Abstract
o-Vanillin and trimethoprim are two main raw materials for the synthesis of Valen Schiff bases which have been proved to possess established biological activities and may be used as the antibacterial agent candidates. The standard molar enthalpy of combustion and the standard molar enthalpy of formation of a substance are some of the most fundamental thermodynamic properties, and closely related to other physical, biological, physiological, and chemical properties. However, up to now, no reports about those thermodynamic properties of the title compounds were found in literatures. In this paper, the constant-volume energies of combustion of o-vanillin and trimethoprim at T = 298.15 K and p = 3.01 MPa were measured by a precision oxygen-bomb combustion calorimeter to be Δc U [o-vanillin(s), 298.15 K] = −(24,971.89 ± 22.02) J g−1 and Δc U [trimethoprim(s), 298.15 K] = −(26,366.77 ± 21.96) J g−1, respectively. According to the definition of combustion enthalpy, the standard (p Θ = 0.1 MPa) molar enthalpies of combustion of o-vanillin and trimethoprim were determined to be \( \Delta_{\text{c}} H_{\text{m}}^{\varTheta } \) [o-vanillin(s), 298.15 K] = −(3,800.55 ± 3.35) kJ mol−1 and \( \Delta_{\text{c}} H_{\text{m}}^{\varTheta } \) [trimethoprim(s), 298.15 K] = −(7,657.05 ± 6.38) kJ mol−1, respectively. Finally, the standard (p Θ = 0.1 MPa) molar enthalpies of formation of o-vanillin and trimethoprim were calculated to be \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \) [o-vanillin(s), 298.15 K] = −(490.85 ± 3.51) kJ mol−1 and \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \) [trimethoprim(s), 298.15 K] = −(424.56 ± 6.64) kJ mol−1 from a combination of the experimental values of enthalpies of combustion and some other auxiliary thermodynamic data through a designed thermochemical cycle based on a supposed chemical reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
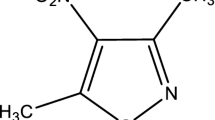
o-Vanillin (2-Hydroxy-3-methoxybenzaldehyde, CAS No. 148-53-8) is an organic solid present in the extracts and essential oils of many plants [1–3]. Its functional groups include aldehyde, ether, and phenol. o-Vanillin, a compound of the formula C8H8O3, is distinctly different from its more prevalent isomer, vanillin. The “o-” prefix refers to position of the compound’s hydroxyl moiety, which is found in the para-position in vanillin (see Fig. 1). Present in a variety of food products, o-vanillin has been proved to have moderate antifungal and antibacterial properties, and thus it has been developed as a potential antibacterial agent candidate in recent years.
Trimethoprim(5-(3,4,5-Trimethoxybenzyl)-2,4-pyrimidinediamine, shown in Fig. 1, CAS No. 738-70-5) is a bacteriostatic antibiotic mainly used in the prophylaxis and treatment of urinary tract infections. The molecular formula of trimethoprim is C14H18N4O3. It belongs to the class of chemotherapeutic agents known as dihydrofolate reductase inhibitors. Trimethoprim was formerly marketed by GlaxoSmithKline under trade names including Proloprim, Monotrim, and Triprim; but these trade names have been licensed to various generic pharmaceutical manufacturers. In clinical use it is often abbreviated as TRI or TMP; its common laboratory abbreviation is W. Trimethoprim is also an antibacterial agent and its curative effect increases when used in combination with sulfonamide drugs such as sulfadiazine or sulfadoxine. It is applicable to many diseases, such as respiratory tract infection, senile chronic bronchitis, bacillary dysentery, urinary tract infection, enteritis, typhia, and malaria, etc [4].
Both of the two compounds mentioned above are the main raw materials for the synthesis of Valen Schiff bases which may possess better antibacterial activities than those of o-vanillin and trimethoprim existence alone. For example, it is reported that the antibacterial activity of o-vanillin is better than that of salicylaldehyde [5]. Likewise, the antibacterial activities of some Valen Schiff bases may also be superior to those of salicylaldehyde Schiff bases [6]. In our previous work, some Valen Schiff bases were designed and synthesized [7, 8]. We have studied the bioactivities of these synthetic Valen Schiff base compounds on biomacromolecules and S. pombe cell lines. The preliminary results indicated that these novel synthetic Valen Schiff base compounds truly possess good bioactivities [9, 10].
The standard molar enthalpy of combustion and the standard molar enthalpy of formation of a substance are some of the most fundamental thermodynamic properties, and closely related to other physical, biological, physiological, and chemical properties [11]. The standard molar enthalpy of formation of a substance is the important data when calculating enthalpy changes, equilibrium constants, and theoretical yields of reactions for which the substance is involved [12]. However, up to now, no reports about those thermodynamic properties of the title compounds were found in literatures. Thus, in the present work, the standard molar enthalpies of combustion and the standard molar enthalpies of formation of the title compounds were investigated by combustion calorimetry.
Experimental
Chemicals
o-Vanillin was purchased from Beishun Chemical Technology Co., Ltd. (Beijing, China). Trimethoprim was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Their labeled mass fraction purities were ≥99.0 and ≥98.0 %, respectively. After purification, the actual mass fraction purities of two samples were determined to be greater than 99.5 % by HPLC method. Furthermore, melt points, NMR, MS, IR, and UV spectra were employed to verify the compositions and structures of the compounds. The analytical results (see the Supplemental Materials) confirmed the reliability of the samples used in this calorimetric research.
Calorimetric experiments
Standardizing the calorimeter
The constant-volume energies of combustion of the samples were obtained from combustion calorimetry with an oxygen-bomb calorimeter (Parr 6400, Parr Instrument Company, Illinois, USA). Benzoic acid (NIST Thermochemical Standard 39j) with a certified massic energy of combustion, under bomb conditions of −(26,434 ± 3) J g−1 [13], was used for calibration of the bomb. The procedure for a standardization test is exactly the same as for testing a fuel sample. Use a pellet of calorific grade benzoic acid weighing not less than 0.9 or more than 1.1 g. The corrected temperature rise, T, is determined from the observed test data and the bomb washings are titrated to determine the nitric acid correction. The energy equivalent of the calorimeter is calculated according to the following equation [14]:
where ε calor is the energy equivalent of the calorimeter in cal K−1; H is the heat of combustion of the standard benzoic acid sample in cal g−1; m is mass of the sample in g. T is the temperature rise in K. e 1 is the correction for heat of formation of nitric acid in cal. The default value in the Parr 6400 is 8 cal. e 2 is the correction for sulfur which is usually 0 cal. e 3 is the correction for heating wire and combustion of cotton thread. The default value in the Parr 6400 is 50 cal. From ten independent calibration experiments, the value of the energy equivalent of the calorimeter was determined as ε calor = (3,893.41 ± 1.32) J K−1.
Determine the constant-volume energies of combustion of the samples
The sample was pressed into pellets (about 0.6 g) for each experiment of combustion and burned under an oxygen pressure of 3.01 MPa in the presence of 0.001 dm3 of distilled water in the bomb to ensure equilibrium in the final state after the combustion. The calorimeter temperatures were measured to ±1 × 10−4 K, at intervals of 10 s, with a quartz crystal thermometer (Hewlett-Packard HP 2804 A), interfaced to a PC. Data acquisition, control of the calorimeter temperature, and the calculation of the adiabatic temperature change were performed using the program LABTERMO [15].
Thermochemical corrections
In the high pressure oxygen environment within the oxygen bomb, nitrogen that was present as part of the air trapped in the bomb is burned to nitric oxide which combines with water vapor to form nitric acid. All of this heat is artificial since it is not a result of the sample burning. The nitric acid correction removes this excess heat from the calculation. Nitric acid is that portion of the total acid in the bomb washings that result when the nitrogen in the air that is trapped in the bomb is burned at high pressure. Since this nitric acid does not result from the sample, and the combustion conditions are reasonably constant from test to test, the amount of nitric acid formed is also constant. In this work, the acid correction is a fixed value set by the operator. The calculation is: e 1 = (nitric acid value) × (acid multiplier) × (heat of formation of nitric acid). For a 1138 bomb, the default nitric acid value is 8 and acid multiplier is 0.0709. The heat of formation of nitric acid is 14.1 cal per milliequivalent so the calculation is: e 1 = 8 × 0.0709 × 14.1 ≈ 8 cal = 33.484 J.
For the fuse correction in the Parr 6400, by default a fixed fuse correction of e 3 = 50 cal is applied to all tests. When using the Parr 6400, there are two components to the fuse correction: (1) The heat introduced by heating the wire used to ignite the cotton thread; (2) The heat of combustion of the cotton thread used to ignite the sample. The semi-permanent heating wire is heated by dissipating an electrical charge from a capacitor. Since this charge is controlled by the size of the capacitor and the charging voltage, and because the capacitor is fully discharged for each test, the energy released can be calculated. In the Parr 6400 calorimeter, this is a fixed correction of 10 cal per test. Cotton has a heat of combustion of 4,000 cal g−1. Ten centimeters of a fine thread supplied by Parr will weigh ~0.010 g which would release 40 cal as it burns. Using the fine thread mentioned above, the fuse correction for the calorimeter would be the 10 cal from electrical heating plus 40 cal from the burning thread for a total of 50 cal (about 209.34 J) per test.
For each compound, (∂U/∂p) T , at T = 298.15 K, was assumed to be −0.21 J g−1 MPa−1, a typical value for organic compounds [16]. Standard state corrections were calculated for the initial states by the procedures given by Hubbard et al. [17] and by Good and Scott [18]. The relative atomic masses of the elements were those recommended by the IUPAC commission in 2011 [19].
Results and discussion
Constant-volume energies of combustion and standard molar enthalpies of combustion of the title compounds
The method for determining the constant-volume energies of combustion of o-vanillin and trimethoprim were the same as that used in the standardization of the combustion calorimeter with benzoic acid. The calculation for the gross heat of combustion is done by the following equation [14]:
where −Δc U is the gross heat of combustion; ε calor is the energy equivalent of the calorimeter being used; Δc T is the observed temperature rise; e 1 is the heat produced by burning the nitrogen portion of the air trapped in the bomb to form nitric acid; e 2 is the correction for sulfur which is usually 0; e 3 is the correction for heating wire and combustion of cotton thread.; and W is the mass of the test samples.
The measured results of the constant-volume energies, −Δc U (J g−1), of combustion of two samples were listed in Tables 1 and 2. At T = 298.15 K, the differential quotient of the constant-volume energy of combustion with the oxygen pressure, (∂U/∂p)T = −0.21 J g−1 MPa−1, a typical value for most solid organic compounds, was assumed [16]. According to the correction procedures for standard state proposed by Hubbard et al. [17, 18], the pressure in the oxygen bomb in the combustion of two samples were corrected from (3.01 to 0.1) MPa (standard pressure), ΔP = −2.91 MPa. Then, the change of the constant-volume energies of combustion of two samples Δ(Δc U) was calculated to be 0.61 J g−1. Therefore, the standard (p Θ = 0.1 MPa) constant-volume energies of combustion of the o-vanillin and trimethoprim were corrected to Δc U Θ [o-vanillin(s), 298.15 K] = Δc U [o-vanillin(s), 298.15 K]+Δ(Δc U) = −(24,971.89 ± 22.02) J g−1+0.61 J g−1 = −(24,971.28 ± 22.02) J g−1 and Δ c U Θ [trimethoprim(s), 298.15 K] = Δc U [trimethoprim(s), 298.15 K] + Δ(Δc U) = −(26,366.77 ± 21.96) J g−1+0.61 J g−1 = −(26,366.16 ± 21.96) J g−1, respectively.
The total uncertainty of the mean value of Δc U Θ(J g−1) has been estimated to be within ±0.5 %, mainly accounting the uncertainties of the energy equivalent, temperature rise, e 1, e 2, e 3, and correction of the standard state value during the combustion of two samples, and so on.
The standard molar enthalpies of combustion of the sample (\( \Delta_{\text{c}} H_{\text{m}}^{\varTheta } \)) referred to the enthalpy change of the following reaction at 298.15 K and 0.1 MPa, respectively.
The standard molar enthalpies of combustion of two samples can be derived from the constant-volume energies of combustion by Eqs. (5) and (6):
where Σn i was the total amount (in moles) of the gases present as products or as reactants. The standard molar enthalpies of combustion of two samples were calculated to be as follows, respectively.
and
Standard molar enthalpies of formation of the title compounds
A reaction scheme used to determine the standard molar enthalpies of formation, \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \) [C8H8O3(s)] and \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \) [C14H18N4O3(s)], of two samples were shown in Tables 3 and 4, respectively.
\( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \)[C8H8O3(s)] was calculated by a designed Hess’ thermochemical cycle according to the reaction (3) as follows:
\( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \)[C14H18N4O3(s)] was calculated by a designed Hess’ thermochemical cycle according to the reaction (4) as follows:
In the Eqs. (7) and (8), the standard molar enthalpies of formation of CO2 (g) and H2O (l), recommended by CODATA [20, 21], \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \)[CO2(g)] = −(393.51 ± 0.13) kJ mol−1 and \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \)[H2O(l)] = −(285.83 ± 0.04) kJ mol−1, were employed in the calculation of \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \)[C8H8O3(s)] and \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \)[C14H18N4O3(s)].
Finally, the standard (p Θ = 0.1 MPa) molar enthalpies of formation of the title compounds can be derived to be:
and
Conclusions
In this work, the standard (p Θ = 0.1 MPa) molar enthalpy of combustion of o-vanillin and trimethoprim at T = 298.15 K were estimated to be \( \Delta_{\text{c}} H_{\text{m}}^{\varTheta } \) [o-vanillin(s), 298.15 K] = −(3,800.55 ± 3.35) kJ mol−1 and \( \Delta_{\text{c}} H_{m}^{\varTheta } \) [trimethoprim(s), 298.15 K] = −(7,657.05 ± 6.38) kJ mol−1, respectively, by using a precision oxygen-bomb combustion calorimeter. And the standard (p Θ = 0.1 MPa) molar enthalpy of formation of these two compounds at T = 298.15 K were calculated to \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \) [o-vanillin(s), 298.15 K] = −(490.85 ± 3.51) kJ mol−1 and \( \Delta_{\text{f}} H_{\text{m}}^{\varTheta } \) [trimethoprim(s), 298.15 K] = −(424.56 ± 6.64) kJ mol−1 from a combination of the experimental values of enthalpies of combustion and some other auxiliary thermodynamic data through a designed thermochemical cycle based on a supposed chemical reaction.
The present work demonstrated that isoperibol oxygen-bomb combustion calorimetry is a very useful tool for thermodynamic research, as it is capable of providing accurate thermodynamic quantities of many important substances in industrial and scientific research. o-Vanillin and trimethoprim are two main raw materials for synthesis of Valen Schiff bases. This work provides some useful thermodynamic data for these two compounds that this information will enrich and develop the fundamental thermodynamic database of Valen Schiff bases and consequently could have theoretical instructive significance and application values for the research and development of Valen Schiff bases complexes.
References
Abou Zeid AH, Sleem AA. Natural and stress constituents from Spinacia oleracea L. leaves and their biological activities. Bull Fac Pharm (Cairo University). 2002;40:153–67.
Barbe JC, Bertrand A. Quantitative analysis of volatile compounds stemming from oak wood. Application to the aging of wines in barrels. J Sci Technol Tonnellerie. 1996;2:83–8.
Brunke EJ, Hammerschmidt FJ, Schmaus G. Das etherische Öl von Santolina chamaecyparissus L. (Santolina chamaecyparissus essential oil). Parfümerie und Kosmetik. 1992;73:617–8, 623–4, 626, 628–30, 632, 634–7.
Goldman JL, Jackson MA, Herigon JC, Hersh AL, Shapiro DJ, Leeder JS. Trends in adverse reactions to trimethoprim-sulfamethoxazole. Pediatrics. 2013;131:e103–8.
Friedman M, Henika PR, Mandrell RE. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2003;66:1811–21.
Jeewoth T, Bhowon MG, Wah HLK. Synthesis, characterization and antibacterial properties of Schiff bases and Schiff base metal complexes derived from 2,3-diaminopyridine. Transit Met Chem. 1999;24:445–8.
Li CH, Tao LM, Xiao SX, Li AT, Jiang JH, Li X, Yao FH, Luo WP, Xie JQ, Peng MN, Pan L, Li QG. Thermochemical study on the Schiff base [H2salen = N,N-bis(salicylidene) ethylenediamine] and its binuclear copper (II) complex. Thermochim Acta. 2013;569:97–103.
Li CH, Song XZ, Jiang JH, Gu HW, Tao LM, Yang P, Li X, Xiao SX, Yao FH, Liu WQ, Xie JQ, Peng MN, Pan L, Xu XB, Jiang C, Wang S, Xu MF, Li QG. Synthesis, crystal structure and thermodynamic properties of a new praseodymium Schiff-base complex. Thermochim Acta. 2014;581:118–22.
Jiang JH, Li X, Xiao SX, Gu HW, Li CH, Yang P, Wei DL, He DG, Li AT, Li X, Yao FH, Li QG. Interaction of 2-{[4-amino-5-(3, 4, 5-trimethoxy-benzyl)-pyrimidin-2-ylimino]-methyl}-6- methoxy-phenol with S. pombe cells and BSA. Chem J Chin Univ. 2014;35:831–8.
Li CH, Song XZ, Jiang JH, Yang P, Yao HF, Li X, Xiao SX, Xie JQ, Peng MN, Pan L, Wu XB, Peng X, Tang CG, Zhang PH, Jiang C, Liu WQ, Li QG. Synthesis, thermochemical and thermokinetic studies on the mononuclear copper Valen Schiff base complex. Sci China Chem. 2014;44:981–8.
Di YY, Yang WW, Kong YX, Shi Q, Tan ZC. Low-temperature heat capacities and standard molar enthalpy of formation of L-3-(3,4-dihydroxyphenyl) alanine (C9H11NO4). J Chem Eng Data. 2008;53:900–4.
Gu HW, Xiao SX, Xiao HY, Xiao Y, Li AT, Hu XL, Li QG. Synthesis, characterization, and thermodynamic properties of the rare earth coordination complex [Sm(C6H4NO2)2·C9H6NO]. Ind Eng Chem Res. 2012;51:4797–803.
Ribeiro da Silva MAV, Santos AFLOM. Thermochemical study of 2-and 3-alkyl substituted thiophenes. J Therm Anal Calorim. 2007;88:7–17.
Operating instruction manual for oxygen bomb calorimeter 6400. Parr Instrument Company.
Santos LMNBF, Silva MT, Schröder B, Gomes L. Labtermo: methodologies for the calculation of the corrected temperature rise in isoperibol calorimetry. J Therm Anal Calorim. 2007;89:175–80.
Almeida ARRP, Matos MAR, Morais VMF, Monte MJS. A calorimetric and computational study of aminomethoxybenzoic acids. J Phys Chem B. 2010;114:11570–5.
Hubbard WN, Scott DW, Waddington G, in Rossini FD (Ed.), Experimental thermochemistry, Vol. 1, New York: Interscience; 1956, Chapter 5.
Good WD, Scott DW, in Skinner HA (Ed.), Experimental thermochemistry, Vol. 2, New York: Interscience; 1962, Chapter 2.
Wieser ME. Atomic weights of the elements 2011 (IUPAC Technical Report). Pure Appl Chem. 2013;85:1047–78.
Report of the CODATA task group on key values for thermodynamics. J Chem Thermodyn. 1978;10:903–906.
Cox JD, Wagman DD, Medvedev VA. CODATA key values for thermodynamics. New York: Hemisphere Publishing Corp; 1984.
Acknowledgements
This work was financially supported by the National Natural Science Foundations of China (No. 21273190), the Educational Committee Foundation of Hunan Province (No. 11A112 and No. 10C1233), and the Science and Technology Department Foundation of Hunan Province (No. 2011TP4016-1, No. 2012TP4021-5 and No. 2013FJ3033) as well as the Construct Program of the Key Discipline in Hunan Province. Prof. Qiang-Guo Li (liqiangguo@163.com) is the chief-director of these fund projects.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Jiang, JH., Gu, HW. et al. Calorimetric determination of the standard molar enthalpies of formation of o-vanillin and trimethoprim. J Therm Anal Calorim 119, 721–726 (2015). https://doi.org/10.1007/s10973-014-4184-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4184-6