Abstract
New bisphenol-based benzoxazines (BBA-a and BBA-bra) were synthesized from bisphenol containing trityl group, paraformaldehyde, aniline and 2,4,6-tribromoaniline by a solventless method. The chemical structure of the monomers was analyzed by FTIR, 1H NMR and elemental analysis. FTIR and differential scanning calorimetry (DSC) indicated that the polymerization of BBA-a and BBA-bra was thermally initiated and occurred via ring opening of benzoxazine monomers. Comparing with that of BBA-a, the curing exothermic peak of BBA-bra shifted from 230 to 245 °C. Thermogravimetry (TG) showed that PBZ from BBA-a and BBA-bra (named as PBBA-a and PBBA-bra, respectively) had superior thermal stability. The char yields of PBBA-a and PBBA-bra at 800 °C were 42.37 and 48.30%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polybenzoxazine (PBZ) is a newly developed high-performance phenolic resin that possesses attractive properties, such as high thermal stability, nearly zero shrinkage upon curing, low water absorption, dimensional stability, no byproduct release upon curing, chemical resistivity and flame resistance. Moreover, the extensive inter- and intramolecular hydrogen bonds in the structure of PBZ can enhance its physical and mechanical properties to meet the application requirements [1–3]. These outstanding characteristics make PBZ a good candidate over traditional thermosetting resins such as phenolic resins, epoxy resins, unsaturated polyester and vinyl ester resin [4–7].
Moreover, it is important that benzoxazines have considerable molecule design flexibility by changing various phenol and amines as raw materials. In the past decades, many types of benzoxazines have been designed and prepared to study the polymerization mechanism of benzoxazine monomers and the relation between structure and properties of PBZ [8–16]. However, a high ring-opening polymerization (ROP) temperature of benzoxazine monomers and brittle characters of the cured resins have limited their application. Various efforts have been made to improve the properties of PBZ via functionality, polymer alloys, fiber-reinforced composites and nanocomposites, etc. [1]. Recently, Lochab et al. [17] synthesized a benzoxazine containing acid functionality based on p-hydroxybenzoic acid. They found that such a monomer could catalyze the ROP, reduce the curing temperature of other benzoxazine monomers and improve the thermal stability of the thermosets. They also reported the synthesis of a novel cardanol-based benzoxazine with high aromatic content. The incorporation of higher aromatic ring in benzoxazine monomers resulted in enhancing the cross-link density of PBZ besides higher functionality to modulate their properties [18]. In our previous studies, we also synthesized many types of benzoxazines and studied their structure and properties [19–24]. For example, we have prepared bisphenol-based benzoxazine–benzoic acid and found that the carboxyl groups on the molecules could catalyze the curing reaction and reduce the curing temperature of benzoxazines [20, 22]. We also synthesized monofunctional and bifunctional brominated benzoxazine monomers. The results of investigation showed that bromination could have a profound effect on increasing char yield and on thermal degradation temperature of PBZ [24]. In recent years, bisphenol containing trityl group (BBA) has been reported to prepare bisphenol novolac epoxy resins. The obtained bisphenol novolac epoxy resins have superior chemical resistances and good mechanical properties due to its unique chemical structure (high content of aromatic rings) [25]. However, to our best knowledge, so far, there have no previous reports on benzoxazines based on BBA.
In the present work, we attempt to prepare a new class of benzoxazines based on bisphenol containing trityl group. In this case, bisphenol having higher content of aromatic rings was prepared and followed by reaction with paraformaldehyde, aniline or 2,4,6-tribromoaniline to prepare benzoxazines through a solventless method. The bromine groups were introduced into the molecular structure of benzoxazines based on bisphenol containing trityl group to further improve the thermal stability of the corresponding PBZ. The chemical structures of benzoxazines based on bisphenol containing trityl group (BBA-a and BBA-bra) were characterized by FTIR, 1H NMR and elemental analysis. The thermally curing behavior of benzoxazine monomers and the thermal properties of the corresponding PBZ were also studied.
Experimental
Materials
Phenol, benzaldehyde, sulfuric acid, hydrochloric acid, paraformaldehyde, aniline, 2,4,6-tribromoaniline, sodium hydroxide, diethyl ether, acetone, tetrahydrofuran, chloroform, toluene, xylene and sodium sulfate were purchased from Shanghai First Reagent Company, China. All chemicals were AR grade and used without further purification.
Synthesis of bisphenol (BBA)
BBA was synthesized according to the previous literature (Fig. 1) [25]. In briefly, a mixture of phenol and benzaldehyde at molar ratio of 4:1 was added into a flask. Then, 25 mL concentrated sulfuric acid and 44 mL concentrated hydrochloric acid were dropped into above mixture gradually. The reaction mixture was stirred for 4 h, left overnight and then filtered. The golden yellow precipitate was washed and crystallized from 50% dilute acid. The product was dried in an oven at 50 °C, and the melting point was measured. The yield of BBA was 92% and m.p. was 153 °C [25].
Synthesis of BBA-based benzoxazines (BBA-a and BBA-bra)
The BBA-based benzoxazine monomer, BBA-a, was synthesized by a solventless method (Fig. 2) [24]. Namely, a mixtures of BBA (20 mmol), aniline (40 mmol) and paraformaldehyde (82 mmol) were placed in a round-bottom flask and stirred at 110 °C for 30 min. After cooling at room temperature, the mixture was dissolved in diethyl ether. The resulting solution was washed five times with 3 N sodium hydroxide aqueous solution and twice with distill water. Then, the diethyl ether solution was dried overnight and filtered, and the solvent was removed by rotary evaporation at reduced pressure. The final product was dried at reduced pressure and room temperature to provide a brown yellow powder. The yield is 78%; m.p.: 63–64 °C. Anal. C35H30O2N2; C 82.35%; H 5.88%; N 5.49%; found: C 82.12%; H 5.62%; N 5.53%. 1H NMR (deuterated DMSO, TMS, δ ppm): 6.65–7.29 (m, 21H, Ar–H), 5.39 (s, 4H, O–CH2–N), 4.58(s, 4H, Ar–CH2–N), 3.36 (s, 1H, (Ar)3–CH, water in DMSO comes at 3.3 ppm).
The other type of BBA-based benzoxazine monomer, BBA-bra, was also prepared from BBA, paraformaldehyde and 2,4,6-tribromoaniline using the same method as mentioned above. The product was dried at reduced pressure and room temperature to provide a white powder. The yield is 83%; m.p.: 88–89 °C. Anal. C35H24O2N2Br6; C, 42.68%; H, 2.24%; N, 2.85%; Br, 48.78% found: C 41.33%; H 2.16%; N 1.98%; Br, 47.98%. 1H NMR (deuterated DMSO, TMS, δ ppm): 6.79–7.92 (m, 15H, Ar–H), 5.43 (s, 4H, O–CH2–N), 4.32(s, 4H, Ar–CH2–N), 3.36 (s, 1H, (Ar)3–CH, water in DMSO comes at 3.3 ppm).
Preparation of PBZ
The PBZ was prepared according to the following method: about 40% solution by weight of BBA-a or BBA-bra in tetrahydrofuran was placed onto a piece of metal plate. After most of the solvent was removed under ambient atmosphere at 60 °C, the metal plate was placed into a vacuum oven at 60 °C for 24 h to remove the residual solvent. The samples were dried at room temperature overnight in vacuum to minimize any trace of solvent. Then the samples were step cured in a closed air-circulating oven for 1 h at 140, 160, 180, 200 and 220 °C, respectively, and finally 2 h at 230 °C. Upon completion of the curing, the samples (named as PBBA-a and PBBA-bra) were allowed to freely cool to room temperature. Individual test samples were cut appropriate dimension to be applied for property evaluation.
Measurements
1H NMR measurement was taken on a Bruker NMR spectrometer at 300 MHz. DMSO was used as a solvent. FTIR spectra were obtained on a Bruker Tensor 2 FTIR spectrometer at a resolution of 4 cm−1 in the region of 400–4000 cm−1. The benzoxazine monomer was dispersed in KBr. Then press the mixture into a disk. Elemental analysis was performed with a VARIO EL III rapid elemental analyzer. DSC or TG was measured on a Mettler Toledo DSC–TG apparatus. All samples were tested under the nitrogen flow rate of 30 mL min−1. In order to investigate the thermal polymerization reactions of benzoxazine monomers, we only chose the DSC data because the simultaneous TG data are insignificant. To study the mass loss behavior of PBZs, we also only chose the TG data of PBZs because the simultaneous DSC data were insignificant. For the obtained DSC data of benzoxazine monomers, about 10 mg of sample was scanned at heating rate of 10 °C min−1. For the obtained TG data of PBZs, about 10 mg of sample was scanned at a heating rate of 5 °C min−1.
Results and discussion
Synthesis and characterization of BBA-based benzoxazines
In order to use the phenol group to replace the methyl group in bisphenol-A, we chose commercial phenol and benzaldehyde as raw materials to synthesize BBA (Fig. 1). As a result, a high yield of BBA (92%) can be obtained. Then, BBA-a was prepared from BBA, paraformaldehyde, aniline and 2, 4, 6-tribromoaniline by a solventless method [24]. The synthesis route of BBA-a and BBA-bra is shown in Fig. 2. BBA-a and BBA-bra were brown yellow and white powder at room temperature, respectively. Both BBA-a and BBA-bra were soluble in common solvents, i.e., acetone, tetrahydrofuran and chloroform, at room temperature. However, BBA-a showed better solubility than BBA-bra in some aromatic solvents such as toluene and xylene.
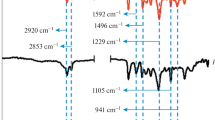
The chemical structure of BBA-a and BBA-bra is further characterized by FTIR and 1H NMR spectra. Figure 3 shows the FTIR spectra of BBA-a and BBA-bra. For BBA-a and BBA-bra, it can be seen that the characteristic absorptions at 943 and 948 cm−1 were assigned to the characteristic modes of the benzene ring with an oxazine ring attached, respectively [3, 19–24]. The band at 1244–1249 and 1026–1076 cm−1 was assigned to asymmetric and symmetric stretching vibrations of C–O–C, respectively [3, 19–24]. The band at 1120 and 820 cm−1 was assigned to asymmetric and symmetric stretching vibrations of C–N–C [3, 19–24]. The absorptions at 1554 cm−1 were associated with the stretching vibrations of the benzene ring (trisubstituted benzene), and the band at 1328–1369 cm−1 are assigned to wagging vibrations of CH2. In addition, the band at 564 cm−1 assigned to Br–C bond in BBA-bra was observed [24].
Figure 4 shows the 1H NMR spectra of BBA-a and BBA-bra. As shown in Fig. 4a, for BBA-a, the resonances appearing at 5.39 ppm and 4.58 ppm were assigned to the methylene protons of O–CH 2 –N and Ar–CH 2 –N of the oxazine ring, respectively. The multi-characteristic peaks at 6.65–7.29 ppm were assigned to the aromatic protons. The trityl group proton in the BBA appeared at 3.36 ppm. For BBA-bra, the resonances appearing at 5.43 ppm and 4.32 ppm were assigned to the methylene protons of O–CH2–N and Ar–CH2–N of the oxazine ring, respectively. The multiple at 6.79–7.92 ppm was assigned to the aromatic protons. The trityl group proton in the BBA also appeared at 3.36 ppm.
Thermal polymerization of BBA-based benzoxazines
In order to understand the thermal polymerization mechanism of BBA-a and BBA-bra, the spectra of BBA-a and BBA-bra before and after the polymerization were recorded by FTIR. Figure 5a shows FTIR spectra of BBA-a at different curing stages. It can be clear seen that the characteristic peaks and regions indicative of benzoxazine ring structure, i.e., 1367, 1218 and 943 cm−1, gradually disappeared with the increase in the curing temperature and time [18, 20]. Moreover, the new characteristic peaks appeared at around 1614 cm−1. This illustrated that ring-opening polymerization of BBA-a had occurred and the Mannich bridge linkage and phenolic hydroxyl groups had produced at elevated temperature [3]. Figure 5b also shows FTIR spectra of BBA-bra at different curing stages. It is also observed that the characteristic peaks and regions indicative of benzoxazine ring structure, i.e., 1367, 1218 and 948 cm−1, gradually disappeared and the new characteristic absorptions appeared at around 1615 cm−1 when the curing temperature increased from 180 to 230 °C. It also indicated that the ring-opening reaction of benzoxazine ring in BBA-bra had occurred [18].
The thermal polymerization reaction of BBA-a and BBA-bra was further monitored by DSC. Figure 6 shows the DSC curves of BBA-a and BBA-bra from room temperature to 300 °C at a heating rate of 10 °C min−1. As shown in Fig. 6, firstly, BBA-a and BBA-bra showed a melting endothermic peaks at 63–64 and 88–89 °C, respectively. Then, a broad exothermic peak around 240 °C associated with the curing reaction was observed, which was ascribed to the polymerization reaction of BBA-a or BBA-bra. Table 1 shows characteristic parameters of the curing exothermic peaks, T onset (onset temperature of the exothermic peak), T p (peak temperature of the exothermic peak), T s (stop temperature of the exothermic peak) and ΔH (exothermic enthalpy under the curing exotherm). It is clear that the temperature of the thermal polymerization (~240 °C) was comparable with that of typical bisphenol-A-based benzoxazines, i.e., ~249 °C [26]. The polymerization reaction enthalpies of BBA-a and BBA-bra were 222 and 238 J g−1, respectively. Furthermore, comparing with BBA-a, the curing exothermic peak of BBA-bra shifted from 230 to 245 °C. This may be due to that 3,4-dihydro-2-H-1,3-benzoxazines exhibit ring/change tautomerism when protonated by migration of the proton from the nitrogen to the oxygen atom, thereby producing iminium ions, which then suffer nucleophilic attack from the aromatic rings. These propagation iminium intermediates should be destabilized in this case by the presence of electron-withdrawing substitutional bromine groups on the phenyl group (Fig. 7) [27].
Thermal properties of the polybenzoxazines
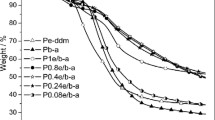
The thermal properties of PBZ from BBA-a and BBA-bra were investigated by TG. Figure 8 shows the TG curves of PBBA-a and PBBA-bra. The results are listed in Table 2. As can be seen that, for PBBA-a, the 5 and 15% mass loss temperatures (T d5 and T d15) were 293 and 372 °C, respectively, whereas for PBBA-a, T d5 and T d15 were 356 and 407 °C, respectively. However, in the same conditions, T d5 of PBZ based on bisphenol-A (PBA-a) was only 274 °C. Moreover, the char yields of PBBA-a and PBBA-bra at 800 °C were 42.37 and 48.30%, respectively, which were much higher than those of commercial PBA-a (32%) [3]. Thus, this meant that the thermal stability of PBBA-a and PBBA-bra was more superior to that of commercial PBA-a. This may be attributed to the fact that the higher content of aromatic rings in PBBA-a and PBBA-bra due to using phenol group to replace methyl groups in bisphenol-A, which restricted the molecular mobility of polybenzoxazine chains [23]. The similar results have been reported by Lochab et al. They synthesized trityl benzoxazines and found that the incorporation of higher aromatic ring in benzoxazine monomers could enhance the thermal properties of the corresponding PBZ [18].
In addition, comparing the T d5 and T d15 of PBBA-a and PBBA-bra, both the values of T d5 and T d15 of PBBA-bra were much higher than those of PBBA-a. The char yield of PBBA-bra at 800 °C (48.30%) was also higher than that of PBBA-a (42.37%). The results are similar to our previous studies. We synthesized monofunctional and bifunctional brominated benzoxazine monomers (P-bra and PB-bra). The study results showed that the thermal degradation temperature and char yields of the brominated PBZ were much high than those corresponding non-brominated PBZ [24]. The main reason may be that the incorporation of bromine groups on benzoxazines had a significant effect on improving thermal stability and char yield of polybenzoxazines.
Conclusions
In this study, new bisphenol-based benzoxazines were successfully synthesized by a solventless method. The chemical structures of the corresponding monomers were confirmed by FTIR and 1H NMR spectra. The thermal polymerization was studied by FTIR and DSC. Both BBA-a and BBA-bra showed the ring-opening curing mechanism with the increasing in temperature. The TG demonstrated that PBZ from BBA-a and BBA-bra exhibited a high thermal stability than the commercial bisphenol-based PBZ due to the high content of aromatic rings. The bromine groups introduced into the backbone of PBZ led to a significant improvement of the temperature of decomposition and the char yield.
References
Ghosh NN, Kiskan B, Yagci Y. Polybenzoxazines-new high performance thermosetting resins: synthesis and properties. Prog Polym Sci. 2007;32:1344–91.
Ning X, Ishida H. Phenolic materials via ring-opening polymerization of benzoxazines: effect of molecular structure on mechanical and dynamic mechanical properties. J Polym Sci Part B Polym Phys. 1994;32:921–7.
Ning X, Ishida H. Phenolic materials via ring-opening polymerization: synthesis and characterization of bisphenol-A based benzoxazines and their polymers. J Polym Sci Part A Polym Chem. 1994;32:1121–9.
Wang H, Zhu R, Yang P, Gu Y. A study on the chain propagation of benzoxazine. Polym Chem. 2016;7:860–6.
Ohashi S, Pandey V, Arza CR, Froimowicz P, Ishida H. Simple and low energy consuming synthesis of cyanate ester functional naphthoxazines and their properties. Polym Chem. 2016;7:2245–52.
Lochab B, Varma I, Bijwe J. Blends cardanol-based bisbenzoxazines. J Therm Anal Calorim. 2012;107:661–8.
Biru I, Damian CM, Gârea SA, Iovu H. Benzoxazine-functionalized graphene oxide for synthesis of new nanocomposites. Eur Polym J. 2016;83:244–55.
Lin CH, Huang CM, Wong TI, Chang HC, Juang TY, Su WC. High-Tg and low-dielectric epoxy thermosets based on a propargyl ether-containing phosphinated benzoxazine. J Polym Sci Part A Polym Chem. 2014;52:1359–67.
Zou T, Li S, Huang W, Liu X. Comparison of two bisbenzoxazines containing carboxylic groups and their thermal polymerization. Des Monomers d Polym. 2013;16:25–30.
Liu Y, Yue Z, Li Z, Liu Z. Thermal degradation behavior and kinetics of polybenzoxazine based on bisphenol-S and aniline. Thermochim Acta. 2011;523:170–5.
Liu Y, Yue Z, Gao J. Synthesis, characterization, and thermally activated polymerization behavior of bisphenol-S/aniline based benzoxazine. Polymer. 2010;51:3722–9.
Choi SW, Ohba S, Brunovska Z, Hemvichian K, Ishida H. Synthesis, characterization and thermal degradation of functional benzoxazine monomers and polymers containing phenylphosphine oxide. Polym Degrad Stabil. 2006;91:1166–78.
Wang J, Fang X, Wu M, He X, Liu W, Shen X. Synthesis, curing kinetics and thermal properties of bisphenol-AP-based benzoxazine. Eur Polym J. 2011;47:2158–68.
Baranek AD, Kendrick LL, Narayanan J, Tyson GE, Wand S, Patton DL. Flexible aliphatic-bridged bisphenol-based polybenzoxazines. Polym Chem. 2012;3:2892–900.
Puchot L, Verge P, Fouquet T, Vancaeyzeele C, Vidal F, Habibi Y. Breaking the symmetry of dibenzoxazines: a paradigm to tailor the design of bio-based thermosets. Green Chem. 2016;18:3346–53.
Yan H, Sun C, Fang Z, Liu X, Zhu J, Wang H. Synthesis of an intrinsically flame retardant bio-based benzoxazine resin. Polymer. 2006;97:418–27.
Lochab B, Varma I, Bijwe J. Blends of benzoxazine monomers. J Therm Anal Calorim. 2013;111:1357–64.
Shukla S, Lochab B. Role of higher aromatic content in modulating properties of cardanol based benzoxazines. Polymer. 2016;99:684–94.
Li S, Yan S, Yu J, Yu B. Synthesis of benzoxazine-based phenolic resins from renewable resources and the properties of their polymers. J Appl Polym Sci. 2011;122:2843–8.
Li S, Zou T. Synthesis, characterization of new carboxylic acid-containing benzoxazine and its cocuring behaviors with bisoxazoline. J Appl Polym Sci. 2012;123:922–8.
Li S, Zou T, Feng L, Liu X, Tao M. Preparation and properties of cardanol-based benzoxazine/SiO2 hybrids by sol–gel technique. J Appl Polym Sci. 2013;128:4164–71.
Li S, Tao M. Preparation and properties of copolymer resins based on phenolphthalein benzoxazine–benzoic acid and bisoxazoline. J Therm Anal Calorim. 2013;113:633–9.
Li S, Yan S. Synthesis and characterization of novel biobased benzoxazines from cardbisphenol and the properties of their polymers. RSC Adv. 2015;5:61808–14.
Li S, Huang W, Liu X, Yu X, Xiao W. Synthesis, characterization and polymerization of brominated benzoxazine monomers and thermal stability/flame retardance of the polymers generated. Polym Adv Technol. 2010;21:229–34.
Atta AM, Abdou MI, Elsayed AA, Ragab ME. New bisphenol novolac epoxy resins for marine primer steel coating applications. Prog Org Coat. 2008;63:372–6.
Santhosh Kumar KS, Reghunadhan Nair CP, Radhakrishnan TS, Ninan KN. Bis allyl benzoxazine: synthesis, polymerization and polymer properties. Eur Polym J. 2007;43:2504–14.
Andreu R, Reina JA, Ronda JC. Studies on the thermal polymerization of substituted benzoxazine monomers: electronic effects. J Polym Sci Part A Polym Chem. 2008;46:3353–66.
Acknowledgements
The project sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, National Technology Support Program (2015BAB07B04), China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Yang, C., Li, C. et al. Synthesis, characterization of new bisphenol-based benzoxazines and the thermal properties of their polymers. J Therm Anal Calorim 128, 1711–1717 (2017). https://doi.org/10.1007/s10973-017-6099-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6099-5