Abstract
This paper describes the thermal investigations and kinetic analysis regarding the solid-state degradation of three compounds used as mental disorder therapeutic agents (antidepressants), namely amitriptyline, desipramine and imipramine. The study was carried according to ICTAC 2000 recommendations, by using three isoconversional methods, namely Flynn–Wall–Ozawa, Kissinger–Akahira–Sunose and Friedman. The differential method of Friedman indicated multistep degradation, which was later confirmed by the nonparametric kinetic method (NPK). NPK method showed that all three tricyclic antidepressants are degraded by two processes. In terms of apparent activation energies for decomposition, the NPK method indicated 123.4 kJ mol−1 for imipramine, 112.3 kJ mol−1 for desipramine and 82.9 kJ mol−1 for amitriptyline, and the results are in good agreement with the ones suggested by isoconversional methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Considering the alarmingly increasing numbers of cases of psychological disorders nowadays, the bioactive substances used in the treatment schemes of these diseases have become a central point in the research field.
Among these substances, tricyclic antidepressants (TCAs) are used since their discovery in 1951, when the first molecule of this class—imipramine—was synthesized [1]. Since then, a number of molecules were discovered and used not only to treat major endogenous depression, but also in the treatment of anxiety states, obsession syndromes [2], neuropathic chronic pain and nocturnal enuresis in children [3].
In order to avoid the decreased levels of neurotransmitters in the synaptic gap, the main cause of depression syndromes, TCAs inhibit the reuptake of serotonin, dopamine or norepinephrine leading to an increase in their levels [2]. In order for the therapeutic effect to appear, a few weeks of treatment are required, despite the fact that the actual absorption of the substances takes about 30 min [4].
Although TCAs have been used a long time, their presence in depression treatment is somewhat controversial mainly because of their severe side effects that appear above the therapeutic range, such as cardiovascular disease, hypotension or even death [5]. However, there are quite a few molecules, such as amitriptyline, imipramine and desipramine, used frequently in therapy at this time.
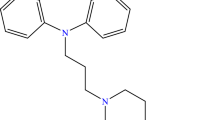
Amitriptyline (AMI) is a dibenzocycloheptene derivative that has a tricyclic ring system and a short hydrocarbon chain linked to the azepine middle ring with a terminal nitrogen atom dimethyl substituted [6]. The molecule has an amphiphilic nature, the tricyclic portion having a hydrophobic character while the tertiary amine is the hydrophilic part. This last one depending on the pH value can become cationic (protonated) at acid pH values and neutral (deprotonated) at basic pH values [7]. Because of these structural characteristics, amitriptyline forms aggregates in solution containing roughly 6–12 monomers [6]. AMI is mainly used as hydrochloride (pKa 9.4). It is a white or almost white crystalline powder, with a melting point of 187–189.5 °C, odorless or with a very delicate smell that has slight anesthetic properties. AMI has good solubility in water (9.71 mg L−1), alcohol, methanol and chloroform, but it is practically insoluble in ether [8].
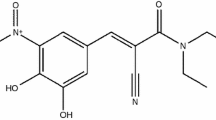
Imipramine (IMI) belongs to the dibenzazepine class. It has two benzene rings connected by a seven-member heterocycle in which one nitrogen atom replaces a carbon atom. The alkylamine side chain due to its hydrophilicity is responsible for the surfactant-like behavior of this chemical bioactive compound. As a result, at different pH values, IMI can acquire a positive or a neutral charge alike with AMI and in water 8–10 monomers can interact forming aggregates [9]. Due to its structural attributes, the unwanted side effects of IMI can be diminished significantly when the side chain of the active substance is encapsulated within a β-cyclodextrin cavity [10]. IMI is a white or slight yellow, sensitive to light, microcrystalline powder that is soluble in water (18.2 mg L−1) and alcohol but is basically insoluble in ether. It has a melting point of 174–175 °C [11].
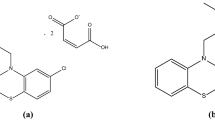
Desipramine (DES) is the active metabolite of IMI, thus belonging in the same organic compound class, dibenzazepine, the only structural difference being the lack of one methyl group at the nitrogen atom from the side chain. Regarding its physicochemical properties, DES comes in the form of white crystals that have a melting point of 214–218 °C, water soluble (58.6 mg L−1) [12].
Structural formulas of investigated compounds are presented in Fig. 1.
The importance of carrying kinetic analysis in the class of bioactive molecules or other compounds used in scientific technology is of great importance nowadays, since it can predict lifetime, stability and the behavior of sample during thermal treatment [13,14,15,16,17,18]. Particular, in pharmaceutical technology, the solid-state stability is crucial in the design and preparation of new pharmaceutical formulation. During processing, a decreased stability of bioactive compounds can lead to decreased biodisponibility or shelf life of the formulation.
Recently, numerous isoconversional kinetic studies were published [13,14,15,16,17,18], but to our knowledge, these three compounds used as antidepressants were not investigated up to the date regarding the kinetic of solid-state decomposition.
According to these considerations and continuing our previous studies on thermal analysis [19,20,21,22,23,24,25,26,27,28], in this paper we present a comparative thermal stability of three similar-structure compounds by kinetic analysis. The strategy is that according to ICTAC 2000 protocol, DTG data obtained at five heating rates (β = 5, 7, 10, 12 and 15 °C min−1) in air were used for the main decomposition process of AMI, IMI and DES. As kinetic methods, Kissinger–Akahira–Sunose, Flynn–Wall–Ozawa, Friedman and NPK were used.
Materials and methods
Samples
Amitriptyline (AMI), imipramine (IMI) and desipramine (DES) were commercial products of Sigma and received as hydrochlorides, with purity as follows: AMI and DES, purity >98%, IMI purity >99%. The samples were used without any a priori purification and kept as requested by the supplier before investigations.
ATR-FTIR spectroscopy
The ATR-FTIR spectra of samples were drawn using a PerkinElmer SPECTRUM 100 device using the UATR technique. The samples were collected after 32 acquisitions, with a resolution of 4 cm−1, on the spectral domain 4000–600 cm−1.
Thermal analysis and kinetic study
Thermoanalytical data TG/DTG/HF were determined using a PerkinElmer DIAMOND TG/DTA instrument. The measurements were carried out in synthetic air atmosphere at a flow rate of 100 mL min−1, using about 7 mg of sample, which were weighted into open aluminum crucibles. The temperature program was chosen to increase linearly from ambient up to 400 °C, at heating rates β = 5, 7, 10, 12 and 15 °C min−1. The kinetic analysis was carried out for the decomposition step that took place between 170 and 330 °C (AMI), 170–310 °C (IMI) and 210–330 °C (DES), which is the main decomposition process of the compound, as suggested by DTG curves.
As kinetic methods, Friedman, Kissinger–Akahira–Sunose, Flynn–Wall–Ozawa and NPK methods were employed. After using linear forms of each isoconversional method, the estimation of E a values was realized for conversion degrees 5% ≤ α ≤ 95%, with a variation step for α of 5%.
Results and discussion
ATR-FTIR analysis
The identity and purity of the compounds were determined by ATR-FTIR spectroscopy. Since all of the compounds have similar structure (Fig. 2), possessing the tricyclic ring and as well similar functional moieties, the FTIR spectra show good resemblance.
The obtained data are in good correlation with the literature [29,30,31], where FTIR band positions (in cm−1) are assigned as presented in Table 1.
Thermal stability study
Thermoanalytical curves are presented for analyzed compounds as follows: the superimposed mass curves (Fig. 3), derivative mass curves (Fig. 4) and heat flow curves (Fig. 5) at selected heating rates. It is clearly visible the effect of modifying the heating rate over the shifting of curves, due to thermal inertia of samples. TG and DTG curves are represented solely on the temperature range 150–350 °C for a better perspective over thermal events.
Thermal decomposition of the studied compounds in air atmosphere begins around 200 °C with DTGpeaks between 230 and 290 °C for AMI, 250–290 °C for IMI and 270–310 °C for DES. An increased thermal stability was observed for all compounds, which was expected due to the presence of the tricyclic moiety in the molecular structure. The literature data mention the melting of these compounds as hydrochlorides at well-defined temperatures, but does not indicate decomposition, or at least, the release of HCl and formation of free bases. In accordance with the chemical structure and by the events indicated by the thermoanalytical curves, it is not expected to observe a classical melting—i.e., a solid–liquid transition, but rather two overlapped events: dehydrochlorination (loss of HCl from the salts and the formation of free bases), followed by the decomposition/thermooxidations of free bases). These events are inseparable during thermolysis of samples and are in good agreement with the melting temperature values reported in the literature that were previously indicated [8, 11, 12].
Each compound exhibits one decomposition stage immediately after melting, with a significant mass loss up to 350 °C (up to 100%).
Even if the thermal behavior is similar, a complete characterization of their stability can be objectively reported solely after performing a complete kinetic analysis.
Kinetic study
As a crucial step in characterization of solid-state stability of compounds under thermal stress and the evaluation of decomposition mechanism, isoconversional kinetic methods offer reliable results [32,33,34].
Isoconversional kinetic methods allow the determination of apparent activation energy without knowing the explicit form of the integral or differential conversion function, but fails in direct revealing of the reaction order (n) and pre-exponential factor (A); however, these methods allow an estimation for the effect of heating rate over the change in decomposition mechanism.
The thermoanalytical data were processed using three well-known and frequently employed isoconversional methods, in agreement with ICTAC 2000 recommendations—the integral methods of Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS) and differential method of Friedman (Fr). For these methods, the theoretical basis and mathematical data were extensively reported in the literature, starting with their first publications [35,36,37,38,39].
The data obtained by isoconversional study were correlated with estimation of kinetic triplet for each compound using the nonparametric kinetics method (NPK), which allowed a concrete separation of parallel steps occurring in thermolysis of compounds, and even attribution to physical versus chemical thermal events. The theoretical aspects regarding the elaboration of NPK method [40, 41] and its modification [42] were previously published.
Since the aim of the paper is to determine the kinetics decomposition for the selected TCAs, and not the theoretical basis of the selected kinetic methods, we briefly present in Table 2 the equations that allow the estimation of E a.
The linear data plotting of data according to the mathematical models presented in Table 2 allowed the estimation of apparent E a values was from the slopes of those lines (Fig. 6), and the results are presented in Table 3.
The analysis of obtained apparent E a at each conversion degree by Friedman method suggests a separation of values into two different groups. According to this, in the case of IMI, the E a values oscillate between 127.4 and 132.3 kJ mol−1 while the conversion degree was below 45%; then, a shifting in the 139.4–159.2 kJ mol−1 range was observed with the advance of conversion up to 95%. These patterns were also revealed by the analysis of values determined for AMI and DES, where irregularities were observed at low conversion degrees versus high conversion degrees. These observations might suggest a modification of degradative mechanism with the modification of temperature, so more than one process of degradation in solid state. In the case of analyzed TCAs, this supposition is pertinent and sustained by the aspect of thermoanalytical curves, where dehydrochlorination is accompanied by thermolysis of organic molecular structure.
In order to determine whether these suppositions are plausible, the NPK method was used as a fourth kinetic method.
As previously mentioned by our research group [20, 25, 26, 33], the NPK method use as starting point the validity of Arrhenius equation, and that the reaction rate can be represented as the product of two functions, f(α) and k(T), which are non-dependant one to another. After creating the reaction rate matrix [20, 33] and its decomposition into a product of two matrixes using the singular value decomposition algorithm [43], and applying a kinetic model suggested by Šesták and Berggren [44], the 3D transformation rate surfaces (Fig. 7) and results presented in Table 4 were obtained.
For IMI, NPK method indicate that the thermal degradation occurs by two processes, the main process having an explained variance λ = 77.0% with both chemical (n = 4/5) and physical contributions (m = 1/4) and the preponderant energetic contribution to the total apparent activation energy (90.73 kJ mol−1). The secondary process has the same reaction orders as main process, but the explained variance is much smaller, i.e., λ = 22.7% and an energetic contribution to the total apparent activation energy of 32.64 kJ mol−1.
In the case of AMI, similar processes are observed, in terms of reaction order. The predominant process has an explained variance λ = 89.8% with both chemical (n = 4/5) and physical contributions (m = 1/4) and an energetic contribution to the total apparent activation energy of 75.97 kJ mol−1. The secondary process has different chemical reaction order as main process (n = 3/2), and an explained variance is considerably smaller (λ = 7.0%), but not negligible, since its contribution to the total apparent activation energy is 6.94 kJ mol−1, which is almost 9% of the medium value.
DES is thermally degraded by two parallel processes, the main contributing process having an explained variance λ = 76.5% with both chemical (n = 4/5) and physical contributions (m = 1/4) as in the case of IMI and AMI. This process has the greatest energetic contribution to the total apparent activation energy (86.29 kJ mol−1). The secondary process is only a chemical decomposition since n = 2 and m = 0 with an explained variance λ = 19.2% and an energetic contribution to the total apparent activation energy of 24.25 kJ mol−1, which is 21.6% of the medium value.
Even if some previous papers, values for explained variance were considered insignificant if λ < 10% and were not taken into account, in the case of AMI were considered, since the medium value for activation energy is not very high (Table 4).
Conclusions
Our study reported the results obtained after investigating the thermal stability and decomposition in solid state of three tricyclic antidepressants, namely amitriptyline, imipramine and desipramine.
The preliminary thermal analysis carried out in oxidative media suggested that the thermal stability of compounds varies in the order IMI < AMI < DES, IMI being stable up to 174 °C, AMI up to 188 °C and DES up to 211 °C.
Isoconversional integral methods suggested single-step degradation by the homogeneity of values E a versus conversion degree, fact that was not confirmed by differential method of Friedman, which suggested a complex route of thermolysis by the variation of E a versus conversion degree. The results indicated by Friedman were later confirmed by the NPK method, which revealed significant differences in the thermal behavior of the three samples: All the compounds are thermodegraded by a process with two significant parallel steps. Concerning the mean values of E a (∑λ·E a), it was observed that IMI and DES show similar stabilities, which were expected due to great structural resemblance, while AMI is less stable, probably due to the presence of C=C bond, more susceptible to oxidations.
Abbreviations
- α :
-
Conversion degree
- T :
-
Temperature
- f(α):
-
Differential conversion function
- g(α):
-
Integral conversion function
- R :
-
Universal gas constant
- β :
-
Heating rate and β = dT/dt (where t is time)
- k(T):
-
A temperature dependence
- A :
-
Pre-exponential factor
- E a :
-
Activation energy given by the Arrhenius equation
References
Undurraga J, Tondo L, Schalkwijk S, Vieta E, Baldessarini RJ. Re-analysis of the earliest controlled trials of imipramine. J Affect Disord. 2013;147(1–3):451–4.
Bermudez-Saldana JM, Quinones-Torrelo C, Sagrado S, Medina-Herndndez MJ, Villanueva-Camanas RM. A micellar liquid chromatographic method for quality control of pharmaceutical preparations containing tricyclic antidepressants. Chromatographia. 2002;56(5/6):299–306.
Cruz-Vera M, Lucena R, Cárdenas S, Valcárcel M. Fast urinary screening for imipramine and desipramine using on-line solid-phase extraction and selective derivatization. J Chromatogr B. 2007;857:275–80.
Nagasawa M, Otsuka T, Yasuo S, Furuse M. Chronic imipramine treatment differentially alters the brain and plasma amino acid metabolism in Wistar and Wistar Kyoto rats. Eur J Pharmacol. 2015;762:127–35.
Jaworska A, Malek K. A comparison between adsorption mechanism of tricyclic antidepressants on silver nanoparticles and binding modes on receptors. Surface-enhanced Raman spectroscopy studies. J Colloid Interface Sci. 2014;431:117–24.
Kabir-ud-Din, Rub MA, Naqvi AZ. Aqueous amphiphilic drug (amitriptyline hydrochloride)-bile salt mixtures at different temperatures. Colloids Surfaces B Biointerfaces. 2011;84(2):285–91.
Kabir-ud-Din, Yaseen Z, Sheikh MS. Modulation of aggregation behavior of amphiphilic drug AMT under the influence of polymer molecular weight and composition. Colloids Surfaces B Biointerfaces. 2011;87(2):340–5.
http://www.drugbank.ca/drugs/DB00321. Accessed 9 May 2016.
Rub MA, Asiri AM, Naqvi AZ, Khan A, Khan AAP, Kabir-ud-Din. Interaction of amphiphilic drug imipramine hydrochloride with gemini surfactants at different temperatures. J Mol Liq. 2014;194:234–40.
Sun T, Shao X, Cai W. Self-assembly behavior of β-cyclodextrin and imipramine. A free energy perturbation study. Chem Phys. 2010;371(1–3):84–90.
http://www.drugbank.ca/drugs/DB00458. Accessed 10 May 2016.
http://www.drugbank.ca/drugs/DB01151. Accessed 10 May 2016.
Karimian M, Schaffie M, Fazaelipoor MH. Determination of activation energy as a function of conversion for the oxidation of heavy and light crude oils in relation to in situ combustion. J Therm Anal Calorim. 2016;125(1):301–11.
Jain A, Anthonysamy S. Oxidation of boron carbide powder. J Therm Anal Calorim. 2015;122(2):645–52.
Duce C, Ciprioti SV, Ghezzi L, Ierardi V, Tinè MR. Thermal behavior study of pristine and modified halloysite nanotubes: A modern kinetic study. J Therm Anal Calorim. 2015;121(3):1011–9.
Budrugeac P. Phase transitions of a parchment manufactured from deer leather. J Therm Anal Calorim. 2015;120(1):103–12.
Krajnikova A, Rotaru A, Gyoryova K, Homzova K, Manolea HO, Kovarova J, Hudekova D. Thermal behaviour and antimicrobial assay of some new zinc(II) 2-aminobenzoate complex compounds with bioactive ligands. J Therm Anal Calorim. 2015;120(1):73–83.
Olszak-Humienik M, Jablonski M. Thermal behavior of natural dolomite. J Therm Anal Calorim. 2015;119(3):2239–48.
Fuliaş A, Vlase G, Vlase T, Şuta L-M, Şoica C, Ledeţi I. Screening and characterization of cocrystal formation between carbamazepine and succinic acid. J Therm Anal Calorim. 2015;121(3):1081–6.
Ivan C, Suta L, Olariu T, Ledeti I, Vlase G, Vlase T, Olariu S, Matusz P, Fulias A. Preliminary kinetic study for heterogenous degradation of cholesterol-containing human biliary stones. Rev Chim. 2015;66(8):1253–6.
Ledeţi I, Vlase G, Ciucanu I, Olariu T, Fuliaş A, Şuta L-M, Belu I. Analysis of solid binary systems containing simvastatin. Rev Chim. 2015;66(2):240–3.
Fuliaş A, Soica C, Ledeţi I, Vlase T, Vlase G, Şuta L-M, Belu A. Characterization of pharmaceutical acetylsalicylic acid-theophylline cocrystal obtained by slurry method under microwave irradiation. Rev Chim. 2014;65(11):1281–4.
Ilici M, Bercean V, Venter M, Ledeti I, Olariu T, Suta L-M, Fulias A. Investigations on the thermal-induced degradation of transitional coordination complexes containing (3 h-2-thioxo-1,3,4-thiadiazol-5-yl)thioacetate moiety. Rev Chim. 2014;65(10):1142–5.
Fuliaş A, Vlase G, Ledeţi I, Şuta L-M. Ketoprofen-cysteine equimolar salt: Synthesis, thermal analysis, PXRD and FTIR spectroscopy investigation. J Therm Anal Calorim. 2015;121(3):1087–91.
Ledeţi I, Ledeţi A, Vlase G, Vlase T, Matusz P, Bercean V, Suta L-M, Piciu D. Thermal stability of synthetic thyroid hormone l-thyroxine and l-thyroxine sodium salt hydrate both pure and in pharmaceutical formulations. J Pharm Biomed Anal. 2016;125:33–40.
Fuliaş A, Vlase G, Vlase T, Soica C, Heghes A, Craina M, Ledeti I. Comparative kinetic analysis on thermal degradation of some cephalosporins using TG and DSC data. Chem Cent J. 2013;7(1):70.
Ledeti I, Fulas A, Vlase G, Vlase T, Doca N. Novel triazolic copper (II) complex: Synthesis, thermal behaviour and kinetic study. Rev Roum Chim. 2013;58(4–5):441–50.
Ledeti I, Fulias A, Vlase G, Vlase T, Bercean V, Doca N. Thermal behaviour and kinetic study of some triazoles as potential anti-inflammatory agents. J Therm Anal Calorim. 2013;114(3):1295–305.
Lv G, Stockwell C, Niles J, Minegar S, Li Z, Jiang WT. Uptake and retention of amitriptyline by kaolinite. J Colloid Interface Sci. 2013;411:198–203.
Ledeti A, Vlase G, Ledeti I, Vlase T, Matusz P, Dehelean C, Circioban D, Stelea L, Suta L-M. Thermal stability of desipramine and imipramine. Rev Chim. 2016;67(2):336–8.
Blessel KW, Rudy BC, Senkowski BZ. Analytical profiles of drug subtances. In: Florey K, editor. Vol 3. New York and London: Academic Press; 1974.
Cui H-W, Jiu J-T, Sugahara T, Nagao S, Suganuma K, Uchida H, Schroder KA. Using the Friedman method to study the thermal degradation kinetics of photonically cured electrically conductive adhesives. J Therm Anal Calorim. 2015;119(1):425–33.
Ledeţi I, Vlase G, Vlase T, Fuliaş A. Kinetic analysis of solid-state degradation of pure pravastatin versus pharmaceutical formulation. J Therm Anal Calorim. 2015;121(3):1103–10.
Soyleyici S, Cilgi GK. Thermal and kinetic analyses of 2,5-bis(2-hydroxyphenyl)thiazolo[5,4-d]thiazole. J Therm Anal Calorim. 2014;118(2):705–9.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci Pol Lett. 1969;7:41–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Research report, Trans joint convention of four electrical institutes. Chiba Inst Technol Sci Technol. 1971;16:22–31.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Pol Lett. 1966;4:323–8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Serra R, Sempere J, Nomen R. The non-parametric kinetics. A new method for the kinetic study of thermoanalytical data. J Therm Anal. 1998;52:933–43.
Serra R, Sempere J, Nomen R. A new method for the kinetic study of thermoanalytical data: The non-parametric kinetics model. Thermochim Acta. 1998;316:37–45.
Vlase T, Vlase G, Birta N, Doca N. Comparative results of kinetic data obtained with different methods for complex decomposition steps. J Therm Anal Calorim. 2007;88:631–5.
Wall ME, Rechtsteiner A, Rocha LM. Singular value decomposition and principal component analysis. In: Berrar DP, Dunitzky W, Granzow M, editors. A practical approach to microarray data analysis. Norwell: Kluwer; 2003. p. 91–109.
Sestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Acknowledgements
This work was supported by the PN-II-RU-TE-2014-4-0515 to Adriana Ledeţi, Gabriela Vlase and Ionuţ Ledeţi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ledeti, A., Vlase, G., Vlase, T. et al. Kinetic study for solid-state degradation of mental disorder therapeutic agents. J Therm Anal Calorim 131, 155–165 (2018). https://doi.org/10.1007/s10973-016-6064-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6064-8