Abstract
In this paper, the kinetic behavior of decomposition of a well-known bioactive substance used for treating dyslipidemia and the prevention of cardiovascular disease drug from statin class, namely pravastatin, was described. The kinetic study was performed on the main decomposition process which occurs at nearly 200 °C, for both pure active substance and a generic commercial formulation that contain 40 mg pravastatin per tablet, using Kissinger, Friedman, Kissinger–Akahira–Sunose, Flynn–Wall–Ozawa and NPK methods. The stability of pravastatin as pure active substance and as tablet was compared by means of kinetic data, and the results suggested that in the solid pharmaceutical formulation, PRV has an increased stability compared to pure active substance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pravastatin (PRV) is an active pharmaceutical ingredient mainly used for the treatment of dyslipidemia and the prevention of cardiovascular disease [1], conditioned in solid dosage forms as sodium salt. PRV is mainly prescribed for treating patients who have or are at risk of arteriosclerotic vascular disease and its consequences and strokes [2]. The literature indicates that PRV provides several health benefits such as decreasing the level of total cholesterol, LDL cholesterol and triglycerides [3], while increasing the level HDL cholesterol [4]. Regarding its activity over cardiovascular system, PRV was reported to reduce the risk of death by decreasing the possibility of heart attacks [5]. Other studies revealed that PRV decreases the risk of acute ischemic strokes and transient ischemic attacks (TIAs or mini-strokes) [6].
Nowadays, PRV is administered to patients on dose range from 10 up to 80 mg day−1. The usual starting dose in adults is 40 mg once daily. The starting dose for patients with major liver or kidney dysfunction is 10 mg daily. PRV is commercialized generically following the Bristol–Myers Squibb’s patent expiration in April 2006 [7] under several brands in solid formulations containing 10, 20, 40 and 80 mg of active pharmaceutical ingredient. Other studies reveals that PRV stimulates angiogenesis in murine models [8] and improves acetylsalicylic acid-mediated blood platelet inhibition, in vitro [9], while it has been suggested that statins appear to have therapeutic benefits in diseases that are unrelated to elevated serum cholesterol levels, such as pain and inflammation [10]. The importance of PRV active substance and statins is also revealed by review studies that indicate new therapeutic perspectives of statins in autoimmune diseases and cancer, antiatherosclerotic, anti-inflammatory, antioxidant, immunomodulatory and antithrombotic effects [11].
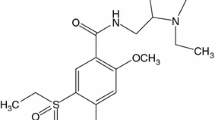
The chemical structure of PRV [(3R,5R)-3,5-dihydroxy-7-((1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-(((S)-2-methylbutanoyl)oxy)-1,2,6,7,8,8a-hexahydronaphthalen-1-yl)heptanoic acid] consists in a hexahydronaphtalene ring functionalized as a methylbutyrate ester [12] (Fig. 1).
In this study, we set our goal in realizing the kinetic study over the decomposition of PRV. This study was realized using the DTG data obtained at five heating rates in air, for the first decomposition step of PRV as pure active substance and from tablet (40 mg PRVTAB), corresponding to β = 5, 7, 10, 12 and 15 °C min−1. A preliminary evaluation for the activation energy was carried out by the use of the Kissinger’s method, followed then by the determination of the kinetic triplet according to Kissinger–Akahira–Sunose, Flynn–Wall–Ozawa, Friedman and NPK methods.
Materials and methods
Pravastatin sodium salt hydrate (PRVAS) was obtained from Sigma-Aldrich (P4498, purity >98 %) and used as received without further purification. The tablet was a generic Romanian commercial product, with 40 mg sodium pravastatin content per tablet. The tablet (PRVTAB) contained as excipients and fillers anhydrous lactose, sodium stearyl fumarate and iron oxide E172. The tablet was homogenized in an agate mortar with a pestle and then kept in a sealed box until experiments were carried out. The active substance was kept in same conditions as the tablet, in order to obtain comparable results.
TG/DTG/HF measurements were taken on a Perkin–Elmer DIAMOND TG/DTA instrument. The experiments were carried out using about 5 mg of sample (for both PRVAS and PRVTAB) which was weighted into an open aluminum crucible. The temperature was programmed to increase under nonisothermal conditions from 35 °C up to 550 °C, linearly at heating rates β = 5, 7, 10, 12 and 15 °C min−1. The experiments were completed in a synthetic air atmosphere at a flow rate of 100 mL min−1, and the kinetic study was carried out for the decomposition step that took place in 170–300 °C temperature range.
Results and discussion
Thermal analysis
The thermoanalytical curves of PRV active substance (PRVAS), obtained during heating at β = 7 °C min−1 in air atmosphere, exhibit a multistadial decomposition route. PRV loss hydration water up to 52 °C (first mass loss of 2.67 %, corresponding to a water content of 0.65 mol), then display a thermal stability up to 177 °C, when a decomposition process occur (temperature range 177–279 °C, with a Δm = 32.33 %). This process is followed by other processes in 279–550 °C temperature range, the last one occurring with a rapid mass loss in the 501–550 °C temperature range. These processes are accompanied by peaks on the DTG curve at 242 °C (first process) and 333, 355, 371, 400 and 514 °C, respectively (Fig. 2a) and can be associated with the advanced thermal destruction of the intermediary organic compounds.
The associated heat effects with these processes are revealed by the analysis of HF curve. The endothermic effect at 174.5 °C is associated with the melting of PRV (MSDS of supplier reported 171.2–173 °C), followed by several exothermic effects, with peaks at 243, 335, 352, 372, 404 and 514 °C (intense), which can be associated with thermo-oxidative breakdown processes of PRV molecule.
A similar thermal profile was observed for the solid pharmaceutical formulation containing 40 mg PRV per tablet (PRVTAB). However, in the case of PRVTAB, thermal stability (according to the TG/DTG, HF curves) is lowered by approx. 15 °C; the degradation process begins at 161 °C, with a corresponding peak on the DTG curve at 203 °C. HF curve reveals an endothermic effect associated with the decomposition process, at a temperature considerably lower in the case of formulation compared with active substance (HFpeak = 191 °C). The endothermic nature of this peak is different with the one observed in the case of active substance at 243 °C and can be considered due to the overlapping thermal effects with the one of the melting of PRV and/or excipients (Fig. 2b).
The analysis of thermoanalytical curves obtained for PRVAS and PRVTAB suggests a lower thermal stability for PRVTAB, compared with the one of PRVAS. In order to evaluate this stability, a kinetic study was realized.
Kinetic study
The importance of performing kinetic studies in the class of bioactive and potential bioactive molecules was previously presented in numerous scientific papers [13–18]. By performing kinetic studied, significant scientific details regarding the stability and decomposition mechanism can be obtained by the analysis of activation energy (E a), reaction order (n) and pre-exponential factor (A).
The kinetic analysis was performed using the thermogravimetrical data obtained in air atmosphere for the first decomposition step, for both active substance (PRVAS) and tablet (PRVTAB).
A first evaluation for the kinetic decomposition was realized by the use of Kissinger method, which states that for an Arrhenius-type dependence of the rate constant on temperature, a mathematical model represented by Eq. 1 can be obtained:
where E a is the activation energy, A is the pre-exponential factor, β is the heating rate, n is the reaction order, α is the conversion degree, T is the temperature and R is the gas constant; index max is used for indicating the maximum of the reaction rate. As 1 − α max is constant for a certain value of n, the evaluation of the E a can be achieved by evaluating the slope of the linear plotting of ln\( (\beta /T_{\hbox{max} }^{2} ) \) versus 1000/T max for experiments carried out at different heating rates [19].
The Kissinger kinetic analysis carried out for PRVAS led to a value of E a = 100.3 kJ mol−1, while for PRVTAB, the value was E a = 127.1 kJ mol−1 (Fig. 3a, b).
Following this, our study was focused on an “in-depth” kinetic analysis, which was performed according to the recommendations made at ICTAC 2000, namely the use of isoconversional methods. Following this, a differential method (Friedman) and two integral were used—Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS), respectively. In order to complete the study and to correlate the obtained results, a fourth method was used, namely the nonparametric kinetics method (NPK), elaborated by Sempere et al. [20, 21] and later modified by Vlase et al. [22, 23]. The main advantages resulting from applying these kinetic methods reside in several aspects, such as detection of complex processes that took place during the decomposition of samples, the estimation of the independence of the value of E a versus conversion degree (α) and as well indicating if the mechanism of thermal decomposition is dependent/independent with the modification of temperature.
As a main advantage, it can be noticed that in the evaluation of the kinetic triplet, the use of isoconversional methods is not implying the known of the explicit equation of the conversion function.
Friedman method
Friedman [24] suggested a logarithmic linearization method for the well-known Arrhenius equation regarding the nonisothermal degradation kinetics in heterogeneous phase, leading to the Eq. 2:
where α is the conversion degree, T is temperature, f(α) is the differential conversion function, R is the gas constant, β is the heating rate β = dT/dt (where t is time), A is the pre-exponential factor and E a is the activation energy given by the Arrhenius equation.
By plotting \( \ln \left( {\beta \frac{{{\text{d}}\alpha }}{{{\text{d}}T}}} \right) \) versus (1/T) for the thermogravimetric data obtained at five heating rates (Fig. 4), linear correlations were obtained, and the estimation of E a values was realized from the slopes of those lines, for 0.05 ≤ α ≤ 0.95, with α variation step of 0.05. The obtained results are presented in Table 1, with determination coefficients higher than 0.972.
Flynn–Wall–Ozawa (FWO) method
Independently, Ozawa [25] and Flynn and Wall [26] suggested an integral isoconversional method based on Doyle approximation, namely the Eq. (3):
where g(α) is the integral conversion function.
As previously stated in Friedman’s method, for different values for α in the [0.05; 0.95] domain, with a α variation step of 0.05, using DTG curves recorded at the same five heating rates, straight lines were obtained when plotting lnβ versus 1/T (Fig. 5). The obtained determination coefficients are superior to 0.986, suggesting a good linear correlation between the analyzed parameters, as well a correct identification and separation of the investigated process on the DTG curve. From the slope of the obtained straight line, the activation energy was estimated (Table 1).
Kissinger–Akahira–Sunose (KAS) method
Following the studies initiated by Kissinger [27] and later by Akahira and Sunose [28], this isoconversional method is obtained as Eq. (4), by using the Murray–White approximation on the integral form of the reaction rate equation. By plotting \( \ln \frac{\beta }{{T^{2} }} \) versus 1/T, the slopes of the obtained straight lines lead to the evaluation of the activation energy value, for each conversion degree (Fig. 6).
where g(α) is the integral conversion function.
The results obtained for this method are presented in Table 1.
According to the three isoconversional models, a nonsignificant variation of the activation energy depending on the conversion degree was observed. This fact suggests that the mechanisms’ decomposition is not influenced by the heating rate and conversion degree.
Nonparametric kinetics method (NPK)
The NPK represents a special kinetic method for processing the kinetic data. This method is based on a single assumption that the reaction rate can be expressed as a product of two independent functions, f(α)—a function of the degree of conversion and k(T)—a temperature dependence, so that the basic relationship for the analysis of kinetic data represents the general rate equation.
The experimental values of reaction rates are arranged in a matrix which is expressed as a product of two vectors containing information on k(T) and f(α). From this matrix, it can be obtained two related to the temperature and conversion functions with only one assumption made, namely the validity of Arrhenius equation. The experimental points obtained at five heating rates form by interpolating a three-dimensional continuous reaction rate surface, with the following coordinates: T, α and reaction rate (Fig. 7). By discretization, from this surface, an n × m matrix M was obtained, which is decomposed, using the singular value decomposition algorithm [29], into the product of matrixes:
The results of NPK analysis are presented in Table 2. These data were obtained by analyzing the vector u (the first column of U) in respect of a kinetic model suggested by Šesták and Berggren [30]:
respectively, the vector v (the first column of V) for an Arrhenius-type temperature dependence.
For PRVAS, the main process with explained variance λ = 78.9 % is a chemical step with a reaction order n = 3/2 accompanied by a physical process (m = 2/3). The second process has a greater value for activation energy, but it contributes to the final energy in a small proportion (λ = 13.8 %).
For PRVTAB, according to Table 2, the degradation process is a complex one, both of steps having a significant value for explained variance (λ ≥ 10 %) with a chemical and physical significance (n ≠ 0 and m ≠ 0).
As been presented, thermoanalytical curves corresponding to the PRVTAB show a complex degradation process due to the presence of excipients which can induce under heating chemical and/or physical interactions. Surprisingly, in this case, the presence of excipients in the drug composition leads to a considerable increase of the activation energy’s value, even if the decomposition temperature is lower compared with the PRVAS. At macroscopic level, this observation can suggest that the pharmaceutical formulation has a longer lifetime than the one of active substance.
For PRVAS, the values of activation energy determined by means of integral isoconversional methods are in good agreement with the value determined by the NPK method for the first step of the decomposition.
Analyzing the values obtained by applying Friedman method, a significant variation of these values (more than 10 %) with the conversion degrees is observed. This fact can be interpreted as a multistage reaction of thermal degradation. Only one isoconversional method (FR) highlighted this complexity of degradation, and it is due to the differential processing of the kinetic data and confirms the results obtained by the NPK method.
Conclusions
The paper described the comparative thermal stability of statin drug pravastatin sodium as pure active pharmaceutical ingredient and as solid formulation (tablet). Thermal analysis revealed that the first step of molecular skeleton degradation of the pure active substance occurs in the 177–279 °C temperature range, while for tablet that occurs in 161–252 °C. The lower thermal stability of the commercial drug suggested by TG/DTG/HF curves registered at a heating rate 7 °C min−1 was further investigated by the means of isoconversional kinetic analysis, in order to evaluate the kinetic triplet for this step of degradation.
Using the DTG data obtained from the multiple heating measurements, the activation energy for the degradation of pravastatin sodium was preliminary evaluated by the use of classical Kissinger method, the results suggesting that in the solid pharmaceutical formulation, PRV has an increased stability compared to pure active substance. For the completion of the study and an accurate determination of this behavior, Friedman, KAS, FWO and NPK methods were used. The obtained results were in good agreement with Kissinger method, and in each case, an increased stability (with approximately 25 %) in the terms of activation energy values was obtained for tablet versus pure pravastatin.
The most accurate method is the NPK, which led to the evaluation of the kinetic parameters without any a priori approximation. The NPK method also permitted a separation of the physical and/or chemical nature of processes that contributed to the thermal degradation of the molecular structure of pravastatin in the studied temperature range. These results are also in a good agreement with the results suggested by the Friedman method, which indicated a complex route of degradation by the variation of E a versus conversion degree.
Our study could be used as a starting point in the field of pharmaceutical technology of generic formulations, regarding the choosing of excipients in the solid tablets containing pravastatin, which can affect the thermal stability of the new drugs. It is clearly that choosing anhydrous lactose, sodium stearyl fumarate and iron oxide E172, the stability of pravastatin is increased.
References
Nishiwaki M, Ikewaki K, Ayaori M, Mizuno K, Ohashi Y, Ohsuzu F, Ishikawa T, Nakamura H. Risk reductions for cardiovascular disease with pravastatin treatment by dyslipidemia phenotype: a post hoc analysis of the MEGA study. J Cardiol. 2013;61:196–200.
Reiner Ž. Statins in the primary prevention of cardiovascular disease. Nat Rev Cardiol. 2013;10:453–64.
Querton L, Buysschaert M, Hermans MP. Hypertriglyceridemia and residual dyslipidemia in statin-treated, patients with diabetes at the highest risk for cardiovascular disease and achieving very-low low-density lipoprotein-cholesterol levels. J Clin Lipidol. 2012;6:434–42.
Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. J Am Med Assoc. 2007;297:499–508.
Pressel S, Davis BR, Louis GT, Whelton P, Adrogue H, Egan D, Farber M, Payne G, Probstfield J, Ward H. Participant recruitment in the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). Control Clin Trials. 2001;22:674–86.
Montecucco F, Quercioli A, Mirabelli-Badenier M, Viviani GL, Mach F. Statins in the treatment of acute ischemic stroke. Curr Pharm Biotechnol. 2012;13:68–76.
FDA News Release. 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108644.html (Accessed 12 Aug 2014).
Orbay H, Hong H, Koch JM, Valdovinos HF, Hacker TA, Theuer CP, Barnhart TE, Cai W. Pravastatin stimulates angiogenesis in a murine hindlimb ischemia model: a positron emission tomography imaging study with 64Cu-NOTA-TRC105. Am J Transl Res. 2014;6:54–63.
Luzak B, Rywaniak J, Stanczyk L, Watala C. Pravastatin and simvastatin improves acetylsalicylic acid-mediated in vitro blood platelet inhibition. Eur J Clin Invest. 2012;42:864–72.
Mirhadi K. Effect of different doses of pravastatin on formalin-induced inflammatory response in mice. Glob Vet. 2012;8:636–41.
Lopez-Pedrera C, Ruiz-Limón P, Valverde-Estepa A, Barbarroja N, Rodriguez-Ariza A. To cardiovascular disease and beyond: new therapeutic perspectives of statins in autoimmune diseases and cancer. Curr Drug Targets. 2012;13:829–41.
Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill; 2006.
Fulias A, Vlase T, Vlase G, Doca N. Thermal behaviour of cephalexin in different mixtures. J Therm Anal Calorim. 2010;99:987–92.
Fuliaş A, Vlase G, Grigorie C, Ledeţi I, Albu P, Bilanin M, Vlase T. Thermal behaviour studies of procaine and benzocaine. Part 1. Kinetic analysis of the active substances under non-isothermal conditions. J Therm Anal Calorim. 2013;113(1):265–71.
Vlase T, Vlase G, Doca N, Ilia G, Fuliaş A. Coupled thermogravimetric-IR techniques and kinetic analysis by non-isothermal decomposition of Cd2+ and Co2+ vinyl-phosphonates. J Therm Anal Calorim. 2009;97:467–72.
Fulias A, Vlase G, Vlase T, Soica C, Heghes A, Craina M, Ledeti I. Comparative kinetic analysis on thermal degradation of some cephalosporins using TG and DSC data. Chem Cent J. 2013;7(1):70.
Ledeti I, Fuliaş A, Vlase G, Vlase T, Doca N. Novel triazolic copper (II) complex: synthesis, thermal behaviour and kinetic study. Rev Roum Chim. 2013;58(4–5):441–50.
Ledeti I, Fuliaş A, Vlase G, Vlase T, Bercean V, Doca N. Thermal behaviour and kinetic study of some triazoles as potential anti-inflammatory agents. J Therm Anal Calorim. 2013;114:1295–305.
Budrugeac P, Segal E. Applicability of the Kissinger equation in thermal analysis. J Therm Anal Calorim. 2007;88(3):703–7.
Serra R, Nomen R, Sempere J. The non-parametric kinetics. A new method for the kinetic study of thermoanalytical data. J Therm Anal Calorim. 1998;52:933–43.
Serra R, Sempere J, Nomen R. A new method for the kinetic study of thermoanalytical data: the non-parametric kinetics method. Thermochim Acta. 1998;316:37–45.
Vlase T, Vlase G, Birta N, Doca N. Comparative results of kinetic data obtained with different methods for complex decomposition steps. J Therm Anal Calorim. 2007;88:631–5.
Anghel M, Vlase G, Bilanin M, Vlase T, Albu P, Fulias A, Tolan I, Doca N. Comparative study on the thermal behavior of two similar triterpenes from birch. J Therm Anal Calorim. 2013;113(3):1379–85.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci. 1965;6C:183–7.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick direct method for determination of activation energy from thermogravimetric data. J Polym Sci B. 1966;4:323–8.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Joint convention of four electrical institutes. Research report Chiba Institute of Technology. Sci Technol. 1971;16:22–31.
Wall ME. Singular value decomposition and principal component analysis. In: Berrar DP, Dubitzky W, Granzow M, editors. A practical approach to microarray data analysis. Dordrecht: Kluwer; 2003. p. 91–109.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Acknowledgements
This work was performed at West University of Timişoara and was supported by the strategic Grant POSDRU/159/1.5/S/137750, Project “Doctoral and Postdoctoral programs support for increased competitiveness in Exact Sciences research” co-financed by the “European Social Fund within the Sectoral Operational Programme Human Resources Development 2007–2013” to Ionuţ Ledeţi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ledeţi, I., Vlase, G., Vlase, T. et al. Kinetic analysis of solid-state degradation of pure pravastatin versus pharmaceutical formulation. J Therm Anal Calorim 121, 1103–1110 (2015). https://doi.org/10.1007/s10973-015-4842-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4842-3