Abstract
High-molecular-weight (HMW) benzoxazines were synthesized by Mannich condensation with various combinations of bisphenols and diamines, and the mechanical and thermal properties of the polybenzoxazines derived from the HMW benzoxazines were measured by tensile tests and thermogravimetric analysis (TGA) to investigate the structure-property relationship. Free-standing precursor films were easily obtained from the solutions of HMW benzoxazines by a cast method on glass plates, and transparent and very tough polybenzoxazine films were obtained by thermally curing the precursor films at a temperature up to 240 °C. The polybenzoxazine films showed higher tensile strength and larger elongation at break than typical polybenzoxazine, i.e., PB-a obtained from low-molecular weight benzoxazine synthesized from bisphenol-A and aniline. In particular, among the HMW polybenzoxazines presented in this work, the PODP-oda film derived from 4,4′-oxydiphenol and 4,4′-oxydianiline showed remarkably good mechanical properties (E = 3.7 GPa, sb = 125 MPa, and eb = 4.5%). Moreover, the PODP-oda film revealed a higher 5 wt% weight loss temperature (Td5 = 332 °C) and char yield at 850 °C (CY850 = 58%) than PB-a (Td5 = 301 °C and CY850 = 42%), as evidenced by TGA, suggesting good thermal stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Polybenzoxazines are a new type of phenolic resin and are obtained by ring-opening polymerization of benzoxazines synthesized from phenols, amines, and formaldehyde [1, 2]. Polybenzoxazines possess advantageous properties associated with traditional phenolic resins, such as good thermal and flame-retardant properties. In addition, polybenzoxazines have other properties that are not found in traditional phenolic resins, such as excellent dimensional stability, low water absorption, low dielectric constants, and low surface free energy [3,4,5,6,7]. Due to these properties, polybenzoxazines are expected to be used as the matrix resin for fiber-reinforced plastics, adhesives, rigid printed circuit boards, and other high-tech applications.
However, the major shortcoming of polybenzoxazines is their lack of toughness, which is a common problem for thermosetting resins. To date, various approaches have been proposed to improve the toughness of polybenzoxazines. One approach is alloying with elastomers such as various siloxanes [8,9,10,11] and liquid rubber [12, 13]. This approach was successful for the toughening of brittle polybenzoxazines without losing their good thermal properties. A second approach is the molecular design of the benzoxazine monomer by introducing flexible linkages such as aliphatic bridges [14, 15]. This approach was effective for the toughening of cured polybenzoxazines but detrimental to the thermal properties. The third approach is the design of high-molecular-weight (HMW) benzoxazines prepared by the Mannich condensation reaction of bisphenol-A and diamine with paraformaldehyde, affording a polymer containing the benzoxazine moiety in the main chain [16]. The polymer containing benzoxazines in the main chain is easily soluble in ordinary solvents and gives flexible and tough uncured films that are easy to handle. Surprisingly, after curing, the films became extremely tough, even though the crosslink density is supposed to be very high [16, 17]. The cured films also showed improved thermal properties. Inspired by the great success of this approach [16,17,18,19,20,21], various HMW benzoxazines have been developed by utilizing synthetic reactions such as azide-alkyne cycloaddition [22, 23], Diels-Alder reactions [24, 25], and hydrosilylation [26].
HMW benzoxazines are easy to prepare via the Mannich reaction, and many bisphenols and diamines can be used as monomers of HMW benzoxazines. We were interested in examining how much the mechanical properties of HMW benzoxazines obtained from the Mannich reaction can be improved. In this paper, we report the elucidation of the structure-property relationship, focusing mainly on the toughening effect. As a result, we found that the polybenzoxazine obtained from the HMW benzoxazine composed of 4,4′-oxydiphenol and 4,4′-oxydianiline, which incorporated ether linkages in both bisphenol and diamine units, showed remarkably good tensile properties with improved thermal stability.
Experimental section
Measurements
1H NMR spectra were recorded with a Varian Mercury 300 spectrometer (300 MHz for 1H) in chloroform-d (CDCl3) at 25 °C using tetramethylsilane as the internal standard. Size exclusion chromatography (SEC) measurements were performed with a Jasco (Hachioji, Japan) PU-1580 liquid chromatograph equipped with a refractive index (Jasco, RI-2031plus) and a UV–visible (254 nm; Jasco UV-1570) detector. Two Shodex (Tokyo, Japan) KF806L SEC columns were connected in series at a flow rate of 1.0 mL/min, and tetrahydrofuran (THF) was used as the eluent. The molecular weight calibration curves were obtained with polystyrene standards (Shodex). Differential scanning calorimetry (DSC) measurements were carried out using a Rigaku (Akishima, Japan) Thermo Plus 2 DSC 8230 instrument, and samples were heated at a heating rate of 10 °C/min under a nitrogen atmosphere. Thermogravimetric analysis (TGA) was performed with a Rigaku Thermo Plus 2 TG8120 system, and samples were heated at a heating rate of 5 °C/min under argon. The tensile properties of films with lengths of ~2 cm were determined with an Imada Seisaku-sho (Toyohashi, Japan) Model SV-3 system operated at a crosshead speed of 0.5 mm/min. The tensile properties of each sample were determined from an average of at least ten tests.
Materials
Bisphenol-A (BPA), 1,2-ethylenediamine (eda), 1,6-hexanediamine (hda), 1,4-dioxane, chloroform, and formalin (37%) were purchased from Kishida Chemicals (Japan). Bisphenol-S (BPS), 4,4′-oxydiphenol (ODP), 4,4′-thiodiphenol (TDP), 4,4′-dihydroxybenzophenone (4,4′-carbonyldiphenol, CDP), 4,4′-oxydianiline (oda), 4,4′-methylenedianiline (mda), and paraformaldehyde were obtained from Tokyo Chemical Industry (Japan). 4,4′-Dihydroxybiphenyl (BP), 1-methyl-2-pyrrolidone (NMP), N,N-dimethylformamide (DMF), and THF were purchased from Wako Pure Chemical Industries (Japan). These reagents and solvents were used as received. B-a, which is a typical bifunctional benzoxazine, was synthesized from bisphenol-A, aniline and formalin according to the literature [3, 5].
Synthesis of high-molecular-weight benzoxazines
A typical experimental procedure used to obtain BPA-hda is described below. Into a 200 mL round-bottom flask equipped with a magnetic stirrer, 50 mL of 1,4-dioxane, 1,6-hexanediamine (1.16 g, 10 mmol), and formalin (37%, 3.24 g, 40 mmol) was added and mixed at room temperature for 20 min. To the suspension, bisphenol-A (2.28 g, 10 mmol) was added at room temperature, and then, the mixture was gradually heated and kept under reflux for 5 h. After the reaction time, the reaction mixture was cooled to room temperature, and the solvent was removed at a reduced pressure by using a rotary evaporator. The residual compound was dissolved in CHCl3, and the insoluble part was removed by filtration. The filtrate was washed three times with 1 N NaHCO3 aq. and several times with pure water until a neutral pH was obtained and then dried with anhydrous sodium sulfate. Chloroform was removed at a reduced pressure by using a rotary evaporator and then by using a vacuum pump at room temperature for 24 h. BPA-hda was obtained as a powder (3.34 g, 8.5 mmol) with a 85% yield. The chemical structure of BPA-hda was confirmed by 1H NMR. 1H NMR (CDCl3, 300 MHz): δ (ppm) = 1.36 (-CH2-), 1.60 (-CH3), 2.73 (-N-CH2-), 3.96 (Ar-CH2-N), 4.83 (O-CH2-N), 6.46-7.15 (aromatic).
Other HMW benzoxazines were prepared similarly. The reaction conditions are summarized in Table 1. The 1H NMR spectra are shown in the Supporting Information.
Preparation and curing of precursor films
A typical procedure used to prepare and cure BPA-hda is described below. A DMF solution of BPA-hda at a concentration of 15 wt% was cast on a silane-treated glass plate, and the solvent was removed by drying at 50 °C for 10 h under vacuum to afford a free-standing yellowish transparent film. The obtained precursor film was flexible and tough. To control the thickness, the film was pressed between glass plates using a spacer. Gradual thermal curing of the film was performed at 100 and 150 °C for 2 h each under reduced pressure and in a vacuum oven, and then at 200 and 240 °C for 2 h each in an air-circulating oven to give a brown-colored tough PBPA-hda film. The other benzoxazines were cured in the same way.
Results and discussion
Synthesis of various high-molecular-weight benzoxazines by combining bisphenol and diamine
The chemical structures of the bisphenols and diamines used in the synthesis of the HMW benzoxazines are shown in Scheme 1. For the bisphenol compounds with different spacers connecting the phenols at the para-position, bisphenol-A (BPA), 4,4′-dihydroxybiphenyl (BP), 4,4′-oxydiphenol (ODP), 4,4′-thiodiphenol (TDP), 4,4′-dihydroxybenzophenone (4,4′-carbonyldiphenol, CDP), and bisphenol-S (BPS) were used. For the diamine compounds, 1,2-ethylenediamine (eda) and 1,6-hexanediamine (hda) of aliphatic type and 4,4′-methylenedianiline (mda) and 4,4′-oxydianiline (oda) of aromatic type were used. The Mannich condensation reaction was performed using various combinations of these bisphenols and diamines in organic solvents to synthesize various HMW benzoxazines (Scheme 1). The reaction conditions and yields are summarized in Table 1. The sample code is abbreviated based on the bisphenol and diamine used, such as BPA-eda.
When BPA was used as the bisphenol compound, the Mannich condensation reaction with the aliphatic diamine proceeded regardless of whether dioxane or chloroform was used as the solvent, and HMW benzoxazines soluble in organic solvents were obtained. On the other hand, when BPA was reacted with aromatic diamines, soluble HMW benzoxazines were obtained in chloroform, but an insoluble gel was precipitated as the main product in dioxane. Therefore, chloroform was used as the solvent for aromatic diamine.
When bisphenols that have relatively flexible spacers, such as BPA, ODP, and TDP, were used, soluble HMW benzoxazines were obtained in good yields, regardless of which diamine was used. However, when bisphenols that have relatively rigid spacers, such as BP, CDP, and BPS, were used, soluble HMW benzoxazines were obtained only by using hda as the diamine compound, and insoluble precipitates were obtained by using eda or oda. The yield of HMW benzoxazines was higher than 85% except when a rigid bisphenol, such as BP, was used. Thus, it was made clear that to obtain soluble HMW benzoxazines, it is necessary to introduce a flexible unit into either the bisphenol or the diamine component, and it is also necessary to appropriately select the solvent for synthesis.
Characterization of high-molecular-weight benzoxazines
The SEC measurement of the HMW benzoxazines using THF as the eluent revealed that the number-average molecular weights (Mns) were 1000–2000, corresponding to oligomers (Table 1). However, the weight average molecular weights (Mws) were 5000–20,000 owing to the wide molecular weight distributions. The chemical structure of HMW benzoxazines was confirmed by 1H NMR measured in chloroform-d (CDCl3) or dimethyl sulfoxide-d6 (DMSO-d6). Fig. 1a shows the 1H NMR spectrum of BPA-eda. The presence of the benzoxazine-ring structure was confirmed by the signals at 4.88 ppm due to O-CH2-N (a) and 3.99 ppm due to the Ar-CH2-N (b) of the oxazine-ring. The signals due to the methylene protons of the eda unit were also observed at 2.98 ppm (c). The integral ratio of the (a) and (c) signals (Aa/Ac = 380/400) did not agree with the theoretical value expected from the chemical structure, suggesting that the HMW benzoxazine obtained by Mannich condensation included some defect structures, such as the ring-opened structure of the benzoxazine unit (Fig. S1 in the Supporting Information), affording phenolic hydroxyl groups [16, 17]. Based on the integration ratio, the ratio of the benzoxazine-ring structure in the chain was estimated to be 95% for BPA-eda. The ratios of other HMW benzoxazines are summarized in Table 1. The ratios of HMW benzoxazines obtained from the aliphatic diamines were relatively high (80–95%), while the ratios of HMW benzoxazines obtained from the aromatic diamines tended to be lower (47–75%).
In addition, the chemical shifts of the oxazine-ring signals obtained for BPA-hda (4.0 and 4.8 ppm in Fig. 1b) were similar to those of BPA-eda. The oxazine-ring signals of BPA-mda and BPA-oda obtained from the aromatic diamines shifted to a lower magnetic field (4.5 and 5.3 ppm, c and d in Fig. 1) than those of BPA-eda. This tendency of the oxazine-ring signals to shift was also observed in other HMW benzoxazines (Figs. S2–S4 in the Supporting Information).
The ring-opening polymerization behavior of the HMW benzoxazines was investigated by DSC. Fig. 2 shows some DSC thermograms of the HMW benzoxazines after drying under reduced pressure, together with that of a typical low-molecular-weight benzoxazine, i.e., B-a obtained from bisphenol-A and aniline. The DSC curve of B-a revealed a sharp exothermic peak corresponding to the ring-opening polymerization of benzoxazine (a in Fig. 2). In contrast, the exothermic peaks of HMW benzoxazines were broadened and shifted toward a lower temperature than that of B-a (b–g in Fig. 2). A small amount of the phenolic hydroxyl group of the ring-opened structure might work as an acid catalyst and lower the polymerization temperature.
The solubility of HMW benzoxazines was examined in chloroform, THF, 1,4-dioxane, DMF, and NMP at a concentration of ca. 17 wt%, and the results are summarized in Table 2. The HMW benzoxazines obtained from the relatively flexible bisphenols, BPA, ODP, and TDP, showed high solubilities in the solvents. The solubility tended to decrease when relatively rigid units such as BP, CDP and BPS were introduced. From the result and the boiling point of the solvents, DMF was selected to prepare the precursor film by a cast method.
Preparation and curing of precursor films
DMF solutions of HMW benzoxazines at a concentration of 15 wt% were cast on silane-treated glass plates. The solvent was removed by drying at 50 °C for 10 h under a reduced pressure to give transparent, yellow-colored films. Note that the free-standing tough films were obtained easily, even though the molecular weights were not so high. Such precursor films could not be obtained from typical low-molecular-weight benzoxazine monomers, such as B-a.
The precursor films were then thermally treated stepwise at 100, 150, 200, and 240 °C for 2 h each. It was confirmed from the DSC measurements of the films that after heat treatment at 240 °C, no exothermic peak was observed, which proves that the temperature was high enough to nearly complete the ring-opening polymerization of benzoxazines (Fig. S5 in the Supporting Information).
After curing at a temperature up to 240 °C, the HMW benzoxazine films gave free-standing yellow- to brown-colored transparent polybenzoxazine films (Fig. 3). The polybenzoxazine films were able to bend easily and possessed an extremely improved toughness compared to the typical polybenzoxazine, PB-a (Fig. S6 in the Supporting Information). The toughness of the films is described in detail later based on the tensile test results.
The appearance of the cured films is shown in Fig. 3. The PBPA-hda film obtained from bisphenol-A and aliphatic diamine was yellow in color (a), while PBPA-oda obtained from aromatic diamine was brown (b). Moreover, when ODP was used as the bisphenol component, the film obtained from the aromatic diamine, PODP-oda, was darker in color (d) than the film obtained using aliphatic diamine (c). The coloring of the film tended to become darker as the aromatic content increased.
Mechanical properties of polybenzoxazines prepared from HMW benzoxazines
The mechanical properties of the polybenzoxazines were investigated by tensile testing of the films. The stress-strain curves are shown in Fig. 4, and the results are summarized in Table 3. The typical polybenzoxazine film, PB-a, showed a high tensile modulus (E = 3.3 GPa) but low tensile strength (sb = 37 MPa) and elongation at break (eb = 1.6%), consistent with the brittleness of the film (Fig. 4A). On the other hand, all the polybenzoxazine films obtained from the HMW benzoxazines revealed significantly higher elongation at break than PB-a accompanied by an increase in tensile strength, showing that the HMW benzoxazines gave tough and flexible polybenzoxazine films.
Stress-strain curves of a PB-a film and the polybenzoxazine films obtained from HMW benzoxazines. a Influence of the bisphenol unit while fixing the diamine as 1,6-hexanediamine (hda). b Influence of the diamine unit while fixing the bisphenol as bisphenol-A (BPA). c Influence of the diamine unit while fixing the bisphenol as 4,4′-oxydiphenol (ODP)
To evaluate the effect of the bisphenol structure, the stress-strain curves of polybenzoxazine films obtained by using hda with various bisphenols are shown in Fig. 4A. Compared with PB-a, the PBPA-hda film obtained from bisphenol-A showed a higher tensile strength of 80 MPa and elongation at break of 3.7%. The slight decrease in the tensile modulus of PBPA-hda (E = 2.5 GPa) compared with that of PB-a was attributed to the long aliphatic diamine unit. The other polybenzoxazines shown in Fig. 4A have relatively rigid bisphenol units, and the tensile modulus was improved to 2.8–3.2 GPa without sacrificing the tensile strength and elongation at break.
To evaluate the effect of diamine components, Fig. 4B shows the stress-strain curves of the HMW polybenzoxazines in which bisphenol-A (BPA) was fixed as the bisphenol unit and various diamines were used. Among the films, PBPA-hda showed the lowest modulus (2.5 GPa) and a high elongation at break (3.7%) because of the long flexible aliphatic hda unit. On the other hand, PBPA-eda showed the highest tensile modulus (4.0 GPa) and lowest elongation at break (2.1%) because of the short eda unit that makes the film relatively rigid. The PBPA-mda and PBPA-oda films obtained from aromatic diamines showed higher tensile strength and elongation at break than the films obtained from aliphatic diamines. In particular, the use of ODA gives the toughest and most flexible films, as evidenced by its having the highest tensile strength (108 MPa) and elongation at break (4.1%). One possible reason for the high toughness of the films composed of aromatic diamines may be the low ratio of ring-closed structures in the HMW benzoxazine chain, as suggested by the 1H NMR analysis. Another possible reason is that the basicity of the aromatic diamines is weaker than that of the aliphatic diamines, leading to weaker intramolecular hydrogen bonding between the nitrogen of the amine and phenolic hydroxyl group and thus increasing the concentration of the intermolecularly hydrogen bonded species. It is considered that the moderately increased intermolecular interaction leads to an increase in the physical crosslinks that make the films tough.
Based on the above results showing that the use of oda-containing ether linkage as the diamine unit gave the toughest and most flexible cured films, ODP, which also contains an ether linkage, was selected as the bisphenol unit and combined with various diamines. The stress-strain curves are shown in Fig. 4C. The four stress-strain curves in Fig. 4B and 4C look very similar, showing that the tendency of diamines found in the case of PBPA is the same as that found in the case of PODP. Among the HMW polybenzoxazine films prepared in this study, the PODP-eda film showed the highest tensile modulus (E = 4.6 GPa), but the tensile strength and elongation at break were not so high. In contrast, the PODP-oda film showed the highest tensile strength (sb = 125 MPa) and elongation at break (eb = 4.5%) and a high tensile modulus (3.7 GPa), showing that this film was the toughest among the films examined. Note that both ODP and oda contain ether linkages.
The toughening effect of the ether linkages has been observed in various polymers. In the case of polyimide, the completely rigid-rod polyimide, PI(PMDA/PDA), which can be prepared from pyromellitic dianhydride (PMDA) and p-phenylene diamine (PDA), was expected to give films with a very high modulus because of the rigidity of the molecular structure. However, PI(PMDA/PDA) was found to be very brittle and almost impossible to handle as a film [27]. However, by introducing ether linkages, for example, into diamine by using oda, the resultant PI(PMDA/ODA) gives surprisingly tough polyimide films with an elongation at break as high as 80–100% [28]. Therefore, PI(PMDA/ODA) films are currently one of the most important commercially available polyimide films.
Thermal stability of polybenzoxazines prepared from HMW benzoxazines
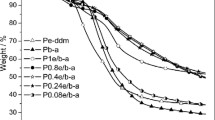
The thermal stability of HMW polybenzoxazine and PB-a films was evaluated by TGA under an argon atmosphere, and the 5% weight loss temperature (Td5) and char yield at 850 °C (CY850) are summarized in Table 4. The Td5 and CY850 of PB-a were 301 °C and 42%, respectively. The observed Td5 value was consistent with a previous report in which the decomposition and elimination of the amine moiety (aniline unit) occurred at ~300 °C in the initial stage of the thermal decomposition of PB-a [29, 30]. In the HMW polybenzoxazines, the diamine moiety is incorporated into the network structure, and a high-temperature shift in the thermal decomposition temperature, such as Td5, is expected. Actually, as shown in the TGA curves (Fig. 5), higher decomposition temperatures were achieved for HMW polybenzoxazines. The exceptions are polybenzoxazines that contain aliphatic diamines (hda and eda).
The effect of the diamine moiety was examined by fixing the bisphenol as BPA and using various diamines. It was clearly shown that the use of aliphatic diamines gave lower Td5 values, i.e., 301 °C (hda) and 285 °C (eda), and the use of aromatic diamines gave higher Td5 values, i.e., 336 °C (mda) and 323 °C (oda). The same trend was also observed for CY850; the use of aliphatic diamines gave lower CY850 values, i.e., 29% (hda) and 36% (eda), and the use of aromatic diamines gave higher CY850 values, i.e., 51% (mda) and 48% (oda). This result indicates that the use of aromatic diamines gives polybenzoxazines with much better thermal stability than those given by the use of aliphatic diamines.
The effect of bisphenols was also examined. Although the effect was not as clear among the bisphenols employed in this study, a difference in CY850 was observed. The CY850 of polybenzoxazine was the lowest when BPA containing the aliphatic moiety was used as the bisphenol; the CY850 of PBPA-hda was 29%. However, when bisphenols that do not contain the aliphatic moiety were used, even when using hda as the diamine, CY850 increased to 38% for PBP-hda and PTDP-hda, 44% for PODP-hda and PCDP-hda, and 46% for PBPS-hda. The difference also suggests that higher CY850 values can be achieved by using bisphenols that do not contain the aliphatic moiety.
It can be concluded that among the HMW polybenzoxazines, PODP-mda (Td5 = 342 °C, CY850 = 61%) and PODP-oda (Td5 = 332 °C, CY850 = 62%) had excellent thermal stability, giving processable and tough polybenzoxazine films. The reason for this is that the amine and bisphenol moieties were incorporated into the network structure of polybenzoxazine through thermally stable aromatic structures. On the basis of the CY850 values, the limiting oxygen index (LOI) of the PODP-mda and PODP-oda films was estimated to be 42 [31], suggesting that the films had good flame retardancy.
Conclusions
We synthesized various soluble HMW benzoxazines via the Mannich reaction with various combinations of bisphenols and diamines and investigated the structure-property relationship of the polybenzoxazine films derived from the HMW benzoxazines. Free-standing and tough precursor films were easily obtained by casting DMF solutions of HMW benzoxazines on glass plates. By thermally curing the precursor films at temperatures up to 240 °C, transparent and very tough polybenzoxazine films were fabricated. In the tensile test, the polybenzoxazine films showed remarkably higher tensile strength and larger elongation at break than PB-a. Comparing the chemical structures shows that the influence of the diamine moiety was greater than that of the bisphenol moiety on the mechanical properties, and the HMW polybenzoxazines composed of aromatic diamines showed higher tensile strength and elongation at break than those composed of aliphatic diamines. In particular, among the HMW polybenzoxazines presented in this study, PODP-oda with ether linkages in both bisphenol and diamine units showed remarkably good mechanical properties (E = 3.7 GPa, sb = 125 MPa, eb = 4.5%) as a highly cross-linked thermoset. We would like to emphasize that the introduction of ether linkages in the main chain of HMW benzoxazines is extremely effective for the toughening of polybenzoxazine films. Moreover, the HMW polybenzoxazines composed of aromatic moieties showed good thermal stability: Td5 = 332 °C and CY850 = 58% for PODP-oda. Bisphenol and diamine compounds are raw materials that can be used for various organic compounds, including polymer materials, and are widely used in industry. We reported here that HMW benzoxazines can be tailored with various combinations of bisphenols and diamines and that the properties of their thermosets can be tuned for different applications. Therefore, the polybenzoxazines derived from HMW benzoxazines can be good candidates for various applications, including for use as matrix resins of advanced composite materials, adhesive films, precision machinery parts, and microelectronics, because of their outstanding mechanical and thermal properties.
References
Ishida H, Froimowicz P. Advanced and emerging polybenzoxazine science and technology. Amsterdam: Elsevier; 2017.
Ishida H, Agag T. Handbook of benzoxazine resins. Amsterdam: Elsevier; 2011.
Ning H, Ishida H. Phenolic materials via ring-opening polymerization: synthesis and characterization of bisphenol-A based benzoxazines and their polymers. J Polym Sci Part A Polym Chem. 1994;32:1121–9.
Ishida H, Allen DJ. Physical and mechanical characterization of near-zero shrinkage polybenzoxazines. J Polym Sci Part B Polym Phys. 1996;34:1019–30.
Shen SB, Ishida H. Synthesis and characterization of polyfunctional naphthoxazines and related polymers. J Appl Polym Sci. 1996;34:1595–605.
Takeichi T, Agag T, Yong G. Synthesis and properties of polybenzoxazine based composites. Recent Res Devel Polym Sci. 2000;4:85–105.
Su Y-C, Chang F-C. Synthesis and characterization of fluorinated polybenzoxazine material with low dielectric constant. Polymer 2003;44:7989–96.
Ardhyananta H, Wahid MH, Sasaki M, Agag T, Kawauchi T, Ismail H, et al. Performance enhancement of polybenzoxazine by hybridization with polysiloxane. Polymer 2008;49:4585–91.
Ardhyananta H, Kawauchi T, Ismail H, Takeichi T. Effect of pendant group of polysiloxanes on the thermal and mechanical properties of polybenzoxazine hybrids. Polymer 2009;50:5959–69.
Ardhyananta H, Kawauchi T, Takeichi T, Ismail H. Preparation and properties of polybenzoxazine/poly(dimethylsiloxane-co-diphenylsiloxane) hybrids as high performance polymers. High Perform Polym. 2010;22:609.
Takeichi T, Agag T, Zeidam R. Preparation and properties of polybenzoxazine/poly(imide-siloxane) alloys: In situ ring-opening polymerization of benzoxazine in the presence of soluble poly(imide-siloxane)s. J Polym Sci Part A Polym Chem. 2001;39:2633–41.
Jang J, Seo D. Performance improvement of rubber-modified polybenzoxazine. J Appl Polym Sci. 1998;67:1–10.
Suwitaningsih DN, Katsuta S, Kawauchi T, Furukawa N, Takeichi T. Preparation and characterization of liquid rubber-modified polybenzoxazine. J Photopolym Sci Technol. 2015;28:137–43.
Allen DJ, Ishida H. Physical and mechanical properties of flexible polybenzoxazine resins: effect of aliphatic diamine chain length. J Appl Polym Sci. 2006;101:2798–809.
Baranek AD, Kendrick LL, Narayanan J, Tyson GE, Wand S, Patton DL. Flexible aliphatic-bridged bisphenol-based polybenzoxazines. Polym Chem. 2012;3:2892–900.
Takeichi T, Kano T, Agag T. Synthesis and thermal cure of high molecular weight polybenzoxazine precursors and the properties of the thermosets. Polymer. 2005;46:12172–80.
Takeichi T, Kano T, Agag T, Kawauchi T, Furukawa N. Preparation of high molecular weight polybenzoxazine prepolymers containing siloxane unites and properties of their thermosets. J Polym Sci Part A Polym Chem. 2010;48:5945–52.
Chernykh A, Liu J-P, Ishida H. Synthesis and properties of a new crosslinkable polymer containing benzoxazine moiety in the main chain. Polymer. 2006;47:7664–9.
Takeichi T, Uchida S, Inoue Y, Kawauchi T, Furukawa N. Preparation and properties of polymer alloys consisting of high-molecular-weight benzoxazine and bismaleimide. High Perform Polym. 2013;26:265–73.
Uchida S, Kawauchi T, Furukawa N, Takeichi T. Polymer alloys of high-molecular-weight benzoxazine and epoxy resin. High Perform Polym. 2014;26:846–55.
Ohara M, Yoshimoto K, Kawauchi T, Takeichi T. Synthesis of high-molecular-weight benzoxazines having azomethine linkages in the main-chain and the properties of their thermosetting resins. Polymer. 2020;202:122668.
Nagai A, Kamei Y, Wang X-S, Omura M, Sudo A, Nishida H, et al. Synthesis and crosslinking behavior of a novel linear polymer bearing 1,2,3-triazol the main chain by and benzoxazine groups in a step-growth click-coupling reaction. J Polym Sci Part A Polym Chem. 2008;46:2316–25.
Chernykh A, Agag T, Ishida H. Synthesis of linear polymers containing benzoxazine moieties in the main chain with high molecular design versatility via click reaction. Polymer. 2009;50:382–90.
Liu Y-L, Chou C-I. High performance benzoxazine monomers and polymers containing furan groups. J Polym Sci Part A Polym Chem. 2005;43:5267–82.
Chou C-I, Liu Y-L. High performance thermosets from a curable Diels-Alder polymer possessing benzoxazine group in the main chain. J Polym Sci Part A Polym Chem. 2008;46:6509–17.
Kiskan B, Aydogan B, Yagci Y. Synthesis, characterization, and thermally activated curing of oligosiloxanes containing benzoxazine moieties in the main chain. J Polym Sci Part A Polym Chem. 2009;47:804–11.
Takeichi T, Endo Y, Kaburagi Y, Hishiyama Y, Inagaki M. Carbonization and graphitization of polyimide films: effect of size of leaving group at imidization. J Appl Polym Sci. 1998;68:1613–20.
Shirai Y, Takahashi K, Kawauchi T, Takeichi T. Preparation and properties of polyimide-polysiloxane hybrids using sol-gel method. J Photopolym Sci Technol. 2013;26:333–40.
Low H-Y, Ishida H. Mechanistic study on the thermal decomposition of polybenzoxazines: effects of aliphatic amines. J Polym Sci Part B Polym Phys. 1998;36:1935–46.
Low H-Y, Ishida H. Structural effects of phenols on the thermal and thermo-oxidative degradation of polybenzoxazines. Polymer 1999;40:4365–76.
van Krevelen DW. Some basic aspects of flame resistance of polymeric materials. Polymer. 1975;16:615–20.
Acknowledgements
TK acknowledges financial support from the Ryukoku University Science and Technology Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Murai, Y., Uemura, T., Chen, Y. et al. Synthesis of high-molecular-weight benzoxazines from various combinations of bisphenols and diamines via Mannich condensation and properties of their thermosets. Polym J 53, 439–447 (2021). https://doi.org/10.1038/s41428-020-00438-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-00438-y
- Springer Nature Limited
This article is cited by
-
Synthesis of thermosets from maleimidobenzoxazines and tetrafunctional thiols and their thermal and mechanical properties
Polymer Journal (2024)
-
Polymer alloys with high thermal properties consisting of polyfunctional benzoxazine derived from an oligonuclear phenolic compound and bismaleimide
Polymer Journal (2024)
-
A Study of the Thermal Properties of Main-Chain Polybenzoxazines Copolymerized with Nitrile-Benzoxazine
Fibers and Polymers (2023)
-
CHCl3/triethanolamine: a new mixed solvent for preparing high-molecular-weight main-chain benzoxazines through Mannich-type polycondensation
Polymer Journal (2022)