Abstract
High-viscosity modified (HVM) asphalt was prepared by the addition of styrene–butadiene–styrene, furfural exact oil (FEO, plasticizer), sulphur (crosslinker). The low-temperature rheological properties of HVM asphalt were investigated by using bending beam rheometer, and different analysis ways including Fourier transform infrared (FTIR) analysis, thermal analysis, 1hydrogen nuclear magnetic resonance (1NMR) analysis, elemental analysis, optical microscopy were used to investigate the structural characteristics of modified asphalts and FEO. Rheological tests demonstrated the effect of each modifier on low-temperature rheological performances of asphalt and displayed the structural characteristics of each binder to some extent. FTIR analysis indicated the effect of ageing and modifier on the distribution of functional groups of modified asphalt before and after ageing. Morphology observation showed the distribution of polymer in asphalt with different modifications or ageing. The thermal analysis showed the effect of each modifier on thermal behaviour of asphalt before and after ageing and confirmed the result of FTIR analysis and morphology observation further. Besides, the constituents of base asphalt and plasticizer were also investigated and compared further by adopting elemental analysis, and 1HNMR and FTIR tests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
HVM asphalt as the binder of draining asphalt pavement is much more predominant in high-temperature performances, toughness and tenacity due to the special aggregate proportion used in pavement. Generally, the open-graded aggregrate with larger particle size is used in road pavement, which leads to the larger and connected voids in pavement for draining. To prevent the pavement deformation and retain internal voids under repeated vehicle loading in hot weather, the asphalt with better deformation resistance and high-temperature performances should be used in the asphalt aggregate preparation. HVM asphalt as a kind of styrene–butadiene–styrene (SBS) compound-modified asphalt owns higher softening point and motive viscosity and is used widely in draining asphalt pavement [1]. Compared with ordinary SBS-modified asphalt, HVM asphalt owns higher SBS content and becomes very thick at 60 °C [2], leading to a better high-temperature loading resistance; meanwhile, the moderate rotational viscosity of HVM asphalt at 135 °C also makes the preparation for the asphalt aggregate become processable [3]. Besides as the binder of the aggregate with larger particle size, HVM asphalt also owns strong toughness and tenacity in order to prevent the aggregates form flying with the strong friction of vehicle tyre [1].
As a technical secret, the proportion research for HVM asphalt is usually not noted in many publications and the present report only focuses on the research for basic physical properties [4,5,6,7]. In our previous work, the modifiers of HVM asphalt including SBS, FEO, sulphur have been pointed out [8, 9] and the corresponding modified asphalts including SBS-modified (SM) asphalt, SBS/FEO-modified (SFM) asphalt and SBS/FEO/sulphur-modified (SFSM or HVM) asphalts were prepared and the detailed research for physical and high-temperature rheological properties of these asphalts also has been finished [8]. However, the structural characteristics and low-temperature rheological properties of these asphalts were not mentioned. In most cases, the low-temperature rheological behaviours of HVM asphalt are also very important and determine the performances of draining pavement in winter. Moreover, the study for the effect of modifier and ageing on the structural characteristics of HVM asphalt is also very necessary for most researchers to understand the compositions and improve the properties further. However the related research is not concerned in our previous work or other publications.
To investigate the low-temperature rheological properties and structural characteristics of HVM asphalt further, the modified asphalts including SM, SFM, SFSM (HVM) asphalts were prepared in the light of the proportion of HVM asphalt [8] and the low-temperature rheological behaviours of these modified asphalts were investigated by using BBR and the structural characteristics were studied by various analysis ways. FTIR, 1HNMR and elemental analysis were used to investigate and compare the constituents of FEO and base asphalt. Optical microscope was used to observe the polymer distribution in asphalt. The thermodynamic behaviour of each binder was analysed and illustrated by scanning calorimetry (DSC), thermogravimetric (TG), derivative thermogravimetry (DTG) curves.
Materials and measurements
Materials
Fuzhou-70 (AH-70) paving asphalt was obtained from the Fuzhou Petroleum Asphalt Factory, China; the physical properties are shown in Table 1 [8]. SBS1301 is linear polymer, containing 30 mass/% styrene, and the average molecule weight is 110,000 g mol−1. Plasticizer is FEO and technically pure reagent, and the major constituent is arene. Crosslinker is sulphur and chemically pure reagent.
Preparation of samples
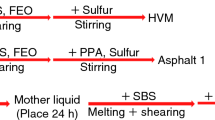
The modified asphalts were prepared by using a high shear mixer (made by Qishuang Machine Co Ltd, China). Firstly, asphalt (500 g) was heated at 180 °C in an iron container until it became fully fluid, and then, the modifiers (plasticizer or SBS based on 100 parts asphalt) were added sequentially and sheared 1 h at the shearing speed of 5000 r min−1; subsequently, the sulphur was added and the blend was stirred by mechanical stirrer at 180 °C for 2 h to make sure the fully swelling and reaction of modifiers in asphalt.
The ageing of asphalt
The short-term thermal ageing of asphalt was performed using the thin-film oven test (TFOT, ASTM D 2872) which simulates the changes in the properties of asphalt during the hot mixing and the lay down process.
Physical properties test
The physical properties of asphalts, including softening point, motive viscosity, penetration, toughness and tenacity, ductility, storage stability, were tested in accordance with ASTM D36, D2171, D5, D5801-95, D113, D5796, respectively.
Low-temperature rheological test
Low-temperature creep tests were carried out at -10 °C using a BBR (Cannon Instrument Company). In tests, the bitumen beam (125 mm long, 12.5 mm wide and 6.25 mm thick) was submerged in a ethyl alcohol bath and kept at the test temperature -10 °C for 1 h. A constant load of 100 g was then applied to the rectangular beam, which was supported at both ends by stainless steel half-rounds (102 mm apart), and the deflection of centre point was measured continuously. Creep stiffness (S) and creep rate (m) of the binders were determined at the loading times of 240 s.
Morphology observation
The morphology of modifier in asphalt was investigated by using optical microscope (Nikon Co., Japan) at a magnification of 400. A small amount of asphalt sample was heated and pressed carefully between two small glass tables at a fine thickness.
Fourier transform infrared (FTIR) spectroscopy
The functional groups of modified asphalt were tested by a FTIR spectrometer, infinity 60 AR (Mattson, resolution 0.125 cm−1) in wave numbers range from 400 to 4000 cm−1. Sample was dissolved in chloroform at 10 mass/% concentration and dropped on KBr table and dried for the FTIR test.
1HNMR spectrophotometry
A Bruker NMR Spectrometer (Avance III 500) was used. Asphalt was dissolved by deuterochloroform (CDCl3) with 10 mass/% concentration. Tetramethylsilane (TMS) was used as internal standard.
Elemental analysis
An elemental analyzer (Vario EL) made in Germany was used to test the elements of sample (100 mg for solid, 1 mL for liquid). The test mechanism is the microcombustion method, and a certain test way named CHNS mode was used to test the four major elements such as carbon, nitrogen, hydrogen, and sulphur.
Thermal analysis
Thermal analysis was performed using a TA Instrument, model SDT 2960, under argon atmosphere, sample mass around 7–10 mg, with the constant heating rate of 10 °C min−1 heated from room temperature to 900 °C. The argon flow during the experiments was 120 mL min−1. The TG, DTG, and DSC versus temperature curves were used to evaluate the thermomechanical behaviour of binder, respectively. All experiments were performed three times for reproducibility.
Results and discussion
Compositions and physical properties of HVM asphalt
According to the previous research, the modifiers of HVM asphalt consisted of SBS, FEO, sulphur and SBS is the major modifier; the two others are the additional ones [8]. The physical properties of SM, SFM, SFSM asphalts are shown in Table 1 [8]. As we discussed previously, FEO promoted the swelling of SBS in asphalt further and improved the ductility; however, as the result of dilution, the softening point, toughness and tenacity of SFM asphalt before and after ageing also declined compared with SM asphalt. Vulcanization increased the softening point; motive viscosity of SFSM asphalt compared to that of SFM asphalt owing to the formation of the crosslinked SBS network in asphalt and the increased ductility of SFSM asphalt before ageing also indicates the improved low-temperature property. However, the declined toughness and tenacity of SFSM asphalt compared with SFM asphalt indicate the weakened stretch strength of the vulcanized SBS in asphalt.
Low-temperature rheological properties
The low-temperature rheological properties of SM, SFM and SFSM asphalts were tested by using BBR at -10 °C, and the results are presented in Fig. 1a and b. Before ageing, as shown in Fig. 1a, SFM asphalt owns lower creep stiffness (S) and higher creep ratio (m) in the whole testing time, showing the improved flexibility by the swelling of FEO. The higher creep S and lower creep m of SFSM asphalt indicates the declined flexibility by the addition of sulphur, which was attributed to the formation of a crosslinked SBS network in asphalt and the polysulfide bonds formed among SBS molecules restricted their movements greatly and declined the crack resistance. After ageing, as shown in Fig. 1b, the lower S and higher m of SFM binder compared to that of SM binder indicate an obvious viscous behaviour, which means the swelled SBS was more susceptible to ageing and degraded severely after TFOT ageing. For SFSM binder, the further elevated m and declined S compared with SFM binder show a more obvious viscous behaviour after ageing. As the result of vulcanization, the swelled and crosslinked SBS particles in asphalt became more feeble, leading to the further increased susceptibility to ageing; this cannot be found only by the physical properties in Table 1.
Morphology observation
The morphology of polymer-modified asphalts (PMAs) was investigated by using optical microscopy, as shown in Fig. 2. For SM asphalt, it can be seen that the dense polymer particles are dispersed in asphalt in Fig. 2a, which can be observed in the whole area of asphalt sample. The morphology of SFM asphalt is shown in Fig. 2b, and it can be seen that the size of SBS particles dispersed asphalt became smaller compared with SM asphalt and a lot of tiny polymer particles are dispersed in asphalt matrix uniformly. Due to the swelling of plasticizer, the SBS became loose and can be sheared into smaller particles easily under the shear force of mixer. For SFSM asphalt, as shown in Fig. 2c, some filamentous polymers are dispersed in asphalt and the tiny polymer particles are very difficult to be found in asphalt again after observing several samples, which means most SBS molecules have reacted with sulphur and were crosslinked together, leading to the obvious change in morphology. After ageing, the morphology of binders is shown in Fig. 2d–f. Ageing prompted the degradation of polymer particles in asphalt greatly; as shown in Fig. 2d and e, the residual SBS particles only can be found in some areas of sample and the outline became dim, which means most particles have been degraded and dissolved in asphalt completely. For SFSM binder, only a few filamentous polymers can be found in some areas of asphalt after observing many samples; as shown in Fig. 2f, the residual crosslinked polymer was very difficult to be found again than before ageing.
Element and 1HNMR analysis
FEO as an additional modifier in the preparation of modified asphalts plays a vital role in determining the properties of HVM asphalt. To investigate the major constituents of FEO further, three tests including element analysis, and 1HNMR and FTIR tests were made.
Element analysis
In this study, FEO as the plasticizer was used firstly in the preparation of HVM asphalt, whose constituents are similar to those of asphalt and consisted of four element asphalt compositions such as asphaltene, aromatics, resins and saturates. To investigate and compare the compositions of FEO and asphalt, the major elements of FEO and asphalt were analysed and are listed in Table 2. It can be seen that the content of four major elements such as carbon, hydrogen is similar, and the major element of FEO and asphalt is still carbon, which indicates both asphalt and FEO own similar constituents to some extent. The extra sulphur content of FEO should be attributed to the use of sulphur in the refinement of FEO.
1HNMR analysis
To investigate and compare the compositions of the plasticizer and asphalt, the 1HNMR of base asphalt and FEO were tested and the diagrams are listed in Fig. 3a and b. It can be seen that the 1 H distribution of FEO and base asphalt is similar and there is no new peak appearing between the two diagrams, which indicates both FEO and base asphalt have similar constituents. The corresponding 1HNMR data are presented in Table 3. The distribution of 1 H can be divided into aromatic protons, (H ar, δ = 6–9) and aliphatic region (δ = 1–4.0), and the aliphatic region is subdivided into three parts further: H α (δ = 2.0–4.0), H β (δ = 1.0–2.0) and H γ (δ = 0.5–1.0) [10,11,12]. The per cent hydrogen distribution of two samples is given in Table 4. It can be seen that the H ar content is similar and there is only a small difference in the distribution of H β and H γ, which indicates the major compositions of base asphalt and FEO are similar.
FTIR analysis
The FTIR spectrum of base asphalt and FEO is shown in Fig. 4a. The strong peaks within 2855–2935 cm−1 region are typical C–H stretching vibrations in aliphatic chains. The peak at 1605.3 cm−1 is attributed to C = C stretching vibrations in aromatics. The C–H asymmetric deforming in CH2 and CH3, and the C–H symmetric deforming in CH3 vibrations are observed at 1454.1 cm−1 and 1375.2 cm−1, respectively. The peak at 1219.3 cm−1 corresponds to the frame vibration of (CH3)3C–R. The small peaks within 667.2–871.2 cm−1 region are typical C–H vibrations of benzene ring [13, 14]. It can be seen that the spectrum of FEO and base asphalt is very similar, indicating the similar constituents.
The FTIR spectra of base and SM asphalts are illustrated in Fig. 4b and c. The new absorption peak at 966.8 cm−1 can be attributed to the bending vibration of C–H in the butadiene double bonds of SBS molecule chain. For SFM and SFSM asphalts, as shown in Fig. 4d, there is no new peak appearing in the FTIR spectra.
The spectra of binder before and after ageing are still similar and can be illustrated by that before ageing. For modified binder, the butadiene bond content (centred around 966.8 cm−1) was usually used to evaluate the butadiene content. By calculating the structural parameter I CH=CH of Eq. (1), we can assess the deterioration of SBS copolymer as a result of further modification or ageing [15, 16].
The calculated result for each binder is listed in Table 5. Before ageing, it can be seen that the I CH=CH of SFM asphalt is lower than that of SM asphalt, which indicates the butadiene content declined by the dilution of FEO. The I CH=CH of SFSM asphalt is lower than that of SFM asphalt, indicating that the crosslinker interacted with the conjugated carbon bond of SBS molecule chain, so the corresponding bending vibration of C–H declined. After ageing, the I CH=CH of each binder is lower than that before ageing, due to the SBS degradation. The influence of sulphur and FEO on I CH=CH is still similar to that before ageing; therefore, the I CH=CH sequence among binders is unchanged.
Thermal analysis
The thermal analysis was used to investigate the structural characteristics of base, SM, SFM and SFSM asphalts before and after ageing in this study. The thermal behaviour of each binder before and after ageing was studied by thermogravimetry at 10 °C min−1 and is illustrated by the TG/DTG/DSC curves, as shown from Figs. 5 and 6. The TG curves show that all binders undergo the major mass loss stage from 350 to 500 °C, and the mass loss is mainly due to the volatilization of the light asphalt constituents such as saturates and aromatics and the decomposition of SBS and hard asphalt constituents [17, 18]. This is the major mass loss stage, as demonstrated by the dramatic event in the DSC and DTG curves.
The comparative DSC curves of all binders before ageing are listed in Fig. 7. The DSC curves show that the thermomechanical behaviour of each binder can be described by the endothermic peak and the area can be calculated by the using tangent [18], as illustrated by Fig. 8; the calculated peak areas of all binders before and after ageing are listed in Table 6. The peak areas can be used to evaluate the distribution of molecular mass or constituents of binder; the broad peak or larger peak area indicate the more complex distribution of molecular mass or constituents, and the contrary conclusion also can be drawn [19, 20]. In Fig. 7, the more elevated curve of SM asphalt with increasing the temperature indicates more energy consumption in degradation, which is confirmed further by the larger endothermic peak of SM asphalt in Table 6. For the original SM asphalt, the SBS particles dispersed in asphalt usually were coarser and larger and led to a higher energy consumption in the decomposition process. The endothermic peak of SFM asphalt is smaller than that of SM asphalt, showing lower energy consumption in the major thermal decomposition. FEO is very thick and owns higher boiling point, which prevented the light asphalt components form volatizing in the major mass loss process to a great extent. The lower peak area of SFSM asphalt than that of SFM asphalt can be attributed to the effect of sulphur. As a result of crosslinking reaction, the morphology of SBS in asphalt changed from coarser particles to filaments, which declined the particle size of SBS obviously and led to a less energy consumption in heating decomposition. In Table 6, the peak area of aged binder declined a little than that of unaged. Owing to the effect of polymer degradation after ageing, the SBS content declined greatly and less residual SBS led to less energy consumption in heating. However, the sequence of peak area among binders is still similar to that before ageing, and the effect of SBS, FEO, sulphur has been confirmed again.
Conclusions
The effect of FEO and sulphur on the low-temperature rheological properties of asphalt was studied by bending creep test. Creep test showed that the flexibility of SM asphalt was improved by the swelling of FEO; however, the vulcanization declined the flexibility due to the restricted sulphur bonds among polymer molecules. Both FEO and sulphur increased the viscous behaviour of SM asphalt after ageing, and the swelled or crosslinked polymers were more susceptible to ageing. Elemental and 1HNMR analyses showed that both base asphalt and FEO own similar compositions, which was also confirmed further by FTIR analysis. The changed butadiene group content in FTIR analysis indicated the effect of modifier and ageing on the structure of modified asphalt. The thermomechanical behaviour of each binder was displayed by the TG/DSC/DTG curves in thermal analysis. The changed enthalpy in the major mass loss process confirmed the effect of modifier and ageing on the structural characteristics of asphalt, which conformed to the results of FTIR analysis to some extent.
References
Xu B. Theory and practices of porous asphalts. 1st ed. Beijing: People communication Press; 2011.
Liu XL. Preparation and properties of high viscosity modified asphalt. Master Dissertation, Wuhan University of Technology, Wuhan City, PR China: 2008.
Standard Test Methods for Bitumen and Bituminous Mixture for Highway Engineering, Communication Ministry of People’s Republic of China; 2006.
Deng XC, Pang LG. Study on the TPS high viscosity modifying asphalt application in OGFC pavement. Shanxi Archit. 2009;35(4):205–6 (in Chinese).
Li WH, Mai YL, Lu Y, et al. Development of high viscosity asphalt modifier HVM-700. Guangdong Chem Ind. 2010;37(9):1–2 (in Chinese).
Jing C, Zhu JH. The contrast research on drain the high gluing pitch road. Shanxi Archit. 2009;35:244–5 (in Chinese).
Deng XC, Pang LG. Study on the TPS high viscosity modifying asphalt application in OGFC pavement. Shanxi Archit. 2009;35(4):205–6 (in Chinese).
Zhang F, Hu CB. Preparation and properties of high viscosity modified asphalt. J Polym Com. 2017;38(5):936–46.
Zhang F, Hu CB. The composition and ageing of high-viscosity and elasticity asphalts. J Polym Com. 2015;. doi:10.1002/pc.23841.
Ahmad I, Shakirullah M, Rehman HU, Ishaq M, Khan MA, Shah AA. NMR analysis of cracking products of asphalt and assessment of catalyst performance. Energy. 2009;34:127–33.
Ma LX, Wu SP, Huang JF. Investigation of chemical properties by NMR during the laboratory aging of asphalt. J Wuhan Univ Technol. 2010;13:43–6.
Zhang F, Hu CB. The research for high-elastic modified asphalt. Appl Polym Sci. 2015;132:42132 (1-14).
Zhang F, Hu CB. The research for structural characteristics and modification mechanism of crumb rubber compound modified asphalts. Constr Build Mater. 2015;76:330–42.
Zhang F, Yu JY. Effect of thermal oxidative ageing on dynamic viscosity, TG/DTA and FTIR of SBS- and SBS/sulfur-modified asphalts. Constr Build Mater. 2011;25:129–37.
Virginie M, Fabienne F, Stanislas B. Ageing by UV radiation of an elastomer modified bitumen. Fuel. 2008;87:2408–19.
Lamontagne J, Dumas P, Mouillet V, Kister J. Comparison by Fourier transform infrared (FTIR) spectroscopy of different ageing techniques: application to road bitumens. Fuel. 2001;80:483–8.
Zhang F, Hu CB. Influence of aging on thermal behavior and characterization of SBR compound-modified asphalt. J Therm Anal Calorim. 2014;115:1211–8.
Zhang F, Hu CB. The research for thermal behaviour, creep properties and morphology of SBS-modified asphalt. J Therm Anal Calorim. 2015;121:651–61.
Gao JL. Analysis and evaluation of bitumen new index and additive index. Doctoral Dissertation, Southeast University, Nanjing City, PR China: 2005.
Gao JL, Huang XM, Li HJ. Evaluation method of differential scanning calorimetry for asphalt performance. J Traff Trans Eng. 2005;5:37–42.
Acknowledgements
This study is financially supported by expensive instrument equipment and open-end foundation of Fuzhou University (Grant Number 2017T038).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, F., Hu, C. & Zhuang, W. The research for low-temperature rheological properties and structural characteristics of high-viscosity modified asphalt. J Therm Anal Calorim 131, 1025–1034 (2018). https://doi.org/10.1007/s10973-017-6569-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6569-9