:119–127
Vermeij MJA, Marhaver KL, Huijbers CM, Nagelkerken I, Simpson SD (2010) Coral larvae move toward reef sounds. PLoS One 5:e10660
Wagner RH, Danchin É (2010) A taxonomy of biological information. Oikos 119:203–209
Wang L, Okin GS, D’Odorico P, Caylor KK, Macko SA (2012) Ecosystem-scale spatial heterogeneity of stable isotopes of soil nitrogen in African savannas. Landscape Ecol. doi:10.1007/s10980-012-9776-6
Waser PM, Brown CH (1986) Habitat acoustics and primate communication. Am J Primatol 10:135–154
Wehrens R, Buydens LMC (2007) Self-and super-organizing maps in R: the Kohonen package. J Stat Softw 21:1–19
Wiens JA (2008) Allerton Park 1983: the beginnings of a paradigm for landscape ecology? Landscape Ecol 23:125–128
Wiens JA, Stenseth CN, Van Horne B, Ims RA (1993) Ecological mechanisms and landscape ecology. Oikos 66:369–380
Wu J (2004) Effects of changing scale on landscape pattern analysis: scaling relations. Landscape Ecol 19:125–138
Yovel Y, Franz MO, Stilz P, Schnitzler HU (2008) Plant classification from bat-like echolocation signals. PLoS Comput Biol 4:e1000032
Acknowledgments
The authors would like to acknowledge the input from Dr. EG Drakou, Dr. AD Mazaris and Dr. I Tsiripidis and Dr. J Tzanopoulos. DB would like to thank E Mandravelli, A Stamellou, V Tsiogka, S Theodoridis and Prof. T Lanaras for input in earlier versions of the paper. This work was partially supported by the Postgraduate Studies Programme “Conservation of Biodiversity and Sustainable Exploitation of Native Plants” at the Biology Department of the Aristotle University of Thessaloniki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bormpoudakis, D., Sueur, J. & Pantis, J.D. Spatial heterogeneity of ambient sound at the habitat type level: ecological implications and applications. Landscape Ecol 28, 495–506 (2013). https://doi.org/10.1007/s10980-013-9849-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-013-9849-1