Abstract
In this study, a new Schiff base ligand [2-{[4-amino-5-(3,4,5-trimethoxy-benzyl)-pyrimidin-2-ylimino]-methyl}-6-methoxy-phenol, C22H24N4O5] and its bismuth(III) complex [BiC22H22N4O5Cl] were synthesized using o-vanillin and trimethoprim in anhydrous solvents. Chemical analysis, elemental analysis, spectrum analysis and TG–DSC analysis were employed to characterize the compositions and structures of the two new compounds. In particular, the metabolic thermogenic curves of Schizosaccharomyces pombe (S. pombe) and Helicobacter pylori (H. pylori) cells treated by the two new compounds at different concentrations were monitored by an isothermal microcalorimeter at 32.00 and 37.00 °C, respectively. On the basis of the metabolic thermogenic curves, some important biothermokinetic parameters such as the microbial growth rate constant (k), inhibition ratio (I) and half inhibition concentration (IC50) were estimated. The experimental results indicated that both the Schiff base ligand and its bismuth(III) complex could inhibit the growth metabolism of S. pombe and H. pylori, but the inhibitory abilities of the two compounds were as follows: the Schiff base ligand > the complex for S. pombe and the Schiff base ligand < the complex for H. pylori, respectively. More importantly, the results also found that the Schiff base bismuth(III) complex exhibited bidirectional biological effect and hormesis effect on H. pylori cell line, i.e., the complex can stimulate the growth metabolism of H. pylori at low concentration, while inhibiting its growth at high concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trimethoprim is a novel oral broad-spectrum antimicrobial agent, whose antibacterial range is similar to that of sulfa drugs [1]. The antibacterial effect can be increased to several times when the drug combined with sulfa drugs. Trimethoprim is suitable for respiratory tract infection in senile chronic bronchitis, urinary tract infections, dysentery, enteritis, typhoid and malaria and other diseases. Valen Schiff bases are a class of compounds synthesized from o-vanillin (or its derivatives) and various amines based on the condensation reaction. Valen Schiff bases exhibit excellent coordination abilities due to the presence of “O” and “N” atoms in their molecules [2]. Accordingly, more kinds of metal complexes with diverse structures can be derived from the Valen Schiff bases. In our previous works, we have designed and synthesized many novel types of Valen Schiff bases and their transition or rare earth metal complexes [3,4,5,6]. The bioactivities of these new Valen Schiff bases and their complexes were also explored. The results demonstrated that these newly synthetic compounds exhibited superior biological activities [3,4,5,6]. However, main group metal complexes of Valen Schiff bases, especially for bismuth (III) complexes, have not been fully studied [7].
Bismuth is one of the most heavy metals in nature, but it has the least toxicity and low radiation and thus can be said to be a green metal. On the other hand, due to a strong deformation and polarization ability of bismuth ion, it can overcome the stereo-hindrance effect during the formation of the complexes. As Lewis acid, it can accept electrons to form a complex of high coordination number. Many organic bismuth compounds have been used in the treatment of gastric ulcer, twelve finger ulcer and H. pylori [8, 9]. More importantly, it was also found that some bismuth compounds could effectively inhibit the growth and reproduction of cancer cells [10]. From the view point of “green chemistry” and “sustainable development,” it is of great scientific values and wide application prospects to develop new types of organic bismuth coordination compounds.
Microcalorimetry provides a continuous monitoring of heat production, which can be applied to directly assess the bioactivities of a living organism. Heat flux is an expression of overall metabolic flux, and the monitoring of small changes in heat production to respond to toxic invade will be a sensitive indicator of altered metabolism. Owing to the characteristics of nondestructive, high accuracy and automaticity, microcalorimetry has been widely used in the field of biological research and pharmacological analysis [11,12,13].
In this work, a novel Schiff base ligand [2-{[4-amino-5-(3,4,5-trimethoxy-benzyl)-pyrimidin-2-ylimino]-methyl}-6-methoxy-phenol, C22H24N4O5] and its bismuth(III) complex [BiC22H22N4O5Cl] were synthesized from the condensation reaction of o-vanillin and trimethoprim in a 1:1 molar ratio. The compositions and structures of the two new compounds were characterized by elemental analysis, chemical analysis, spectrum analysis (including MS, FT-IR, NMR and UV–Vis) and TG–DSC analysis. Then, S. pombe and H. pylori cell lines were selected as ideal biological models to evaluate the bioactivities of the two new compounds by an isothermal microcalorimeter. As a basic study, the purpose of this research is to obtain some helpful biothermokinetic data for the further exploitation and utilization of Schiff bases bismuth(III) complexes.
Experimental
Chemicals and reagents
Trimethoprim (C14H18N4O3, 99.0%), o-vanillin (C8H8O3, 99.0%), ethanol (EtOH, 99.5%) and tetrahydrofuran (THF, 99.0%) were bought from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China); N,N-dimethyl formamide (DMF, > 99.5%) and dimethyl sulfoxide (DMSO, > 99.5%) were purchased from Tianjin Guangfu Chemical Research Institute (Tianjin, China); bismuth chloride (BiCl3, 99%) was purchased from Tianjin Jingke Chemical Research Institutes (Tianjin, China). All other chemicals including silver nitrate (AgNO3, 99.8%), sodium chloride (NaCl, 99.5%) and ethylenediamine tetraacetic acid (EDTA, 99.0%) were obtained from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals and reagents were used as received without further purification. The water used was double-distilled.
Physical measurements
1H and 13C NMR spectra were measured by a Bruker 400 MHz advance nuclear magnetic resonance spectrometer with trimethyl silicane (TMS) as internal standard and DMSO-d6 as solvent. Mass spectra were recorded on a Thermo Finnigan MAT95XP high-resolution mass spectrometer with methanol as solvent. The elemental analysis of C, H and N was performed on a PerkinElmer 2400 elemental analyzer. The contents of Bi3+ and Cl− in Schiff base bismuth(III) complex were analyzed by the EDTA titration and Mohr method, respectively. FT-IR spectra (400–4000 cm−1) were determined using a Thermo Nicolet Avatar 360 Fourier transform IR spectrometer with a KBr pellet. All absorption spectra were measured by a Hitachi U-3010 UV–Vis spectrophotometer. TG and DSC curves were determined by a HENVEN HCT-3 integrated thermal analyzer in flowing air with a heating rate of 10 °C min−1 between 50 and 1500 °C. The molar conductance was determined by a DDS-12DW conductivity meter (Shanghai Lida Instrument Factory, China). Microcalorimetric measurements were taken on a 3116-2/3239 TAM Air isothermal heat conduction microcalorimeter (Thermometric AB, Sweden). The microcalorimeter equipped with eight twin calorimetric channels with one side for the tested samples and the other for static references. The principles and structures of the microcalorimeter have been described detailedly in our previous work [14].
Cell lines and culture conditions
S. pombe cell line (ACCC20047) was obtained from the Agricultural Culture Collection of China. It was cultured in the Edinburgh minimal medium (EMM). The formula of EEM (natural pH) per liter was as follows: 2.2 g Na2HPO4, 3 g K2HPO4, 20 g glucose, 5 g NH4Cl, 1.0 mL vitamin, 0.1 mL minerals and 20 mL inorganic salt. Prior to use, the EEM medium was sterilized at 121 °C under high pressure for 30 min.
H. pylori cell line (ATCC43504) was bought from Shanghai Beinuo Biological Technology Co., Ltd. The formula of H. Pylori culture medium (HB8647) was 10.0 g cattle brain infusion power, 10.0 g proteose peptone, 9.0 g cattle heart infusion power, 2.0 g glucose, 2.5 g Na2HPO4, 5.0 g NaCl, pH = 7.4 ± 0.2, at 25 °C. 3.85 g HB8647 was dissolved in 93 mL distilled water, heated and stirred until dissolution. Then, the resulting solution was sterilized at 121 °C under high pressure for 30 min. The stock solution of HB8647-HP was prepared by adding 1 HP bacteriostatic agent and 7 mL sterile defibrinated sheep blood into the above HB8647 solution.
Synthesis of the Schiff base ligand
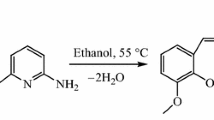
The Schiff base ligand, 2-{[4-amino-5-(3,4,5-trimethoxy-benzyl)-pyrimidin-2-ylimino]-methyl}-6-methoxy-phenol, was prepared as follows: 1.452 g (5 mmol) trimethoprim was dissolved in a three-neck round flask with 70 mL of ethanol, and it was placed in a water bath, and then heated to 55 °C, and stirred until the solid was dissolved completely. 0.761 g (5 mmol) o-vanillin was dissolved in 25 mL of ethanol and transferred into a separating funnel. Then, the ethanol solution of o-vanillin was added dropwise into the ethanol solution of trimethoprim; the color of the solution changed from colorless to pale yellow and finally became light red during the reaction process. After continuously reacted for 6 h, plenty of precipitates were generated. Subsequently, the precipitates were filtrated and recrystallized with ethanol–methylene chloride mixed solution (1:1, v/v). The product was dried under vacuum at 50 °C until the weight of the crystals became constant, with a yield of 68%. The synthetic scheme of the Schiff base ligand is shown in Fig. 1.
Synthesis of the Schiff base bismuth(III) complex
The reaction was carried out in a glove box. 2.79 g (5 mmol) Schiff base ligand was dissolved in 150 mL of THF; then, the solution was heated to 55 °C and stirred using s magnetic stirrer until the solid was dissolved completely. Another 1.58 g (5 mmol) BiCl3 was dissolved in the 50 mL of THF. Then, the solution of BiCl3 was slowly added to the solution of the Schiff base ligand. The reaction mixture was heated with stirring and refluxing for 3 h, cooling and crystallization; plenty of yellow precipitates were obtained. The precipitates were filtered and washed with THF-ethanol mixed solution (1:1, v/v) for several times. The product was dried in a vacuum drying oven, with a yield of 66%. The synthetic scheme of the Schiff base bismuth(III) complex is shown in Fig. 2.
Determination of metabolic thermogenic curves of S. pombe and H. pylori cells treated by the Schiff base ligand and its bismuth(III) complex
The metabolic thermogenic curves of S. pombe and H. pylori cells treated by the Schiff base ligand and its bismuth(III) complex at different concentrations were determined by an isothermal heat conduction microcalorimeter, respectively. The optimal temperature of growth metabolism of S. pombe and H. pylori is 32.00 and 37.00 °C, respectively. Processes of the determination were briefly described as follows: the microcalorimeter was adjusted to the optimal temperature (32.00 or 37.00 °C), and the measurement was taken with the ampoule method. Baselines were acquired before each measurement, and the microcalorimeter was calibrated electrically. Once the instrument had attained a stable baseline, 5 mL EMM-sterilized (or HB8647-HP) culture medium was pipetted to the sterilized sample ampoules. S. pombe (or H. pylori) cell lines were inoculated with an initial density of 1 × 106 CFU mL−1. The compounds with different concentrations were added into the cell suspension, respectively. All the ampoules containing cell suspension of S. pombe (or H. pylori) were sealed up and put into 8-channel calorimeter block. The data were acquired continuously by the proprietary software. All the microcalorimetric experiments were repeated three times in parallel, and the obtained results were identical.
Results and discussion
Characterization of the Schiff base ligand and its bismuth(III) complex
The Schiff base was a light red powder with a melting point of 165 ± 1 °C. It is very stable in the atmosphere. The powders are easily soluble in some organic solvents such as tetrahydrofuran (THF), dimethyl sulfoxide (DMSO), N,N-dimethyl formamide (DMF) and trichloromethane, but cannot dissolved in water, methanol and ethanol. The molar conductance of Schiff base ligand in DMSO at 25 °C was determined to be 3.93 S cm2 mol−1, suggesting that the Schiff base ligand is a nonelectrolyte and exists as a neutral molecule in DMSO solvent.
The Schiff base bismuth(III) complex was a yellow powder with a melting point of 242 ± 1 °C. The dissolubility experiment demonstrated that the complex cannot be dissolved in water, methanol, dichloromethane, trichloromethane, THF and diethyl ether, whereas it can be dissolved in DMSO and DMF, especially easily dissolved in the mixed solution of THF and DMSO (v/v = 1:1). The molar conductance of the complex in DMSO at 25 °C was determined to be 55.37 S cm2 mol−1, indicating that the complex is an electrolyte in DMSO solvent.
The Schiff base ligand and its bismuth(III) complex were characterized by chemical analysis, elemental analysis, mass spectra, FT-IR, 1H and 13C NMR spectra, UV–Vis spectra and TG–DSC analysis. The analytical results of the Schiff base and its bismuth(III) complex are provided in Tables S1–S6 in “Supplementary Materials,” respectively.
As can be seen from Table S1, the chemical and elemental analysis data are consistent with their theoretical values. Based on these results, we can infer the molecular formula of the Schiff base ligand and its bismuth(III) complex was C22H24N4O5 (abbreviated as H2L, L = C22H22N4O5) and BiC22H22N4O5Cl (abbreviated as BiLCl), respectively, which will be further confirmed by MS, FT-IR, NMR, UV–Vis and TG–DSC analyses.
The mass spectra of the ligand and the complex (see Table S1) showed that the M + = [H2L]+ = 424.2 and M + = [BiLCl] + = 666.1 for EI source in positive mode, respectively. These experimental results are in a good accordance with their theoretical values M[H2L] = 424.5 and M[BiLCl] = 666.9, which further verified the molecular formula of the Schiff base ligand and its complex.
As can be seen from Table S2, there are four strong absorption peaks appearing at 1605, 1503, 1455 and 1254 cm−1 in the FT-IR spectra of the Schiff base ligand H2L, belonging to the ν(C=N) stretching vibration of azomethine group [15], ν(C=N) stretching vibration of pyridine group, ν(C–N) stretching vibration of azomethine group [16] and ν(C–O) stretching vibration of phenolic hydroxyl group, respectively. After the complex formation, the peak corresponding to ν(C=N) stretching vibration of azomethine group has shifted to a high wave number 1611 cm−1, Δν = (νcomplex − νSchiff base) = 54 cm−1, which implied that the nitrogen atoms of azomethine group in the complex participated in the coordination with the Bi3+ ion [17]. In addition, the ν(C–O) phenolic hydroxyl group of the free ligand displayed a strong absorption peak at 1254 cm−1. However, two absorption peaks appeared at 1238 and 526 cm−1 for the complex, which was an indication of O–Bi3+ and N–Bi3+ bond formation and provided further evidence for coordination via the deprotonated phenolic oxygen atom and the amino nitrogen atom. The absorption peak attributed to ν(C=N) stretching vibration of pyridine group disappeared after the complex formation, which could confirm that the nitrogen atom of pyridine group in the complex was involved in the coordination. The wide peak appeared at 3395 cm−1 because of the superposition of the ν(Ar–NH2) and ν(Ar–OH) stretching vibration in the ligand. When the complex was formed, the absorption peak disappeared, which further indicated that the phenolic hydroxyl group and amino group took part in the coordination.
As can be seen from Tables S3 and S4, the 1H NMR peaks of the complex were very similar to those of the Schiff base ligand except the disappearance of a hydroxyl proton and an amino proton at δ5.13 ppm, which indicated that the hydroxyl group and the amino group from the Schiff base ligand were involved in the coordination to form the complex. 13C NMR peaks of the Schiff base ligand appeared at 162.10 ppm due to the imine carbon, at 56.08 (or 60.79 and 77.24) ppm due to OCH3 carbon and at 196.63 ppm due to the pyridine carbon.
As can be seen from Table S5, Schiff base ligand presented a wide and strong absorption peak 337 nm due to the p–π conjunction and n–π* transition between the lone pair electrons of nitrogen atom in C=N group and the big π bond of benzene ring. Besides, there is another strong absorption peak at 270 nm, which belonged to the p–π conjugation and n–π* transition between the lone pair electrons of the phenolic hydroxyl oxygen atom and benzene ring. With regard to the absorption peak at 245 nm, it was derived from the π–π* transition of the big conjugated bond in the benzene ring. The experimental results showed that the product is a Schiff base ligand. After the bismuth(III) complex formation, the two n–π* transition absorption bands have shifted, which indicated that the phenolic hydroxyl oxygen atom and the nitrogen atom of C=N were coordinated to the Bi3+ ion. Meanwhile, the absorption bands at 370 and 270 nm have blueshifted to 346 and 272 nm, respectively.
As can be seen from Fig. 3 and Table S6, the thermal decomposition and mass losses of the bismuth(III) complex can be divided into two steps. The first step ranges from 50 to 766.5 °C with a mass loss of 27.49%, which resulted from the removal of 1 mol of Cl− and 1 mol of the ligand (C8H7O3) and agreed with the theoretical value 28.06%. The composition of the residue was (BiOC14H15N4O3). The second step was from 766.5 to 1500.0 °C with a mass loss of 42.91%, which owed to the loss of 1 mol of the ligand (C14H15N4O3), and was in agreement with the theoretical value 43.52%. The composition of the residue was Bi2O3 (or BiO1.5).
Based on the results of characterization above, we can finally conclude that the compositions of Schiff base ligand and its bismuth(III) were C22H24N4O5 and BiC22H22N4O5Cl, respectively. The chemical structures and 3D structures of the two newly synthetic compounds are demonstrated in Figs. 4 and 5.
Biothermokinetics of S. pombe and H. pylori cells treated by the Schiff base ligand and its bismuth(III) complex
The growth rate constant (k) of S. pombe and H. pylori
Microcalorimetry was employed to estimate the effects of the ligand and its complex on the growth metabolism of S. pombe and H. pylori. The metabolic thermogenic curves of S. pombe and H. pylori cells treated by the free Schiff base ligand and its bismuth(III) complex at different concentrations were monitored by an isothermal heat conduction microcalorimeter at 32.00 and 37.00 °C, respectively. All microcalorimetric experiments were repeated three times in parallel. The measured metabolic thermogenic curves of S. pombe and H. pylori are plotted in Figs. 6 and 7, respectively.
As shown in Figs. 6 and 7, the shapes of the metabolic thermogenic curves of S. pombe (or H. pylori) are similar to each other except the differences of peak positions and heights caused by the Schiff base ligand and its bismuth(III) complex at different concentrations. The process of the growth metabolism of S. pombe (or H. pylori) can be abstracted into a mathematical model as shown in Figs. 6a and 7a, respectively. The metabolic thermogenic curves could be divided into four phases, i.e., a lag phase (ab), a log phase (bc), a stationary phase (cd) and a decline phase (de). During the log phase, the metabolic thermogenic curves follow the exponential equation below:
where t is the time after the start of log phase, t0 is the start time of log phase, and nt and n0 are the germ or cell number at time t and t0, respectively. If the output power of each germ or cell was w, then
If we defined that \(P_{\text{t}} = n_{\text{t}} w,\;P_{0} = n_{0} w\), then
or
or
Equation (5) is a linear equation, in which Pt is the heat output power of the S. pombe or H. pylori cell at time t and k is the growth rate constant of the S. pombe or H. pylori cell at specified conditions, whose size stranded for the growth speed. Using this equation, the growth rate constant k of S. pombe or H. pylori can be calculated and the results are summarized in Tables 1 and 2, respectively.
From Fig. 6 and Table 1, we can find that the growth rate constant (k) of S. pombe decreased with the increase in concentration of the ligand and the complex, which revealed that both the ligand and the complex exhibited inhibitory effects on the growth metabolism of S. pombe. Similarly, we can see from Fig. 7 and Table 2 that the growth rate constant (k) of H. pylori decreased with the increase in concentrations of the ligand and the complex, suggesting that both the ligand and the complex also owned inhibitory effects on the growth metabolism of H. pylori.
Inhibition ratio (I) and half inhibition concentration (IC50)
The inhibition ratio (I) of the growth metabolism of S. pombe and H. pylori treated by drugs can be calculated as follows:
where k0 is the control rate constant (without any drug treatment) of S. pombe or H. pylori and kc is the growth rate constant of S. pombe or H. pylori treated by an inhibitor at concentration of c. When the inhibition ratio (I) was 50%, the corresponding drug concentration was called as the half inhibition concentration (IC50). The values of I and IC50 are collected in Tables 1 and 2, respectively.
The results showed that both the Schiff base ligand and its complex had significantly inhibitory effects on the growth metabolism of S. pombe and H. pylori cell lines. The IC50 values of the ligand and the complex on the growth metabolism of S. pombe and H. pylori cell lines were found to be 0.2861 and 0.5580 mmol L−1, 1.1939 and 0.4569 mmol L−1, respectively. The inhibitory abilities of the two new compounds on the growth metabolism of cell lines have been observed to follow the order: the Schiff base ligand > the complex for S. pombe and the Schiff base ligand < the complex for H. pylori, respectively. More importantly, the complex could stimulate the growth metabolism of H. pylori cell lines at low concentration, while inhibited its growth at high concentration, which indicated that the complex possessed the bidirectional biological effect and hormesis effect.
Conclusions
In this paper, studies of synthesis and antimicrobial activities of a new Schiff base ligand and its bismuth(III) complex were reported. The antimicrobial activities of the two new compounds on S. pombe and H. pylori cell lines were determined by isothermal heat conduction microcalorimetry. The preliminary results revealed that both the Schiff base ligand and its bismuth(III) complex inhibited the growth of S. pombe and H. pylori, and the half inhibition concentration (IC50) of the two new compounds on the growth metabolism of S. pombe cell lines was 0.2861 and 0.5580 mmol L−1, respectively. In contrast, the values of IC50 of the two new compounds on the growth metabolism of H. pylori cell lines were 1.1939 and 0.4569 mmol L−1, respectively. However, the action mechanism of the Schiff base ligand and its bismuth(III) complex on S. pombe and H. pylori cell lines need to be further studied.
References
Wang Z. Chemical dictionary. Beijing: Chemical Industry Press (CIP); 2010. p. 445.
Li X, Jiang JH, Gu HW, Xiao SX, Li CH, Ye LJ, Li X, Li QG, Xu F, Sun LX. Calorimetric determination of the standard molar enthalpies of formation of o-vanillin and trimethoprim. J Therm Anal Calorim. 2015;119:721–6.
Li X, Jiang JH, Xiao SX, Gu HW, Li CH, Ye LJ, Li X, He DG, Yao FH, Li QG. Synthesis, thermodynamic properties and BSA interaction of a new Valen Shiff base derived from o-vanillin and trimethoprim. Thermochim Acta. 2014;575:291–9.
Xie JQ, Li CH, Dong JX, Qu W, Pan L, Peng ML, Xie MA, Tao X, Yu CM, Zhu Y, Zhang PH, Tang CG, Li QG. The standard molar enthalpy of formation of a new copper(II) Schiff-base complex and its interaction with bovine serum albumin. Thermochim Acta. 2014;598:7–15.
Li X, Jiang JH, Han BX, Gu HW, Xie ZF, Chen L, Xiao SX, Li CH, Li AT, Li X, Yao FH, Wang Q, Li QG. Synthesis and biological activities of o-vanillin-histidine Schiff-base and lanthanum Schiff-base complex. Chem J Chin Univ. 2015;36:856–63.
Li CH, Jiang JH, Li X, Tao LM, Xiao SX, Gu HW, Zhang H, Jiang C, Xie JQ, Peng MN, Pan LL, Xia XM, Li QG. Synthesis, crystal structure and biological properties of a bismuth(III) Schiff-base complex. RSC Adv. 2015;5:94267–75.
Galić N, Cimerman Z, Tomišić V. Spectrometric study of tautomeric and protonation equilibria of o-vanillin Schiff base derivatives and their complexes with Cu(II). Spectrochim Acta, Part A. 2008;71:1274–80.
Li X, Li CH, Jiang JH, Gu HW, Wei DL, Ye LJ, Hu JL, Xiao SX, Guo DC, Li X, Zhang H, Li QG. Synthesis and microcalorimetric determination of the bioactivities of a new Schiff base and its bismuth(III) complex derived from o-vanillin and 2,6-pyridinediamine. J Therm Anal Calorim. 2017;127:1767–76.
Phipps MA, Mackin LA. Application of isothermal microcalorimetry in solid state drug development. Pharm Sci Technol Today. 2000;3:9–17.
Li MX, Yang M, Niu J, Zhang LZ, Xie SQ. A nine-coordinated bismuth(III) complex derived from pentadentate 2,6-diacetylpyridine bis(4 N-methylthiosemicarbazone): crystal structure and both in vitro and in vivo biological evaluation. Inorg Chem. 2012;51:12521–6.
Backes GL, Neumann DM, Jursic BS. Synthesis and antifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides. Bioorg Med Chem. 2014;22:4629–36.
Friedman M, Henika PR, Mandrell RE. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2003;66:1811–21.
Jeewoth T, Bhowon MG, Wah HLK. Synthesis, characterization and antibacterial properties of Schiff bases and Schiff base metal complexes derived from 2,3-diaminopyridine. Transit Metal Chem. 1999;24:445–8.
Li X, Jiang JH, Chen QQ, Xiao SX, Li CH, Gu HW, Zhang H, Hu JL, Yao FH, Li QG. Synthesis of nordihydroguaiaretic acid derivatives and their bioactivities on S. pombe and K562 cell lines. Eur J Med Chem. 2013;62:605–13.
Zhou YM, Ye XR, Xin FB, Xin XQ. Solid state self-assembly synthesis of cobalt(II), nickel(II), copper(II) and zinc(II) complexes with a bis-Schiff base. Transit Metal Chem. 1999;24:118–20.
Ilhan S, Temel H, Yilmaz I, Şekerci M. Synthesis and characterization of new macrocyclic Schiff base derived from 2,6-diaminopyridine and 1,7-bis(2-formylphenyl)-1,4,7 trioxaheptane and its Cu(II), Ni(II), Pb(II), Co(II) and La(III) complexes. Polyhedron. 2007;26:2795–802.
Kaya İ, Bilici A, Gül M. Schiff base substitute polyphenol and its metal complexes derived from o-vanillin with 2,3-diaminopyridine: synthesis, characterization, thermal, and conductivity properties. Polym Adv Technol. 2008;19:1154–63.
Acknowledgements
The authors would like to acknowledge the financial supports from the National Natural Science Foundation of China (Grant Nos. 21273190 and 20973145). Prof. Qiang-Guo Li is the chief-director of these fund projects.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors claim that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Jiang, JH., Gu, HW. et al. Synthesis and biothermokinetic study of a new Schiff base and its bismuth(III) complex on the growth metabolism of S. pombe and H. pylori cell lines. J Therm Anal Calorim 132, 1913–1922 (2018). https://doi.org/10.1007/s10973-018-7101-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7101-6