Abstract
The co-combustion characteristics of municipal sewage sludge and bituminous coal in air and O2/CO2 atmospheres were assessed by using a thermogravimetric analysis approach between heating rates of 20 and 80 K min−1. The combustion characteristics of sewage sludge/bituminous coal blend (50% blending ratio) in air were also compared with its oxy-fuel behavior. In air atmosphere, the ignition temperatures for sludge/coal blends were between those for their parent samples, which ranged from 238 to 418 °C. In O2/CO2 atmosphere with oxygen content of 30%, the ignition temperatures of 50%sewage sludge/50%coal blend were between 260 and 275 °C, which were less than those (268–280 °C) in air. The ignition and burnout indexes of the sample in 30%O2/70%CO2 atmosphere were very close to those in air. The temperatures with respect to maximum combustion rates of the 50%sewage sludge/50%coal blend in 40%O2/60%CO2 were lower than those in air. The average value of the activation energy for the 50%sewage sludge/50%coal blend in 30%O2/70%CO2 was less than that in air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sewage sludge is the residue produced by the wastewater treatment [1]. Especially in recent years, the production amounts of the sewage sludge around worldwide from wastewater treatment plants increase greatly [2]. The annual production of municipal wastewater and dewatered sewage in China increases about 5% on average [3], and there are more than four million tons (dry weight) of municipal SS produced annually in China [4]. The average production of dry sewage sludge through treatment in Europe is about 90 g per person each day [1]. Sewage sludge is a heterogeneous mixture made up of microorganisms, undigested organics, inorganic materials and moisture [5].
The co-combustion technique of sewage sludge with coal may be a secure outlet and can give rise to high profits from energy [6]. The co-combustion of sludge together with coal in existing infrastructures should be an interesting option, since the facilities are already equipped with appropriate devices for emission control [7]. McIlveen-Wright et al. [8] stated that the co-combustion of coal with sludge can contribute to lower CO2 emissions and fuel costs compared with the combustion from the coal individual. Much investigation on co-combustion behavior of sewage sludge and coal has been conducted [9,10,11].
Moreover, oxy-fuel combustion technology as one of the new promising technologies has been widely adopted in coal-fired power plants [12, 13], because it can lead to high CO2 content (over 95%) in flue gas, which is helpful to capturing CO2 [14, 15]. Also, the oxy-fuel combustion manner can lead to high combustion efficiency due to low heat loss [12], which can be realized easily in existing combustion system. Contreras et al. [16] noted that the temperatures where maximum combustion rates present for several kinds of biomass and coal in 40%O2/60%CO2 are lower than those in conventional air combustion. Sewage sludge combustion can cause high NOx and SO2 emissions due to its high nitrogen and sulfur contents [17,18,19]. In particular, thermal NOx and prompt NOx are affected by the presence of N2 atmosphere and temperature. The oxy-fuel combustion manner can reduce NOx and SO2 emissions in the combustion process of sewage sludge. Shao et al. [18] addressed that sludge combustion under an 80%CO2/20%O2 atmosphere might simultaneously reduce SO2 and NOx emissions compared with that under 80%N2/20%O2 atmosphere. Based on non-isothermal thermogravimetric analysis, majority of investigations are focused on the oxy-fuel combustion behaviors of different solid fuels such as coal [20,21,22], biomass [22, 23], sewage sludge [24], petrochemical wastewater sludge [25] and other materials like plastic, rubber and leather [26]. Yi et al. [20] highlighted the oxy-fuel combustion characteristics of 28 kinds of Chinese coals under three O2 concentrations of 21, 30 and 100%, and then stated that O2 concentration in coal combustion with a high fuel ratio has to be increased to more than 30% to promote combustion. Chen et al. [25] indicated that oxy-fuel combustion rates and combustion performance indexes of petrochemical wastewater sludge sample grow up with an increasing of the oxygen content, and stated that the most appropriate kinetic models for various decomposition steps are the order reaction models based on Coats–Redfern approach. Zhou et al. [27] also indicated that the increasing of O2 concentration in CO2/O2 atmosphere can significantly improve the combustion performance of lignite, especially at the O2 concentrations below 60%. They also pointed out that the activation energy for the lignite combustion in O2/N2 atmosphere is higher than that in O2/CO2 atmosphere according to the Kissinger–Akahira–Sunose isoconversional method.
Coimba et al. [7] detected the combustion behavior of one bituminous coal and one pulp mill sludge and their blend (sludge 10 mass%) by using a TG technique, and estimated their apparent activation energy based on the application of the Vyazovkin kinetic model. Folgueras et al. [28] also using the TG technique addressed the co-combustion of one bituminous coal and dried sewage sludge (50 mass%) and noted that in the lower-temperature region (T < 350 °C), the reactivity for the blend is similar to that of sewage sludge, while that in the higher-temperature region (T > 350 °C) is close to that of the coal. Yanfen and Xiaoqian [29] revealed the co-combustion kinetics of semi-anthracite coal and paper sludge in a differential thermogravimetric analyzer at three heating rates (10, 20 and 30 K min−1). And then they stated that the value of activation energy for paper sludge is less than that of semi-anthracite coal at low conversion degree, while there is an opposite trend in the main combustion stage, and that the blends show the integrative thermal profiles of two individuals, which depend on the blending ratio of sludge. Kijo-Kleczkowska et al. [30] stated that adding sewage sludge into hard coal and lignite can shorten the combustion time compared with coal individual. Sewage sludge is characterized by high moisture and ash contents, and low calorific value and significant concentrations of harmful substances (chlorine, sulfur, metals, etc.) [30]. The optimized fuel combustion and co-combustion technique with coal can reduce pollution emissions of sewage sludge as a fuel. Sludge samples with moisture contents up to 40–50 mass% are acceptable in coal-firing boilers [31].
So far, comprehensive evaluation about the influence of blending ratio, heating rate on co-combustion performance and kinetics of municipal sewage sludge with coal is still insufficient. In particular, the oxy-fuel combustion kinetics of sewage sludge blending with coal has not been reported. Majority of works about the oxy-fuel combustion behavior of solid fuels are focused on coal samples.
The current work presents co-combustion behavior of typical Chinese municipal sewage sludge and bituminous coal with different blending ratios (20, 50, 80% for the sludge) at certain heating rates (20–80 K min−1). In addition, the oxy-fuel combustion characteristics for sewage sludge/coal blend are compared with those in air atmosphere. The purpose is mainly to address co-combustion performance and kinetics of sewage sludge and bituminous coal.
Methods
Experimental facility and test samples
A thermogravimetric analyzer (TGA/SDTA851, Mettler Toledo) with a precision of 0.001 mg was utilized to conduct the combustion tests. The raw municipal sewage sludge through mechanical dewatering treatment was obtained from a wastewater treatment plant (Beijing, China). The initial moisture content of the sample was about 82.6% (wet base). A kind of Chinese bituminous coal (Nei Monggol, China) was collected from a power plant (Tianjin, China), and then it was through-air-dried for a long period before testing. The raw sewage sludge was put into an oven (KUNTIAN; 101-00B, China) for 12 h. After that, the lump coal and sewage sludge each was crushed to 0.1–0.4 mm. The individual samples were sieved through a standard screen (80 meshes). The sludge/coal blends were shaken thoroughly in a box with sludge mass percentages of 20, 50 and 80%, respectively. In this work, 20SS80C, 50SS50C and 80SS20C refer to 20%sewage sludge/80%coal blend, 50%sewage sludge/50%coal blend, and 80%sewage sludge/20%coal blend, respectively. Also, 100SS and 100C refer to sewage sludge and coal individuals. The sample (10 mg) was placed in the crucible. The temperature range for all tests was set between 25 and 1000 °C at four heating rates—20, 40, 60 and 80 K min−1. The combustion behaviors of all samples in air atmosphere were carried out, and the flow rate of air was set at 100 mL min−1. The combustion behaviors of sludge/coal blend with 50% blending percentage in CO2/O2 atmosphere were conducted. The total flow rate of carbon dioxide and oxygen was set at 100 mL min−1, in which oxygen flow rate was taken as 21, 30 and 40 mL min−1, respectively.
The proximate analyses of the samples were evaluated according to ASTM D 3172-89 standard. The ultimate analyses of the samples were examined in an Element Analyzer (Vario EL III, ELEMENTAR). The heating values for the two samples were determined through an oxygen bomb calorimeter (Parr 1281, PARR Instrument, America). The proximate and ultimate analyses are given in Table 1.
The uncertainties of temperature and mass in this work were calculated as follows:
Temperature: \(\pm \,\frac{\delta T}{T} = \pm \,\frac{0.01/2\sqrt 3 }{30} = \pm \,0.01\%\)
Mass: \(\pm \,\frac{\delta m}{m} = \pm \,\frac{0.001/2\sqrt 3 }{10} = \pm \,0.003\%\)
Combustion performance
Ignition index (D i) and burnout index (D b) can be used to evaluate combustion characteristics of solid fuels, which can be given by [33, 34]
where DTGmax is the maximum burning rate (mg min−1), t i the moment regarding to the ignition (min), t p the moment regarding to the maximum combustion rate (min), t b the moment regarding to the burnout (min), ∆t 1/2 the time zone of DTG/DTGmax = 1/2 (min).
Also, the effects of the heating rates and the blending ratios of sewage sludge on the combustion performance for samples, and the effects of the oxygen contents on the oxy-fuel combustion performance of 50SS50C, were analyzed by applying a two-way analysis of variance (ANOVA) [35].
Kinetic analysis
The kinetic parameters are determined by the Ozawa–Flynn–Wall method along with Coats–Redfern equation [36, 37]:
where β indicates the heating rate (K min−1), A the pre-exponential factor (s−1), E a the activation energy (J mol−1), g(α) the integral mechanism function, α the conversion degree of the sample, R the universal gas constant (J mol−1K−1), and T the absolute temperature (K). The specific method for evaluating the kinetic parameters is the same with the previous work [38].
Results and discussion
TG and DTG profiles
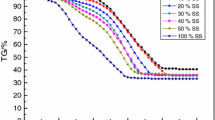
TG (mass fraction of samples versus temperature) and DTG (burning rate of samples versus temperature) profiles for combustion processes of sewage sludge, bituminous coal and their blend at air and oxy-fuel atmospheres are obtained, as shown in Fig. 1.
From Fig. 1a, there is obvious distinction between combustion behavior of sewage sludge and bituminous coal. Between heating rates of 20 and 80 K min−1, the total mass conversions for the sewage sludge during the combustion are much less than those of the coal sample, which can be attributed to high ash content in the sewage sludge sample. There are two obvious peaks in the main combustion region of the sewage sludge and sludge/coal blends with blending ratios of sludge over 20%, while only one peak for the coal sample and 20SS80C blend, which can be attributed to high volatile substance content in the sewage sludge compared with the coal sample. The first peak occurred for the samples could be caused by the decomposition of complex organic structures [39]. Fixed carbon compositions play a major role during pulverized coal combustion [40]. Yu and Li [40] and Folgueras et al. [28] also obtained the similar results on combustion behavior of dried sludge and bituminous coal. As expected, the conversion rates of sewage sludge/coal blend at the first peaks located at the temperature range of 200–400 °C go up with increasing the blending ratio of sewage sludge, while those at the second peaks within the temperature range of 400–700 °C decrease. The reason could be attributed to that burning of volatile materials combustion would cause more ash formed by volatile combustion in the blends to be packed on the outer surface of the fixed carbon. According to a thermogravimetric assessment on co-combustion of an anthracite coal and sewage sludge, Otero et al. [41] also gave the similar trend within a narrow blending ratio range of 2–10% for the sewage sludge.
From Fig. 1b, for sewage sludge/coal blend with blending ratio of 50%, the oxy-fuel burning process of the sample at high oxygen content in O2/CO2 atmosphere is ahead of that at low oxygen content. For the reason, at certain total atmosphere pressure, CO2 partial pressure decreases with increasing the O2 partial pressure due to increasing the oxygen content, which would lead to smaller temperature reduction for the sample because of less thermal energy taken away by CO2 [25]. The other results are also similar with those of sewage sludge individual reported in the previous work [38]. In comparison with 40%O2/60%CO2 atmosphere, the combustion process of the blend sample in air is delayed. However, the combustion profile of the blend in air is very similar to that in 30%O2/70%CO2 atmosphere. In comparison with air atmosphere, the burning process of the blend in O2/CO2 atmosphere at analogous oxygen content is delayed, which is attributed to the fact that the CO2 energy absorption reduces flame temperature in the chamber [23, 34]. Between heating rates of 20 and 80 K min−1, the average maximum combustion rate of the blend in air is about 30% higher than that in 21%O2/79%CO2. Yuzbasi and Selçuk [14, 42] also addressed the similar trend on oxy-fuel co-combustion characteristics of biomass and lignite.
Moreover, from Fig. 1, the TG profiles shifts to a lower temperature with increasing the blending ratio of sewage sludge in sewage sludge/high-rank bituminous coal and O2/CO2 ratio, which is similar to that noted by Zhang et al. [24].
Combustion indices
The combustion performance parameters of the samples are shown in Table 2 and Fig. 2. According to the ANOVA method, the values for the influence degrees of main factor on the combustion performance of the samples are depicted in Table S1 and Table S2.
As seen in Table 2, in air atmosphere, the ignition temperatures for the sewage sludge are between 235 and 253 °C, while those for the bituminous coal are between 420 and 438 °C, which are much higher than those of the sludge due to its high volatile substance contents [40]. Just as expected, the ignition temperatures for sludge/coal blends are between those for their parent samples, which range from 238 to 418 °C. According to investigation on the co-combustion of semi-anthracite coal and paper mill sludge, Yanfen and Xiaoqian [29] also gave similar results and emphasized that the low ignition temperature of the sludge sample could be caused by de-volatilization and combustion of the organic components in it. Also, by comparison with the coal, the burnout temperature of the sewage sludge is low due to low reactivity of char caused by its small fixed carbon content [23]. As expected, the values for the burnout temperature of sludge/coal blends decrease with increasing the blending ratio of the sludge. Consequently, the ignition and burnout indexes of sludge/coal are improved with increasing both the blending ratio of sludge and heating rate. Chen et al. [25] also revealed that the higher heating rate could result in a better ignition and burnout performance of a petrochemical wastewater sludge. In addition, between heating rates of 20 and 80 K min−1, the average values for the ignition and burnout indexes of the sludge individual in air atmosphere are 1.3 and 4.5 times higher than those for the coal individual, respectively. As for 50SS50C blend, between heating rates of 20 and 80 K min−1, its average ignition and burnout indexes in air atmosphere increase by 20 and 40% compared with those for the coal individual, respectively. Also seen from Table 2, the ignition and burnout temperatures for 50SS50C decrease with increasing the oxygen content in O2/CO2 atmospheres, while the ignition and burnout temperatures in 21% O2/79%CO2 atmosphere are higher than those in air. In O2/CO2 atmospheres with oxygen content of 30%, the ignition temperatures of 50SS50C are between 260 and 275 °C, which are less than those (268–280 °C) in air. Chen et al. [43] also indicated that the ignition and burnout temperatures of microalgae Chlorella vulgaris decrease gradually as the oxygen content in O2/CO2 atmospheres increases. According to investigation on the oxy-fuel combustion behavior of lignite, Zhou et al. [27] stated that increasing the O2 concentration is more effective way on the volatile matter combustion. In addition, as seen in Table 2 the temperatures with respect to maximum combustion rates of the 50SS50C blend present in 40%O2/60%CO2 are lower than those in conventional air combustion, and the temperature reductions are in the order of 15–50 °C. Contreras et al. [16] also gave the similar trend based on examining the oxy-fuel combustion behavior of one sub-bituminous coal and two Spanish biomasses (olive grove and thistle) in lab- and bench-scale combustors. From Fig. 2, the ignition and burnout indexes of 50SS50C are improved with increasing the oxygen content. The ignition and burnout indexes of the sample in 40%O2/60%CO2 atmosphere are about 4 and 1 times higher than those in 21%O2/79%CO2 atmosphere, respectively, and the ignition and burnout indexes of the sample in 30%O2/70%CO2 atmosphere are about 2 and 0.5 times higher than those in 21%O2/79%CO2 atmosphere, respectively. Chen et al. [25] also addressed the similar results according to investigating the oxy-fuel combustion of a petrochemical wastewater sludge and stated that higher O2 concentration could enhance the combustion of remaining chars. In addition, the ignition and burnout indexes of the sample in 30%O2/70%CO2 atmosphere are very close to those in air, while the ignition and burnout indexes of the sample in air are 1.5 and 0.5 times higher than those in 21%O2/79%CO2 atmosphere. In addition, Zhang et al. [24] also indicated that the combustion property indexes of sewage sludge/coal increase with increasing SS blended ratio and O2/CO2 ratio.
From Table S1 and Table S2, the influences of both heating rates and blending ratios on ignition and burnout and ignition temperatures of the samples are important (P < 0.05). In particular, the effect of the blending ratios on the ignition temperatures of the samples is much significant (P ≪ 0.05), while the effect of the heating rates on the burnout temperatures is much significant (P ≪ 0.05). Moreover, the influence of heating rates on the ignition and burnout indexes is also important (P < 0.05). As for the oxy-fuel combustion, the influences of both heating rates and oxygen contents on ignition and burnout and ignition temperatures of the samples are important (P < 0.05). The influence of heating rates on both the ignition and burnout indexes is also important (P < 0.05), while the effect of the oxygen contents on the two indexes is not important.
Kinetic parameters
Figure 1 presents that the temperature ranges to pre-peak periods around maximum combustion values (DTG) in the char combustion for 50SS50C at 20, 40, 60 and 80 K min−1 are 440–500, 460–535, 470–560 and 475–575 °C, respectively, while conversion degrees are 0.35–0.62, 0.35–0.63, 0.35–0.63 and 0.35–0.62. Therefore, conversion degrees intersection at the pre-peak periods for the four heating rates can be taken as 0.35–0.62 (440–500 °C for 20 K min−1), and then the average conversion degree is determined as 0.49. The linear fitting is shown in Fig. 3a by plotting lg (β) versus 1/T based on Eq. (3) at α = 0.49. The reference value of activation energy of the sample is obtained, which is 123.34 kJ mol−1. According to Eq. (4), by plotting ln(g(α)/T 2) versus 1/T as shown in Fig. 3b, the dominant mechanism is fitted to order-based reaction equation (n = 4), g(α) = [(1−α)−3−1]/3. Therefore, the real activation energy and pre-exponential factor of the sample are gained, 127.91 kJ mol−1 and 1.79 × 107 s−1. The method can be applied to the other cases. The results are presented in Table S3. The average values for the activation energy of the releasing and combustion of volatile matters and those for the char combustion of all samples are listed in Table 3. And the average values for the activation energy of the releasing and combustion of volatile matters, and those for the char combustion of the 50SS50C blend at each O2/CO2 atmosphere are also given in Table 3.
From Table S3, the dominant mechanisms related to release and combustion kinetics for volatile matters of sewage sludge, 80SS20C and 50SS50C are described by the Avrami–Erofeev equations (m = 3, 4), while those for their char combustion kinetics are also described by the Avrami–Erofeev equations (m = 2, 3, 4) or order-based reaction equation (n = 4) or Jander model (3D diffusion, m = 2) or G-B equation (3D diffusion). The Avrami–Erofeev equations are controlled by the random nuclear producing and growing process, which belong to sigmoidal rate models [44] and represent processes whose initial and final stages demonstrate, respectively, the accelerating and decelerating behaviors so that the process rate reaches its maximum at some intermediate values of the extent of conversion [45]. The initially generated germ nucleus at some imperfection in the reactants may develop into a growth nucleus, which grows in size by the advancing of the reactant–product interface into the crystallite [46, 47]. In the order-based reaction equations, the reaction rate is proportional to the concentration or fraction of remaining reactants and the rate-determining step is the chemical reaction [48], in which the reaction rate can be described by certain simple reaction order [46]. The Jander equations are used in diffusion-controlled solid-state reaction kinetics in a sphere, where diffusion in all three directions is all-important [49], and the reaction rate decreases with the increasing thickness of the product barrier layer, and the control equation for diffusion mechanism is related to the mass of reactants that go through the product layer. The G-B model is also a function for a diffusion-controlled reaction starting on the exterior of a spherical particle [49]. The dominant combustion mechanisms related to char of the coal or 20SS80C at pre-peak periods for heating rates of 60 and 80 K min−1 are described by the Avrami–Erofeev equations (m = 2, 3), while those for heating rates of 20 and 40 K min−1 are described by Valensi model (2D diffusion) or order-based reaction model (n = 4) or parabola rule model (1D diffusion) or Avrami–Erofeev equations (m = 2). The Valensi model is for a two-dimensional diffusion-controlled process into a cylinder [49]. The 1D diffusion is governed by a parabolic, which means that no concentration gradient can occur in any other dimension. Also, the 1D diffusion mechanism describes a process where the interface area is constant and the diminution of reaction rate is a consequence of increasing thickness of the diffusion barrier [50]. The dominant combustion mechanisms related to char of the coal or 20SS80C at post-peak periods are described by spherical symmetrical model, Valensi equation or order-based reaction equation (n = 0, 1) or Jander model. The spherical symmetrical equation is controlled by shrinking core process. The spherical symmetrical model known as geometric contraction is used for a sphere (a three-dimensional phase boundary reaction), which reacts from the surface inward. Its mechanism is sometimes assumed to be the governing conversion model in the combustion of some carbonaceous materials [50]. The dominant mechanisms related to releasing and combustion kinetics for volatile matters of 50SS50C in oxy-fuel atmospheres are described by the Avrami–Erofeev equations (m = 4). The dominant mechanisms related to char combustion kinetics are mainly described by Avrami–Erofeev equations (m = 2, 3) or spherical symmetrical equation or Mampel single law (m = 1) or order-based reaction model (n = 4, 3/4) or G-B equation (3D diffusion) or Jander equation. The Mampel single law is also controlled by the random nuclear producing and growing process [47], in which the formation of nuclei that grew in two dimensions on a surface can be seen as comparable with random placement of circular disks area on a planar surface. The fraction of surface unoccupied by such disks is specified with (1−α).
In addition, from Table 3, the average activation energies of the sewage sludge, 80SS20C, 50SS50C, 20SS80C and bituminous coal in the main combustion stage are 165.80, 139.24, 118.21, 83.62 and 68.07 kJ mol−1, respectively, which means that the average activation energies of the samples in main combustion process increase with increasing the blending ratio of sewage sludge. The reason could be related to much high molecular organisms involved in sewage sludge. Otero et al. [51] also found a similar trend on an anthracite coal and sewage sludge within blending ratio range of 2−10% for the sewage sludge.
Also, the average activation energies of the 50SS50C blend at the dominant combustion stage in 21%O2/79%CO2, 30%O2/70%CO2 and 40%O2/60%CO2 are 105.41, 113.34 and 125.12 kJ mol−1, respectively, which means that the average activation energies of the sample in the dominant combustion process increase with increasing the oxygen content in O2/CO2 atmospheres. Fang et al. [52] also gave a similar trend on oxy-fuel combustion of wood and stated that the activation energy of cellulosic materials is affected by decreasing activated molecule concentration, diffusion limitation and organic impurities during the combustion process of sample, and that heat release from semi-coke oxidization increases and thus surface temperature of semi-coke increases as oxygen concentration increases. Werther and Ogada [31] suggested that semi-coke structure expands the higher size of grain and increases ash content with the increase in the final temperature. The activation energy thus increases with increased oxygen concentration. In addition, the average value of the activation energy of the 50SS50C in 30%O2/70%CO2 is less than that in air.
A linear relation between E a and lnA for the samples by analyzing the data in Table S3 is observed, as given in Figs. 4 and 5. There are certain kinetic compensation effects for both blending ratios and different oxygen contents due to the high correlation coefficients (R 2). The compensation effects usually occur in a group of related reactions for which the influence of variations in pre-exponential factor (A) caused from changing the blending ratios or oxygen contents on the reaction rate is offset to a greater or lesser extent by a sympathetic variation in activation energy (E a). That implies that temperature coefficients in reaction rates in solids are not necessarily an acceptable basis for determining the energy requirement in the activation step [47], and there are also the possibilities that diffusion and equilibrium in reversible reactions or heat transfer processes may act as some limitation over the magnitudes of the kinetic parameters.
Conclusions
There were two obvious peaks in the main combustion region of the sewage sludge and sludge/coal blends with blending ratios of sludge over 20%, while only one peak for the coal sample and 20%sewage sludge/80%coal blend. In air atmosphere, the ignition temperatures of the sewage sludge ranged from 235 to 253 °C, while those of the bituminous coal were between 420 and 438 °C. The ignition and burnout indexes of sludge/coal were improved with increasing both the blending ratio of sludge and heating rate, and the ignition and burnout indexes of 50%sewage sludge/50%coal blend were improved with increasing the oxygen content. The ignition and burnout indexes of the sample in 30%O2/70%CO2 atmosphere were about 2 and 0.4 times higher than those in 21%O2/79%CO2 atmosphere, respectively. The average activation energies of the 50%sewage sludge/50%coal blend at the main combustion stage in 21%O2/79%CO2, 30%O2/70%CO2 and 40%O2/60%CO2 were 105.41, 113.34, and 125.12 kJ mol−1, respectively. The dominant mechanisms associated with combustion kinetics for volatile matters and char of 50%sewage sludge/50%coal blend in oxy-fuel atmospheres were described by the Avrami–Erofeev equations.
Abbreviations
- α :
-
Conversion degree
- A :
-
Pre-exponential factor (s−1)
- C:
-
Char
- D :
-
Combustion index
- E a :
-
Apparent activation energy (J mol−1)
- m :
-
Mean; empirical constant in integral function
- n :
-
Reaction order
- R :
-
Universal gas constant (J mol−1K−1)
- T :
-
Temperature (K/ °C)
- VM:
-
Volatile matter
- β :
-
Heating rate (K min−1)
- b:
-
Burnout
- i:
-
Ignition
- max:
-
Maximum
- p:
-
Peak
- ∆:
-
Increment
References
Fytili D, Zabaniotou A. Utilization of sewage sludge in EU application of old and new methods—a review. Renew Sustain Energy Rev. 2008;12(1):116–40. doi:10.1016/j.rser.2006.05.014.
He Q, Xie D, Xu R, Wang T, Hu B. The utilization of sewage sludge by blending with coal water slurry. Fuel. 2015;159:40–4. doi:10.1016/j.fuel.2015.06.071.
Chen H, Yan S-H, Ye Z-L, Meng H-J, Zhu Y-G. Utilization of urban sewage sludge: Chinese perspectives. Environ Sci Pollut R. 2012;19(5):1454–63. doi:10.1007/s11356-012-0760-0.
Duan F, Zhang L, Sun X, Huang Y. Comparison of thermal behavior for modified calcium magnesium acetate blended separately with peanut shell and sewage sludge at different atmospheres. J Therm Anal Calorim. 2017;127(3):2417–25. doi:10.1007/s10973-016-5829-4.
Tyagi VK, Lo S-L. Sludge: a waste or renewable source for energy and resources recovery? Renew Sustain Energy Rev. 2013;25:708–28. doi:10.1016/j.rser.2013.05.029.
Xiao H, Ma X, Liu K. Co-combustion kinetics of sewage sludge with coal and coal gangue under different atmospheres. Energy Convers Manage. 2010;51(10):1976–80. doi:10.1016/j.enconman.2010.02.030.
Coimbra RN, Paniagua S, Escapa C, Calvo LF, Otero M. Combustion of primary and secondary pulp mill sludge and their respective blends with coal: a thermogravimetric assessment. Renew Energy. 2015;83:1050–8. doi:10.1016/j.renene.2015.05.046.
McIlveen-Wright DR, Huang Y, Rezvani S, Wang Y. A technical and environmental analysis of co-combustion of coal and biomass in fluidised bed technologies. Fuel. 2007;86(14):2032–42. doi:10.1016/j.fuel.2007.02.011.
Tsai M-Y, Wu K-T, Huang C-C, Lee H-T. Co-firing of paper mill sludge and coal in an industrial circulating fluidized bed boiler. Waste Manage. 2002;22(4):439–42. doi:10.1016/S0956-053X(02)00027-2.
Vamvuka D, Salpigidou N, Kastanaki E, Sfakiotakis S. Possibility of using paper sludge in co-firing applications. Fuel. 2009;88(4):637–43. doi:10.1016/j.fuel.2008.09.029.
Areeprasert C, Scala F, Coppola A, Urciuolo M, Chirone R, Chanyavanich P, et al. Fluidized bed co-combustion of hydrothermally treated paper sludge with two coals of different rank. Fuel Process Technol. 2016;144:230–8. doi:10.1016/j.fuproc.2015.12.033.
Buhre BJP, Elliott LK, Sheng CD, Gupta RP, Wall TF. Oxy-fuel combustion technology for coal-fired power generation. Prog Energy Combust. 2005;31(4):283–307. doi:10.1016/j.pecs.2005.07.001.
Escudero AI, Espatolero S, Romeo LM, Lara Y, Paufique C, Lesort A-L, et al. Minimization of CO2 capture energy penalty in second generation oxy-fuel power plants. Appl Therm Eng. 2016;103:274–81. doi:10.1016/j.applthermaleng.2016.04.116.
Yuzbasi NS, Selçuk N. Air and oxy-fuel combustion behaviour of petcoke/lignite blends. Fuel. 2012;92(1):137–44. doi:10.1016/j.fuel.2011.08.026.
Gładysz P, Ziębik A. Life cycle assessment of an integrated oxy-fuel combustion power plant with CO2 capture, transport and storage—Poland case study. Energy. 2015;92(Part 3):328–40. doi:10.1016/j.energy.2015.07.052.
Contreras ML, García-Frutos FJ, Bahillo A. Study of the thermal behaviour of coal/biomass blends during oxy-fuel combustion by thermogravimetric analysis. J Therm Anal Calorim. 2016;123(2):1643–55. doi:10.1007/s10973-015-5067-1.
Normann F, Andersson K, Leckner B, Johnsson F. Emission control of nitrogen oxides in the oxy-fuel process. Prog Energy Combust. 2009;35(5):385–97. doi:10.1016/j.pecs.2009.04.002.
Shao L-M, Fan S-S, Zhang H, Yao Q-S, He P-J. SO2 and NOx emissions from sludge combustion in a CO2/O2 atmosphere. Fuel. 2013;109:178–83. doi:10.1016/j.fuel.2013.01.027.
Wang X, Ren Q, Li L, Li S, Lu Q. TG–MS analysis of nitrogen transformation during combustion of biomass with municipal sewage sludge. J Therm Anal Calorim. 2016;123(3):2061–8. doi:10.1007/s10973-015-4712-z.
Yi B, Zhang L, Huang F, Xia Z, Mao Z, Ding J, et al. Investigating the combustion characteristic temperature of 28 kinds of Chinese coal in oxy-fuel conditions. Energy Convers Manage. 2015;103:439–47. doi:10.1016/j.enconman.2015.06.053.
Abbasi-Atibeh E, Yozgatligil A. A study on the effects of catalysts on pyrolysis and combustion characteristics of Turkish lignite in oxy-fuel conditions. Fuel. 2014;115:841–9. doi:10.1016/j.fuel.2013.01.073.
Ahn S, Choi G, Kim D. The effect of wood biomass blending with pulverized coal on combustion characteristics under oxy-fuel condition. Biomass Bioenergy. 2014;71:144–54. doi:10.1016/j.biombioe.2014.10.014.
López R, Fernández C, Fierro J, Cara J, Martínez O, Sánchez ME. Oxy-combustion of corn, sunflower, rape and microalgae bioresidues and their blends from the perspective of thermogravimetric analysis. Energy. 2014;74:845–54. doi:10.1016/j.energy.2014.07.058.
Zhang Y, Zhang L, Duan F, Jiang X, Sun X, Chyang C. Co-combustion characteristics of sewage sludge with different rank bituminous coals under the O2/CO2 atmosphere. J Therm Anal Calorim. 2015;121(2):729–36. doi:10.1007/s10973-015-4582-4.
Chen J, Mu L, Cai J, Yao P, Song X, Yin H, et al. Pyrolysis and oxy-fuel combustion characteristics and kinetics of petrochemical wastewater sludge using thermogravimetric analysis. Biores Technol. 2015;198:115–23. doi:10.1016/j.biortech.2015.09.011.
Tang Y, Ma X, Lai Z, Fan Y. Thermogravimetric analyses of co-combustion of plastic, rubber, leather in N2/O2 and CO2/O2 atmospheres. Energy. 2015;90(Part 1):1066–74. doi:10.1016/j.energy.2015.08.015.
Zhou Z, Hu X, You Z, Wang Z, Zhou J, Cen K. Oxy-fuel combustion characteristics and kinetic parameters of lignite coal from thermo-gravimetric data. Thermochim Acta. 2013;553:54–9. doi:10.1016/j.tca.2012.11.030.
Folgueras MB, Díaz RM, Xiberta J, Prieto I. Thermogravimetric analysis of the co-combustion of coal and sewage sludge. Fuel. 2003;82(15–17):2051–5. doi:10.1016/S0016-2361(03)00161-3.
Yanfen L, Xiaoqian M. Thermogravimetric analysis of the co-combustion of coal and paper mill sludge. Appl Energy. 2010;87(11):3526–32. doi:10.1016/j.apenergy.2010.05.008.
Kijo-Kleczkowska A, Sroda K, Kosowska-Golachowska M, Musial T, Wolski K. Experimental research of sewage sludge with coal and biomass co-combustion, in pellet form. Waste Manage. 2016;53:165–81. doi:10.1016/j.wasman.2016.04.021.
Werther J, Ogada T. Sewage sludge combustion. Prog Energy Combust. 1999;25(1):55–116. doi:10.1016/S0360-1285(98)00020-3.
García G, Arauzo J, Gonzalo A, Sánchez JL, Ábrego J. Influence of feedstock composition in fluidised bed co-gasification of mixtures of lignite, bituminous coal and sewage sludge. Chem Eng J. 2013;222:345–52. doi:10.1016/j.cej.2013.02.073.
Li XG, Lv Y, Ma BG, Jian SW, Tan HB. Thermogravimetric investigation on co-combustion characteristics of tobacco residue and high-ash anthracite coal. Biores Technol. 2011;102(20):9783–7. doi:10.1016/j.biortech.2011.07.117.
López R, Fernández C, Cara J, Martínez O, Sánchez ME. Differences between combustion and oxy-combustion of corn and corn–rape blend using thermogravimetric analysis. Fuel Process Technol. 2014;128:376–87. doi:10.1016/j.fuproc.2014.07.036.
Moore DS, McCabe GP. Introduction to the practice of statistics. New York: WH Freeman/Times Books/Henry Holt & Co; 1989.
Islam MA, Auta M, Kabir G, Hameed BH. A thermogravimetric analysis of the combustion kinetics of karanja (Pongamia pinnata) fruit hulls char. Bioresour Technol. 2016;200:335–41. doi:10.1016/j.biortech.2015.09.057.
Alvarez A, Pizarro C, Garcia R, Bueno JL, Lavin AG. Determination of kinetic parameters for biomass combustion. Bioresour Technol. 2016;216:36–43. doi:10.1016/j.biortech.2016.05.039.
Niu S, Chen M, Li Y, Xue F. Evaluation on the oxy-fuel combustion behavior of dried sewage sludge. Fuel. 2016;178:129–38. doi:10.1016/j.fuel.2016.03.053.
Magdziarz A, Werle S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manage. 2014;34(1):174–9. doi:10.1016/j.wasman.2013.10.033.
Yu LY, Li PS. Thermogravimetric analysis of coal and sludge co-combustion with microwave radiation dehydration. J Energy Inst. 2014;87(3):220–6. doi:10.1016/j.joei.2014.03.009.
Otero M, Gomez X, Garcia AI, Moran A. Effects of sewage sludge blending on the coal combustion: a thermogravimetric assessment. Chemosphere. 2007;69(11):1740–50. doi:10.1016/j.chemosphere.2007.05.077.
Yuzbasi NS, Selçuk N. Air and oxy-fuel combustion characteristics of biomass/lignite blends in TGA-FTIR. Fuel Process Technol. 2011;92(5):1101–8. doi:10.1016/j.fuproc.2011.01.005.
Chen C, Lu Z, Ma X, Long J, Peng Y, Hu L, et al. Oxy-fuel combustion characteristics and kinetics of microalgae Chlorella vulgaris by thermogravimetric analysis. Biores Technol. 2013;144:563–71. doi:10.1016/j.biortech.2013.07.011.
Ochoa A, Ibarra Á, Bilbao J, Arandes JM, Castaño P. Assessment of thermogravimetric methods for calculating coke combustion-regeneration kinetics of deactivated catalyst. Chem Eng Sci. 2017;171:459–70. doi:10.1016/j.ces.2017.05.039.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1):1–19. doi:10.1016/j.tca.2011.03.034.
Liu X, Chen M, Wei Y. Kinetics based on two-stage scheme for co-combustion of herbaceous biomass and bituminous coal. Fuel. 2015;143:577–85. doi:10.1016/j.fuel.2014.11.085.
Bamford CH, Tipper CFH. Comprehensive chemical kinetics: reactions in the solid state. Amsterdam: Elsevier Scientific Publishing Company; 1980.
Ochoa A, Ibarra Á, Bilbao J, Arandes JM, Castaño P. Assessment of thermogravimetric methods for calculating coke combustion-regeneration kinetics of deactivated catalyst. Chem Eng Sci. 2017;171(Supplement C):459–70. doi:10.1016/j.ces.2017.05.039.
Gil MV, Casal D, Pevida C, Pis JJ, Rubiera F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Biores Technol. 2010;101(14):5601–8.
Yorulmaz SY, Atimtay AT. Investigation of combustion kinetics of treated and untreated waste wood samples with thermogravimetric analysis. Fuel Process Technol. 2009;90(7–8):939–46. doi:10.1016/j.fuproc.2009.02.010.
Otero M, Calvo LF, Gil MV, Garcia AI, Moran A. Co-combustion of different sewage sludge and coal: a non-isothermal thermogravimetric kinetic analysis. Bioresour Technol. 2008;99(14):6311–9. doi:10.1016/j.biortech.2007.12.011.
Fang MX, Shen DK, Li YX, Yu CJ, Luo ZY, Cen KF. Kinetic study on pyrolysis and combustion of wood under different oxygen concentrations by using TG-FTIR analysis. J Anal Appl Pyrol. 2006;77(1):22–7. doi:10.1016/j.jaap.2005.12.010.
Acknowledgements
This work was supported by the National Natural Science Foundation of China under No. 51376017.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niu, S., Chen, M., Li, Y. et al. Co-combustion characteristics of municipal sewage sludge and bituminous coal. J Therm Anal Calorim 131, 1821–1834 (2018). https://doi.org/10.1007/s10973-017-6716-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6716-3