Abstract
Azo compounds (azos) are widely used as radical initiators in the polymerization industry. Nonetheless, due to the azo group molecular structure, azos gravitate toward thermal decomposition and lead to thermal runaway accidents. In this paper, the thermal decomposition behaviors of 2-(1-cyano-1-methylethyl)azocarboxamide (CABN) under the dynamic and adiabatic environments were investigated using differential scanning calorimetry and accelerating rate calorimeter. Several safety assessment parameters such as time to maximum rate under adiabatic condition (TMRad), temperature of no return, and self-accelerating decomposition (SADT) temperature were calculated based on thermokinetic analysis as well as curve fitting. The results indicated that CABN decomposes at low temperatures (90.0–100.0 °C) and releases huge volumes of gaseous products, which may set off a fire, deflagration, or even explosion if the decomposition occurs uncontrolled in a confined space. Compared with commonly used azos, the shorter TMRad, lower SADT, and more heat from thermal decomposition reflect the potential thermal explosion hazards of CABN. To investigate emergency response procedure in terms of industrial applications, the oxygen-balance method was further used to evaluate the explosion hazard of CABN, and several recommendations on alleviating the thermal hazards of CABN were established to prevent catastrophic accidents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Azo compounds (azos) are widely used as radical initiators in the polymerization industry, such as acryl resins for paints, water-absorbent resins, polymer coagulants, adhesives, and paper finishing agents. Compared with other initiators, the thermal degradation of most azo initiators can be regarded as the first-order reaction, and the impurity has little effect on its thermal stability [1,2,3]. Nonetheless, the unique properties of azo initiators bring not only the necessary chemical trait for the industry but also series potential thermal hazards to the storage and reaction system. Due to the presence of the azo group in its molecule structure, azos gravitate toward thermal decomposition and release considerable amounts of heat in production, storage, and transportation [4]. Hence, the thermal hazard and thermokinetics of azo initiators have attracted much attention. For example, Chen et al. [5] investigated the coupling effect of azobisisobutyronitrile decomposition, and developed two decoupling methods for nonisothermal DSC results. Liu et al. [6] built up a thermokinetic-based numerical simulation approach to evaluate the thermal hazards of four commonly used azos. Zhu et al. [7] explored the decomposition mechanism of 1,2,4-triazolone using FTIR-TGA-MS. Although the special decomposition mechanism and thermal hazards of azos have attracted the interest of researchers and attention of the industry, the investigation of thermal behavior for new-type azos is still rare in the literature.
2-(1-Cyano-1-methylethyl)azocarboxamide (CABN) is a newly developed azo initiator mainly used in living radical polymerizations [8]. Table 1 lists several commonly used organic-solvent soluble type and water-soluble type azo initiators. Compared with commonly uses azo initiators, the asymmetric structure (Fig. 1) makes CABN dissolvable in both oil and water. This trait enables the polymerization industry to improve polymerization efficiency and narrow down production costs [9]. Nevertheless, as a type of azo, CABN shares the self-reactive property found in other azos. To forestall accidents and take full advantage of the unique structure and properties of CABN, thermal hazard evaluation for CABN is salient.
In this paper, differential scanning calorimetry (DSC) and accelerating rate calorimeter (ARC) were used to analyze the thermal behaviors of CABN under dynamic and adiabatic conditions. A few safety assessment parameters, such as time to maximum rate under adiabatic condition (TMRad), temperature of no return (TNR), maximum temperature (Tmax), the temperatures at which the TMRad is 8 or 24 h (TD8 or TD24) [10], and self-accelerating decomposition temperature (SADT) were obtained based on thermokinetic analysis and curve fitting. The research results would provide references for the further study of azos, the development of new azo initiators, the optimization of production processes, and the establishment of feasible emergency response plans.
Experimental and theoretical methods
Material
Powdered crystal CABN (98.00 mass%) was purchased from Beijing Hwrk Chemical Co., Ltd., and stored at a low temperature (4.0 °C) to prevent its degradation.
Nonisothermal tests by differential scanning calorimetry (DSC)
From the guideline for the transportation of dangerous goods set out by the United Nations (UN), DSC was recommended as a technique for determining the thermal hazards of energetic materials [11,12,13,14]. Experiments were carried out with approximately 3.0 mg samples by Mettler Toledo’s high pressure DSC 2+ (Switzerland), and the results were evaluated using Mettler Toledo’s STARe software. Alumina crucibles with 70.0 μL volume were used for all DSC experiments using pure N2 as a purge gas at a flow rate of 80.0 mL min−1. The heating rates (β) were set at 0.5, 1.0, 2.0, 4.0, and 8.0 °C min−1 with the heating range of 30.0 to 300.0 °C.
Adiabatic analysis by accelerating rate calorimeter (ARC)
The worst case scenario was simulated by ARC under adiabatic conditions, which means there is no heat exchange between bomb and the environment, the heats generated from the thermal decomposition of the sample are all used to heat up itself [15, 16]. Thus, ARC can be used to evaluate the thermal hazards of materials more accurately in extreme environments. The ARC from Thermal Hazard Technology Co., Ltd. (United Kingdom) was used for the adiabatic test. Roughly, 1.0 g of sample was placed in a 1/4-inch Hastelloy bomb and sealed with an air atmosphere. A standard heat-wait-search (H-W-S) procedure was selected to implement the adiabatic test. According to the results of DSC test, the experimental conditions for ARC tests were as follows: The heating step was regulated at 5.0 °C intervals with start and end temperature were set at 80.0 and 450.0 °C, respectively, and waiting time was set to 15.0 min with the slope sensitivity at 0.02 °C min−1.
Determination of apparent activation energy by Flynn–Wall–Ozawa method
Flynn, Wall, and Ozawa (FWO) proposed the isoconversional method using DSC curves to obtain kinetic parameters of thermal decomposition reactions [17, 18]. On the basis of the Doyle approximation [19], the FWO equation can be given as Eq. (1):
where T is the temperature at the arbitrary time, g(α(T)) is the conversional function, A is the pre-exponential factor, Ea is the apparent activation energy, and R is the universal gas constant.
Kinetics under adiabatic conditions
The extensively used thermokinetic method under adiabatic conditions was suggested by Townsend and Tou [20]. For n-order reaction with a single reaction, the self-heating rate can be defined as Eq. (2):
where mT is the self-heating rate at the arbitrary temperature, T0 and Tf is the measured initial and final exothermic temperatures, ΔTad is the measured adiabatic temperature rise, n is the reaction order, and k is the rate constant which can be expressed as Eq. (3).
Based on the Arrhenius equation, Eq. (4) was obtained to determine the value of Ea and A.
Time to maximum rate under adiabatic conditions (TMR ad)
TMRad indicates the time taken for a reaction at the given temperature from the initial reaction to the maximum rate. This means an interposing measure is still possible within this period. For a thermal decomposition reaction with high Ea, the chief part of reaction time is TMRad [20]. By employing the boundary condition that beyond the temperature at the maximum rate (Tm), the reaction rate decreases rapidly, TMRad can be expressed as Eq. (5) [20, 21]:
Self-accelerating decomposition temperature (SADT)
SADT is characterized as the lowest temperature at which a self-accelerating decomposition will take place in substance in different packaging specifications within 1 week [22, 23]. Adiabatic kinetics parameters from the ARC test were used for the computation of SADT for CABN in different packing conditions. Initially, the time constant (τ) is obtained by Eq. (6) [21]:
where Cp is the heat capacity of the reactant, S is the wetted surface area, and U is the heat transfer coefficient.
Then, the no return temperature (TNR) can be obtained by feeding τ into the fitted function of TMRad and T (Eq. 7). Afterward, the SADT for various packaging specification can be determined through Eq. (7) [21].
Perimetry explosion hazard evaluation by oxygen-balance (OB) method
The oxygen-balance (OB) method is invariably used to estimate the explosion hazard of organic compounds which mainly contain C, H, N, and O in their molecule structure, and extensively applied in the explosive industry [24]. To estimate the explosion hazards for CABN, the OB method was adopted in this paper. The following formula was recommended for calculating OB [25]:
where X is the number of atoms of carbon, Y is the number of atoms of hydrogen, Z is the number of atoms of oxygen, and M is the molecular weight.
Results and discussion
Preparative thermal analysis by DSC
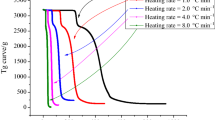
An elementary thermal hazard analysis of CABN by DSC was established under dynamic conditions. Figure 2 shows the DSC curves of CABN. Taking the result at the heating rate of 4.0 °C min−1 as an example: From 75.0 to 90.0 °C, an endothermal peak appears due to the melting of the CABN. With the increase in system temperature, an exothermic signal distributed across 110.0 to 190.0 °C is detected, which is caused by the thermal decomposition of the CABN, and the corresponding heat of decomposition (ΔHd) is 1244.0 J g−1 (Table 2). Compared with commonly used azos (2,2′-azobis(2-methylpropionitrile) (AIBN), 2,2′-azobis(2-methylbutyronitrile) (AMBN), and dimethylvaleronitrile (ABVN) are 1035, 781, and 786 J g−1, respectively) [26, 27], the preponderant ΔHd of CABN indicated that CABN will be more destructive and dangerous once a runaway reaction occurred.
Thermal runaway reaction by ARC
Self-heating rate, pressure rise rate, and initial exothermic temperature are prominent parameters used to quantify thermal risks of runaway reaction when handling energetic materials. As shown in Fig. 3, after H-W-S periods, the self-decomposition reaction of CABN occurred at 96.1 °C after an elapsed time of 145.7 min, during which the pressure rise rate and temperature rise rate maintained an upward trajectory with the maximum rate approximately at 329.3 bar min−1 and 17,849.4 °C min−1. Subsequently, the self-heating rate decreased with the consumption of reactants (Fig. 4).
The high pressure and temperature rise rate indicated that the released energy from the runaway reaction of CABN will be eminently numerous, and may set off a fire, deflagration, or even explosion if the decomposition occurs uncontrolled in a confined space. The maximum temperature (Tmax) and maximum pressure (Pmax) of the runaway reaction were 218.6 °C and 56.9 bar, respectively. Both the pressure and temperature rise rates as a function of reaction time are depicted in Fig. 4, which shows that the value of self-heating rate and pressure rise rate was small at the outset, but increased abruptly as the temperature exceeded 140.0 °C. The pressure rise rate stepped up slowly below 140.0 °C, this may be caused by the following reasons: When the air in the bomb becomes hot, the pressure rise is mainly due to the expansion of heated and compressed air, gaseous products have little contribution to the pressure rise. Nevertheless, when the temperature exceeds 140.0 °C, the sample decomposes promptly, and yields large volumes of gaseous products, thus leading to a sharp increase in pressure.
Determination of thermokinetic parameters
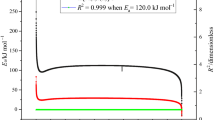
The evaluation of the thermokinetics of an energetic material, apparent activation energy (Ea) is one of the main prerequisites for the determination of SADT. In this paper, nonisothermal and adiabatic thermokinetics were both applied to determine the value of Ea. According to DSC tests, Ea determined by FWO equation (Eq. 1) is 134.7 kJ mol−1 from the line’s slope by plotting lg β versus 1/T (Fig. 5). In the adiabatic kinetic analysis, the Ea and A can be obtained by plotting lnk versus 1/T (Eq. 4). When the reaction order is chosen correctly, the plot is supposed to be a straight line [28]. As shown in Fig. 6, the first-order reaction is selected for its highest linear correlation coefficient (0.9983). Subsequently, Ea and A were calculated from the plot.
It is noteworthy that the Ea calculated from the adiabatic test (190.0 kJ mol−1) is higher than the value obtained from the dynamic test (134.7 kJ mol−1), which may be caused by the elevated pressure in the adiabatic test. According to Wahl [29], Hammond [30], and Bickel et al. [31], the thermal decomposition of CABN is set out at the formation of free radicals. As shown in Fig. 7, nitrogen was also released when two kinds of carbon radicals were formed. This implied that the concentration and density of nitrogen would be increasingly high in the airtight bomb. From the viewpoint of collision theory [32], the increase in the reaction products will result in a reduced reaction rate, which is because the higher concentration of reaction products will lead to fewer collisions of reactant. This could be a suitable explanation for the huge gap between Ea obtained under different experimental methods.
Evaluation of safety parameters
Prediction of TMR ad with extrapolation method
From adiabatic thermokinetic analysis, the thermal decomposition reaction for CABN is a first-order reaction. Thus, Eq. (9) is derived from Eq. (5) with n = 1:
Based on numerical integration and curve fitting by Origin 2017 (Originlab Co., US), the extrapolated TMRad versus temperature curve (Fig. 8) and fitted function (Eq. 10) are obtained from Eq. (9):
As shown in Fig. 8, with an increase in ambient temperature, the TMRad decreased exponentially. Taking TMRad = 24 h (TD24) as an example, the corresponding temperature was 78.2 °C, meaning that when the ambient temperature reached 78.2 °C, the emergency program should be taken within 24 h. Compared with other commercial used azos, the TD24 of CABN is in the middle of ABVN, dimethyl-2,2′-azobis(2-methylpropionate) (AIBME), AMBN, AIBN, and N,N′-dinitro-4,4′-azobis(1,2,4-triazolone) (DNZTO) (30, 32, 44, 64, and 120 °C, respectively) [6, 7]. Nevertheless, the higher ΔHd enables CABN to create greater temperature rise of the reaction or storage system once the runaway reaction occurs, which may induce more severe consequences to both industry and society.
SADT of CABN at various packaging specifications
The packaging size is crucial for the determination of SADT. According to Roduit [33] and Malow et al. [34], the SADT mainly depends on the packaging size, once the storage/transport environments are determined. In this research, different packaging specifications including 55 gallon drum, UN 25 kg package, and 0.51 Dewar vessel were selected for the SADT calculation with the required heat transfer property-related parameters presented in Table 3 [22]. From Eqs. (6) and (7), the calculated SADT for 55 gallon drum, UN 25 kg package, and 0.51 Dewar vessel are 77, 81, and 76 °C, respectively. It should be noted that the lowest value of SADT comes from 0.51 L Dewar vessel. This is due to the lower heat transfer coefficient will bring the inefficient heat exchange between system and the environment. From Liu [6] and Cao [35], the SADT for CABN is higher than commonly used azos, which demonstrates the good thermal stability of CABN. Howbeit, the short TMRad at operation temperature, abrupt temperature rise rate, and relatively high pressure rise rate in adiabatic tests indicates that CABN could readily decompose and release enormous amounts of heat and gaseous products after prolonged exposure to elevated temperatures.
Explosion hazard evaluation
According to the theory of spontaneous ignition put forth by Frank-Kamenetskii [36], a sufficient temperature increase would lead to a self-accelerating reaction front propagating through energetic substances, the reaction front can propagate in energetic substances at a velocity less (deflagration) or more (detonation) than the speed of sound. With the employment of oxygen-balance (OB) method, the potential thermal explosion hazard of CABN can be evaluated.
Figure 9 shows the calculated OB versus hazard for selected substances. As shown, the OB of CABN is fairly close to 2,4,6-trinitrotoluene (TNT). This does not mean the explosive power of CABN is adjoin to TNT, but it does indicate that the potential explosion hazard of CABN is relatively high when compared with other commonly used azos. To maximize safety, the potential explosion hazards of CABN should be considered in the process design. Explosion-proof devices are needed in the establishment of safety facilities.
Conclusions
The thermal behavior and thermokinetic of CABN has been investigated in this paper. Experimental results showed that the initial exothermic temperature (T0), the maximum temperature (Tmax) and pressure (Pmax) of CABN were 96.1 °C, 218.6 °C, and 56.9 bar, respectively, which indicates that CABN could readily decompose and release enormous amounts of heat and gaseous products after prolonged exposure to elevated temperatures. The safety assessment parameters such as time to maximum rate under adiabatic conditions (TMRad), temperature of no return (TNR), maximum temperature (Tmax), TD8, TD24, and SADT of CABN for various packaging specifications including 55 gallon drum, UN 25 kg package, and 0.51 Dewar vessel were predicted based on the adiabatic analysis. In addition, the explosion hazard of CABN was evaluated by the oxygen-balance method. Compared with other commonly used azos, the potential explosion hazard of CABN is relatively high. To prevent a self-accelerating reaction front propagating through CABN and touching off a fire, deflagration or even explosion accident, strict safety measures should be set and adhered in production, applications, storage, and transportation. In general, the thermal parameters produced above can be used to determine some of these measures.
Abbreviations
- A :
-
Pre-exponential factor (min−1)
- C p :
-
Heat capacity of the reactant (kJ kg−1 K−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- k :
-
Rate constant (mol1−n Ln−1 s−1, n is reaction order)
- m :
-
Mass of CABN in different packaging specifications (kg)
- m T :
-
Self-heating rate at arbitrary temperature (°C min−1)
- M :
-
Molecular weight (g mol−1)
- n :
-
Reaction order
- OB:
-
Oxygen-balance
- P max :
-
Maximum pressure (bar)
- R :
-
Universal gas constant (J mol−1 K−1)
- S :
-
Wetted surface area (m2)
- SADT :
-
Self-accelerating decomposition temperature (°C)
- T :
-
Temperature at arbitrary time (K)
- T 0 :
-
Measured initial exothermic temperature (°C)
- T D24 :
-
The temperatures at which the TMRad is 24 h (°C)
- T D8 :
-
The temperatures at which the TMRad is 8 h (°C)
- T f :
-
Measured final exothermic temperature (°C)
- T m :
-
Temperature at maximum rate (K)
- T max :
-
Maximum temperature (°C)
- TMR ad :
-
Time to maximum rate under adiabatic conditions (min)
- T NR :
-
Temperature of no return (K)
- T onset :
-
Onset temperature (°C)
- T p :
-
Peak temperature (K)
- U :
-
Heat transfer coefficient (kJ min−1 m−2 K−1)
- X :
-
Number of atoms of carbon
- Y :
-
Number of atoms of hydrogen
- Z :
-
Number of atoms of oxygen
- β :
-
Heating rate (°C min−1)
- ΔH d :
-
Heat of decomposition (J g−1)
- ΔT ad :
-
Measured adiabatic temperature rise (°C)
- τ :
-
Time constant (min)
References
Idage BB, Vernekar SP, Ghatge ND. Decomposition rate studies of azo initiators in solution. J Polym Sci Polym Chem Ed. 1983;21:2145–55.
Yang Y, Tsai YT. Evaluation on thermal stability and kinetics of 2,2′-azobis(2,4-dimethyl)valeronitrile in aerobic and anaerobic conditions under isothermal process. J Therm Anal Calorim. 2018;132:1961–8.
Chiang CL, Liu SH, Cao CR, Hou HY, Shu CM. Multiapproach thermodynamic and kinetic characterization of the thermal hazards of 2,2′-azobis(2-methylpropionate) alone and when mixed with several solvents. J Loss Prev Process Ind. 2018;51:150–8.
Liu SH, Lin WC, Hou HY, Shu CM. Comprehensive runaway kinetic analysis and validation of three azo compounds using calorimetric approach and simulation. J Loss Prev Process Ind. 2017;49:970–82.
Zhang CX, Bin LuG, Chen LP, Chen WH, Peng MJ, Lv JY. Two decoupling methods for non-isothermal DSC results of AIBN decomposition. J Hazard Mater. 2015;285:61–8.
Liu SH, Cao CR, Lin WC, Shu CM. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J Hazard Mater. 2019;365:164–77.
Zhu J, Jin S, Yu Y, Zhang C, Li L, Chen S, et al. Evaluation of thermal hazards and thermo-kinetic parameters of N,N′-dinitro-4,4′-azo-bis(1,2,4-triazolone) (DNZTO). Thermochim Acta. 2016;623:58–64.
Nakamura Y, Kitada Y, Kobayashi Y, Ray B, Yamago S. Quantitative analysis of the effect of azo initiators on the structure of α-polymer chain ends in degenerative chain-transfer-mediated living radical polymerization reactions. Macromolecules. 2011;44:8388–97.
Mathias LJ, Lee S, Wright JR, Warren SC. Improvement of wood properties by impregnation with multifunctional monomers. J Appl Polym Sci. 1991;42:55–67.
Vernières-Hassimi L, Dakkoune A, Abdelouahed L, Estel L, Leveneur S. Zero-order versus intrinsic kinetics for the determination of the time to maximum rate under adiabatic conditions (TMRad): application to the decomposition of hydrogen peroxide. Ind Eng Chem Res. 2017;56:13040–9.
United Nations. Committee of experts on the transport of dangerous goods. Recommendations on the transport of dangerous goods. UN. 1995.
Halim NA, Ali A, Abidin ZHZ, Ahmad AB, Sulaiman AZ, Ibrahim ZA. Thermal analysis of organically modified Ca2+-montmorillonite using DSC and TSC techniques. J Therm Anal Calorim. 2017;128:135–40.
Alarcon RT, Gaglieri C, de Oliveira AR, Bannach G. Use of DSC in degree of conversion of dimethacrylate polymers: easier and faster than MIR technique. J Therm Anal Calorim. 2018;132:1423–7.
Ferencz A, Lőrinczy D. DSC measurements of blood plasma on patients with chronic pancreatitis and operable and inoperable pancreatic adenocarcinoma. J Therm Anal Calorim. 2017;127:1187–92.
Pakkirisamy SV, Mahadevan S, Paramashivan SS, Mandal AB. Adiabatic thermokinetics and process safety of pyrotechnic mixtures: atom bomb, Chinese, and palm leaf crackers. J Therm Anal Calorim. 2012;109:1387–95.
Lei B, Zhao W, Ziebert C, Uhlmann N, Rohde M, Seifert H. Experimental analysis of thermal runaway in 18650 cylindrical Li-ion cells using an accelerating rate calorimeter. Batteries. 2017;3:14.
Li R, Guo X, Zhang J, Zhang H, Wu C. Investigation on the thermal behavior of nitroguanidine, potassium perchlorate, ammonium perchlorate and their mixtures by DSC and TG, DEStech Transactions on Computer Science and Engineering. 2018, p. 298–307. http://www.dpi-proceedings.com/index.php/dtcse/article/view/291144
Trache D, Maggi F, Palmucci I, DeLuca LT. Thermal behavior and decomposition kinetics of composite solid propellants in the presence of amide burning rate suppressants. J Therm Anal Calorim. 2018;132:1601–15.
Doyle CD. Series approximations to the equation of thermodynamic data. Nature. 1965;207:290–1.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Fisher HG, Goetz DD. Determination of self-accelerating decomposition temperatures using the accelerating rate calorimeter. J Loss Prev Process Ind. 1991;4:305–16.
Yang D, Koseki H, Hasegawa K. Predicting the self-accelerating decomposition temperature (SADT) of organic peroxides based on non-isothermal decomposition behavior. J Loss Prev Process Ind. 2003;16:411–6.
Sun JH, Lu SX, Sun ZH. Study on thermal risk evaluation of reactive substance. China Saf Sci J. 2003;2003:44–7.
Shanley ES, Melhem GA. A review of ASTM CHETAH 7.0 hazard evaluation criteria. J Loss Prev Process Ind. 1995;8:261–4.
Shanley ES, Melhem GA. The oxygen balance criterion for thermal hazards assessment. Process Saf Prog. 1995;14:29–31.
Liu SH, Shu CM. Advanced technology of thermal decomposition for AMBN and ABVN by DSC and VSP2. J Therm Anal Calorim. 2015;121:533–40.
Guo S, Wan W, Chen C, Chen WH. Thermal decomposition kinetic evaluation and its thermal hazards prediction of AIBN. J Therm Anal Calorim. 2013;113:1169–76.
Cisneros LO, Rogers WJ, Mannan MS. Effect of air in the thermal decomposition of 50 mass% hydroxylamine/water. J Hazard Mater. 2002;95:13–25.
Wahl RUR, Zeng L, Madison SA, DePinto RL, Shay BJ. Mechanistic studies on the decomposition of water soluble azo-radical-initiators. J Chem Soc Perkin Trans. 1998;2:2009–18.
Hammond GS, Chin-Hua SW, Trapp OD, Warkentin J, Keys RT. The mechanism of decomposition of azo compounds. II. Cage effects in the decomposition of α,α′-azoisobutyronitrile and related compounds. J Am Chem Soc. 1960;82:5394–9.
Bickel AF, Waters WA. The decomposition of aliphatic azo-compounds. I. Recl des Trav Chim des Pays-Bas. 1950;69:312–20.
Eliason MA, Hirschfelder JO. General collision theory treatment for the rate of bimolecular, gas phase reactions. J Chem Phys. 1959;30:1426–36.
Roduit B, Hartmann M, Folly P, Sarbach A, Brodard P, Baltensperger R. Determination of thermal hazard from DSC measurements. Investigation of self-accelerating decomposition temperature (SADT) of AIBN. J Therm Anal Calorim. 2014;117:1017–26.
Malow M, Wehrstedt KD, Manolov M. Thermal decomposition of AIBN part A: decomposition in real scale packages and SADT determination. Thermochim Acta. 2015;621:1–5.
Cao W, Guo J, Chen X, Ding Z, Xu K, Song J, et al. Synthesis, characterization, thermal properties and theoretical investigation on Bis (guanidinium) 4,4′-azo-1h-1, 2,4-triazol-5-one. J Mol Struct. 2017;1147:754–62.
Yang LF, Sheng JJ. Feasibility of using the Frank-Kamenetskii theory to predict the spontaneous ignition of crude oil-sand mixture. Energy Fuels. 2019;33:4816–25.
Acknowledgements
The authors would like to express their appreciation to the Anhui Provincial Natural Science Foundation, China, for its financial support of this study under the Contract No. 1908085ME125.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, YM., Liu, SH. & Shu, CM. Evaluation of thermal hazards based on thermokinetic parameters of 2-(1-cyano-1-methylethyl)azocarboxamide by ARC and DSC. J Therm Anal Calorim 138, 2873–2881 (2019). https://doi.org/10.1007/s10973-019-08827-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08827-z