Abstract
This work presents densities, ρ, speeds of sound, u, and molar heat capacities, C p, of 1-butyl-3-methylimidazolium tetrafluoroborate (1) + cyclopentanone or cyclohexanone (2) mixtures as a function of composition at 293.15, 298.15, 303.15, 308.15 K and excess molar enthalpies, H E of same mixtures at 298.15 K. The measured ρ, u and C p values were used to calculate excess molar volumes, V E, excess isentropic compressibilities, \(\kappa_{\text{S}}^{\text{E}}\) and excess heat capacities, \(C_{\text{P}}^{\text{E}}\). The V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) have been tested in terms of Graph theory. The analysis of V E data and IR spectroscopic data has suggested that (1 + 2) mixtures are characterized by interactions between hydrogen and oxygen atom of cyclopentanone or cyclohexanone with fluorine atom of [BF4]− anion and proton of –CH3 group attached to imidazolium ring of [Bmim]+ cation. The quantum mechanical calculations also support the proposed molecular entities in pure as well as mixed states along with observations inferred from IR data of the studied mixtures. The V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) values estimated by Graph theory are in agreement with experimental values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) due to their unique physical properties such as negligible vapor pressure at room temperature, wide liquid range, solvating properties for diverse substances, high thermal and chemical stability [1–4] have attracted much attention as a promising alternative to the conventional organic solvents involved in various technological and separation processes in industries processes. Due to their significant and beneficial properties, these have also been utilized in sustainable processes for organic synthesis, catalysis, extraction and separation [5–8]. These imidazolium cations-based ILs are considered to be efficient for various processes such as high absorption of carbon dioxide, diesel extractive desulfurization (EDS), oxidative desulfurization (ODS), catalytic desulfurization (ECODS) [9–12]. Over the past few years, a considerable amount of experimental data and modeling work has been reported on thermodynamic properties of imidazolium-based ILs with different alkyl groups substituted on the ring of imidazolium cation and organic solvents. Such studies have yielded positive results in the extraction of aromatic sulfur compounds. Imidazolium-based ILs containing tetrafluoroborate anion [BF4] are considered to have good efficiency for the extraction of organic compounds and also act as good oxidizing agents [13–15]. Excess molar enthalpies, H E, and excess heat capacities, \(C_{\text{P}}^{\text{E}}\), data of liquid mixtures play important role to utilize them as working fluid in refrigerator, solar cells, etc. [16–19]. The knowledge of thermodynamic properties such as V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) of imidazolium-based IL or mixture of IL with organic solvents is therefore required to utilize them for various applications in industries or to replace conventional organic solvents. Cyclopentanone and cyclohexanone are being used as solvent for polymers, lacquers, oils, resins in the synthesis of pharmaceuticals and in cosmetic industries [20–22]. In continuation of our recent studies, focusing on thermodynamic properties of binary mixtures containing imidazolium-based cations with organic solvents, we report here densities, ρ, speeds of sound, u, molar heat capacities, \(C_{\text{P}}^{{}}\) and excess molar enthalpies, H E, of 1-butyl-3-methylimidazolium tetrafluoroborate (1) + cyclopentanone or cyclohexanone (2) mixtures.
Experimental
1-Butyl-3-methylimidazolium tetrafluoroborate [Bmim][BF4] (Fluka, mass fraction: ≥0.988) was used without further purification. The content of water in IL was checked time to time using Karl Fischer titration and found to be less than 340 ppm [23]. Cyclopentanone (Fluka, mass fraction: 0.992) and cyclohexanone (Fluka, mass fraction: 0.994) were purified by standard methods [24]. Details of studied chemicals, their CAS number, the source, purification method, purity and analysis method are presented in Table 1. The vibrating tube densimeter and sound analyzer manufactured by Anton Paar (Model DSA 5000) with a built-in thermostat was used for the measurement of densities, ρ, and speeds of sound, u, of pure liquids and their binary mixtures [25, 26]. These measurements are based on measuring the period of oscillation of a vibrating U-shaped hollow tube filled with the liquid samples. The calibration of the equipment was done with the doubly distilled, deionized and degassed water before each series of measurements. The ρ and u values of IL and purified liquids are reported in Table 2 and also compared well with their literature values [27–40]. The mole fraction in each mixture was obtained by measuring the masses of the components of mixtures using an electric balance (Mettler AX-205 Delta) with an uncertainty of ±1 × 10−5 g. The standard uncertainty in mole fraction is ±1 × 10−4. The uncertainties of the ρ and u measurements are ±1.2 kg m−3 and 0.1 m s−1, respectively. Further, the uncertainty in calculated V E values is ±0.1 % and in temperature measurement is ±0.01 K.
The excess molar enthalpies, H E, of investigated mixtures and molar heat capacities, C p, of pure liquids and their binary mixtures were measured by using micro differential scanning calorimeter Micro DSC (Model–μDSC 7 Evo) supplied by M/S SETARAM instrumentation, France, in the manner as described elsewhere [41, 42]. The calibration of calorimeter (controlled by the software) was done by the Joule effect method and checked by measuring the heat of fusion of naphthalene 148.21 J g−1 which is comparable to literature value 148.7 J g−1 [43]. The molar heat capacities, C p, of pure liquids are listed and compared with literature values in Table 2 [27, 30, 32, 44–47]. The uncertainties in measured \(C_{\text{P}}^{{}}\) and H E are ±0.3 and ±1 %, respectively. The uncertainty in temperature measurement is ±0.02 K.
Results
The densities, ρ, speeds of sounds, u and molar heat capacities, C p of [Bmim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures at 293.15, 298.15, 303.15 and 308.15 K and excess molar enthalpies, H E at 298.15 K measured over entire composition range are listed in supplementary Tables S1, S2 and S3, respectively. The excess molar volumes, V E, and isentropic compressibilities, \(\kappa_{\text{S}}\), were calculated by utilizing the experimental data using the following equations:
where x 1, x 2 are mole fractions; M 1, M 2 are molar masses and ρ 1, ρ 2 are densities of [Bmim][BF4] (1) and cyclopentanone or cyclohexanone (2), respectively. The ρ and u are densities and speeds of sound values of mixtures.
The excess isentropic compressibilities, \(\kappa_{\text{S}}^{\text{E}}\) and excess heat capacities, \(C_{\text{P}}^{\text{E}}\) were obtained using:
where (C p)mix denotes the molar heat capacities of the mixtures, (C p)1, (C p)2 are molar heat capacities of pure [Bmim][BF4] (1) and cyclopentanone or cyclohexanone (2), respectively. The \(\kappa_{\text{S}}^{{\text{id}}}\) values were estimated using Benson and Kiyohaya relation [48]
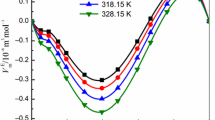
where \(\varphi_{\text{i}}\), \(\kappa_{{\text{S},\text{i}}}\),\(v_{\text{i}}\), \(\alpha_{\text{i}}\) and \((C_{\varvec{P}} )_{\varvec{i}}\) (i = 1 or 2) are the volume fraction, isentropic compressibility, molar volume, thermal expansion coefficient and molar heat capacity, respectively, of pure component (i) (i = 1 or 2). The α value for the studied liquids was calculated using density data in the manner as described elsewhere [49]. The excess molar enthalpies, H E, values at desired compositions for (1 + 2) mixtures were provided directly by the software supplied by the M/S SETARAM. The V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) values of the (1 + 2) mixtures are presented in supplementary Tables S1, S2 and S3, and graphically shown in Figs. 1–4, respectively.
Excess isentropic compressibilities, \(\left( {\kappa_{\text{S}}^{\text{E}} } \right)_{{\text{12}}}\) for (I) [Bmim][BF4] (1) + cyclopentanone (2) expt. (green line); graph (green diamond) (II) [Bmim][BF4] (1) + cyclohexanone (2) expt. (pink line); graph (pink star) at 298.15 K. (Color figure online)
Thermodynamic properties V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) were related to mole fraction of IL, x 1 by fitting with Redlich–Kister equation [50]:
where Y represents V or \(\kappa_{\text{S}}\) or H or C P. The Y n (n=0−2) (Y = V or \(\kappa_{\text{S}}\) or H or C P) are binary adjustable parameters and were obtained by the method of least squares. The standard deviations, σ (Y E) (Y = V or \(\kappa_{\text{S}}\) or H or C P) in YE (Y = V or \(\kappa_{\text{S}}\) or H or C P) were calculated using
where m is the number of data points, n is the number of parameters in Eq. (7). The obtained binary parameters along with σ for V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) are summarized in Table 3.
Discussion
The V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) data of [Bmim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures are not available in the literature for comparison with experimental results. The V E values of [Bmim][BF4] (1) + cyclohexanone (2) and \(\kappa_{\text{S}}^{\text{E}}\) of [Bmim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures are negative across full range of composition at investigated temperatures. However, sign of V E values changes from negative to positive with increase in concentration of [Bmim][BF4] for the [Bmim][BF4] (1) + cyclopentanone (2) mixture. Further, the sign and magnitude of H E and \(C_{\text{P}}^{\text{E}}\) data of [Bmim][BF4] (1) + cyclohexanone (2) mixtures are dictated by the relative proportion of components (1)/(2) in mixed state. However, for [Bmim][BF4] (1) + cyclopentanone (2) mixture, while H E values are negative; \(C_{\text{P}}^{\text{E}}\) data are positive over entire mole fraction range.
The results reported in supplementary Table S1 indicate that ρ values decrease with an increase in temperature for both the mixtures. Also the ρ values increase with increase in mole fraction of IL. The increase in ρ values with increase in concentration of IL may be due to the strengthening of interactions among IL and cyclopentanone or cyclohexanone in the mixed state. The sign and magnitude of V E values is an inductive to difference in packing efficiency and interaction intensity among the constituent molecules. The negative V E values for [Bmim][BF4] (1) + cyclohexanone (2) mixture suggest a more efficient packing or attractive interactions in mixed state. The packing effect may be due to difference in molar volume (at T/K = 298.15) of [Bmim][BF4] (188.52 × 10−6 cm3 mol−1) and cyclohexanone (104.08 × 10−6 cm3 mol−1) which leads to the accommodation of cyclohexanone in the interstices of IL network and hence leads to contraction in volume of mixtures. The V E values of [Bmim][BF4] (1) + cyclohexanone (2) mixture are more negative than those of [Bmim][BF4] (1) + cyclopentanone (2) mixture. It may be due to reason that cyclohexanone possesses chain form with almost no strain and so can be easily accommodated in the interstices of [Bmim][BF4] as compared to cyclopentanone (89.06 × 10−6 cm3 mol−1). Further, the comparison of V E values of [Bmim][BF4] (1) + cyclopentanone or cyclohexanone (2) with [Emim][BF4] (1) + cyclopentanone or cyclohexanone (2) [32] has revealed that V E values of [Emim][BF4] (1) + cyclopentanone or cyclohexanone (2) are more negative than those of corresponding [Bmim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures, thereby indicating that packing efficiency is more in mixtures containing [Emim][BF4]. It may be due to larger alkyl chain attached to imidazolium ring of [Bmim]+ cation which in turn restrict the approach of cyclopentanone or cyclohexanone toward [Bmim]+ cation in comparison with [Emim]+ cation. The \(\partial V^{\text{E}} /\partial T\) and \(\partial \left( {\kappa_{\varvec{S}}^{\varvec{E}} } \right) /\partial T\) for the present mixtures are negative which suggest strong interactions exist among the unlike molecules in the mixtures. This is also supported by decrease in speeds of sound values with increase in temperature, also suggesting strong interactions between the components of the mixtures.
The sign and magnitude of H E values of liquid mixtures reflects the interactions between like and unlike molecules. The positive H E data suggest that interactions between unlike molecules are weaker than between like molecules, whereas negative H E data view to the opposite. The H E of present (1 + 2) mixtures may be explained, if we assume that (i) [Bmim][BF4] is characterized by cohesion forces and exist as monomer; cyclopentanone or cyclohexanone are characterized by dipole–dipole interactions and exist as associated entities; (ii) addition of (1) to (2) or vice versa ruptures cohesion forces in [Bmim][BF4] and dipole–dipole interactions in cyclopentanone or cyclohexanone to give (1) and (2) molecules; and (iii) (1) and (2) molecules undergo interactions to form 1:2 molecular complex. The negative H E values of [Bmim][BF4] (1) + cyclopentanone (2) mixtures indicate that contribution to H E due to factor (iii) is more than due to factor (ii). However, positive H E data of [Bmim][BF4] (1) + cyclohexanone (2) at x 1 ≤ 0.6315 suggest that contribution to H E due to destruction of cohesive forces in IL or dipole–dipole interactions in cyclopentanone is higher in comparison with like molecule interaction. However, at x 1 > 0.6315 the unlike interactions dominate over like interactions. Cyclohexanone is more basic in character than cyclopentanone and also possesses chain form with no strain. Consequently, cyclohexanone must interact strongly with [Bmim][BF4] in comparison with cyclopentanone. Thus, H E for [Bmim][BF4] (1) + cyclohexanone (2) mixture should be less than those for [Bmim][BF4] (1) + cyclopentanone (2) mixture. This is not true. It may be due to fact that cyclohexanone is larger in size than cyclopentanone and so requires more energy to break cohesion forces in [Bmim][BF4]. The H E values of [Emim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures have been reported in the literature [32]. The comparison of H E data for [Bmim][BF4] or [Emim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures has revealed that H E values for [Bmim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures are lesser than [Emim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures. It may be due to larger alkyl chain attached to imidazolium ring of [Bmim]+ cation which in turn restrict the approach of cyclopentanone or cyclohexanone molecules toward [Bmim]+ in comparison with [Emim]+.
The positive \(C_{\text{P}}^{\text{E}}\) values for [Bmim][BF4] (1) + cyclopentanone (2) mixture suggest that contribution to \(C_{\text{P}}^{\text{E}}\) due to formation of 1:2 molecular complex (possessing non-random structure) dominate over contribution due to rupture of cohesion forces in IL and dipole–dipole interactions in cyclopentanone. Further, for [Bmim][BF4] (1) + cyclohexanone (2) mixture, \(C_{\text{P}}^{\text{E}}\) values are both positive and negative. The negative \(C_{\text{P}}^{\text{E}}\) values at x 1 ≤ 0.5352 suggest that contribution to \(C_{\text{P}}^{\text{E}}\) due to complexity among constituent molecules is less than contribution due to rupture of cohesion forces in IL and dipole–dipole interactions in cyclohexanone. However, positive \(C_{\text{P}}^{\text{E}}\) values indicate the domination of \(C_{\text{P}}^{\text{E}}\) due to formation of 1:2 molecular complex possessing non-random structure. The \(\partial \left( {C_{\text{P}}^{\text{E}} } \right)/\partial T\) for the studied mixtures are positive. This may be due to the destructions of associated molecular entities cyclopentanone or cyclohexanone and ionic interactions in IL which suggests that the interactions between like molecules are more difficult than between unlike molecules which in turn leads to more packed structure in mixed state.
The V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) data for the present mixtures were next analyzed in terms of Graph theory.
Graph theory
Graph theory has been utilized [51–54] successfully to obtain information about state of components in pure and mixed state along with nature and extent of interaction existing in mixtures. The theory involves the topology of the constituent molecules which in turn is described by connectivity parameters of third degree of a molecule, \({}^{3}\xi^{{}}\). As the topology of the constituent molecules in pure state changes in mixed state and excess molar volumes, V E reflects packing effect, the V E data of (1 + 2) mixtures were analyzed in terms of Graph theory. According to Graph theory [55, 56], excess molar volumes, V E, is given by
where x 1 is the mole fraction of IL and α 12 is a constant characteristic of (1 + 2) mixtures. The α 12 parameter for the present mixtures has been determined by employing V E values at x 1 = 0.6. The (3 ξ i) (i = 1 or 2); (3 ξ i)m (i = 1 or 2) are connectivity parameters of third degree of 1/2 molecule and are defined by
where \(\delta_{\text{m}}^{\upnu}\), etc. is related to the maximum valency, Z m, and number of hydrogen atoms, h, attached to mth vertex by relation: \(\delta^{\upnu}\) = Zm − h [57]. The (3 ξ i) (i = 1 or 2), (3 ξ i)m (i = 1 or 2) values were determined by employing V E data of (1 + 2) mixtures to Eq. (8) and only those values were considered that yielded V E values comparable to experimental data. Such V E values along with experimental values, (3 ξ i) (i = 1 or 2); (3 ξ i)m (i = 1 or 2), α 12 parameters for the investigated mixtures are recorded in Tables 4, 5, respectively. Examination of Table 4 has indicated that V E values for [Bmim][BF4] (1) + cyclohexanone (2) mixture are comparable with experimental values. Also V E values for [Bmim][BF4] (1) + cyclopentanone (2) mixture compare well at x 1 ≤ 0.6942. The failure of theory to correctly predict V E data at x 1 > 0.6942 is due to estimation of α 12 parameter by employing V E values at one composition.
A number of structures were then assumed for [Bmim][BF4], cyclopenanone and cyclohexanone. Structure or combination of structures providing (\({}^{3}\xi^{{\prime }}\)) values (using structural consideration, i.e., Eq. 9) closest to (3 ξ) values (determined via Eq. 8) was taken to be representative structure of [Bmim][BF4], cyclopenanone and cyclohexanone. It was assumed that [Bmim][BF4], cyclopenanone and cyclohexanone exist as molecular entities I, II–IV and V–VII, respectively. The (\({}^{3}\xi^{{\prime }}\)) values for these molecular entities were found to be 2.698, 0.984, 1.347, 1.386, 1.411, 1.597, 1.935, respectively (Scheme 1). In evaluating (\({}^{3}\xi^{{\prime }}\)) for [Bmim][BF4], it was assumed that cohesion forces exist between (1) hydrogen atom of C–H (edge) of imidazolium ring and two fluorine atoms of [BF4]−; (2) proton of –CH3 group of imidazolium ring and fluorine atom of [BF4]−. Since [Bmim][BF4] has (3 ξ) value of 2.396 (Table 5), the present analysis revealed that [Bmim][BF4] exists as molecular entity I. Further, (3 ξ) values of 1.287 and 2.105 for cyclopentanone and cyclohexanone (Table 5) suggest that cyclopentanone and cyclohexanone exist as a mixture of open and cyclic dimers. The (\({}^{3}\xi_{\text{1}}^{{\prime }}\))m were then estimated to obtain information about the state of [Bmim][BF4] in cyclopentanone and cyclohexanone. We assumed that present (1 + 2) mixtures may contain molecular entities VIII–IX which in turn are characterized by interactions between hydrogen and oxygen atoms of cyclopentanone or cyclohexanone with fluorine atom of [BF4]− anion and proton of methyl group attached to imidazolium ring of [Bmim]+ cation. The (\({}^{3}\xi_{\text{1}}^{{\prime }}\))m value for these molecular entities was then calculated to be 2.358. The (3 ξ 1)m value of 2.396 (Table 5) suggest the presence of molecular entities VIII–IX in [Bmim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures.
The existence of molecular entities VIII–IX in the present (1 + 2) mixtures advocated that addition of cyclopentanone or cyclohexanone to [Bmim][BF4] must influence (i) C–H vibrations of [Bmim]+ cation; (ii) B–F stretching of [BF4]− anion; (iii) in plane bending vibrations of –CH3 group in [Bmim][BF4]; and (iv) > C=O vibrations of cyclopentanone or cyclohexanone. The IR studies of pure [Bmim][BF4], cyclopentanone, cyclohexanone and their equimolar (1 + 2) mixtures has revealed that [Bmim][BF4], cyclopentanone, cyclohexanone exhibited characteristic vibrations [27, 58–60] at 3156 cm−1 (C–H vibrations); 528 cm−1 (B–F stretching); 1042 cm−1 (in plane bending vibrations of –CH3); and 1730, 1720 cm−1 (>C=O vibrations of cyclopentanone or cyclohexanone) which were shifted to 3154, 3150 cm−1 (C–H vibrations); 520, 522 cm−1 (B–F stretching); 1057, 1060 cm−1 (in plane bending vibrations of –CH3); and 1716, 1708 cm−1 (>C=O vibrations of cyclopentanone or cyclohexanone). The IR studies thus lend additional support to the postulation of molecular entities VIII–IX in the mixed state.
The postulation of molecular entities I–VII in pure and VIII–IX in mixed states was further confirmed by predicting inter-nuclear distances between the interacting atoms of the components of mixtures by quantum mechanics calculations using density functional theory [61–63]. All quantum mechanical calculations were performed with Gaussian program package 09 [64].
The full optimization of structures of [Bmim][BF4], cyclopentanone, cyclohexanone (molecular entities I–VII) and their mixtures (molecular entities VIII–IX) was carried out on the B3LYP/6-311++G (d, p) level of theory [64, 65]. The various inter-nuclear distances between interacting atoms in pure [Bmim][BF4], cyclopentanone, cyclohexanone and their mixed state (molecular entities I–IX) are labeled in Scheme 1. The inter-nuclear distances between hydrogen and fluorine atoms (2.28–2.96 Å) in pure [Bmim][BF4] (molecular entity I) suggest interactions between protons of carbon (C2 in imidazolium ring) and –CH3 group attached to imidazolium ring of [Bmim]+ cation with fluorine atoms of [BF4]−. Also inter-nuclear distances of 3.16 and 3.19 Å between carbon and oxygen atoms of cyclopentanone (molecular entities II–IV); and cyclohexanone (molecular entities V–VII), respectively, indicate the presence of open and cyclic dimer. It has also been observed in molecular entities VIII–IX that inter-nuclear distances between the proton of –CH3 group attached to imidazolium ring of [Bmim][BF4] and oxygen atom of cyclopentanone or cyclohexanone are 2.84 and 2.83 Å, respectively, and inter-nuclear distances between proton of cyclopentanone or cyclohexanone and fluorine atom of [BF4]− are 3.67 and 2.92 Å. Such inter-nuclear distances between interacting atoms support (i) proposed molecular entities I–IX in pure and mixed states; (ii) assumptions made for the estimation of connectivity parameters of third degree, \({}^{3}\xi^{{\prime }}\), (via structural consideration) of molecular entities I–IX; and (iii) observations inferred from the analysis of IR spectral data of the present mixtures.
Excess molar volumes, excess isentropic compressibilities, excess molar enthalpies and excess heat capacities
The estimated V E data for [Bmim][BF4] (1) + cyclopentanone (2) mixtures (by Graph theory) are not comparable well with experimental results at x 1 > 0.6942. It was, therefore, worthwhile to analyze V E data of [Bmim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures using Moelwyn–Huggins concept along with topology of the constituent molecules [66, 67]. The \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) data of the investigated mixtures were also tested in terms of Graph theory by taking into consideration the various processes involved in the mixture formation. The analysis of V E data in terms of Graph theory has suggested that [Bmim][BF4] is characterized by cohesion forces and exists as monomer; cyclopentanone and cyclohexanone exist as dimer. The studies of (1 + 2) mixtures formation were then assumed to involve processes; (i) formation of unlike 1–2n contacts; (ii) unlike contact formation leads to rupture of cohesion forces in [Bmim][BF4] as well as 2n–2n dipole interactions and results into depolymerization of 2n to form monomer which in turn enhance randomness; (iii) monomers of (1) and (2) molecules then undergo ion–dipole interactions to form 1:2 molecular complex possessing non-random structure. If χ 12, χ 1, χ 2, \(\chi_{12}^{*}\) are molar volumes, molar compressibilities, molar interaction parameters for establishment of 1–2 contacts; rupture of cohesion forces in IL and 2n–2n dipole interactions and increase in randomness; and molecular interactions between (1) and (2) to form 1:2 molecular complex; and increase in non-randomness, respectively; then change in thermodynamic properties, ∆Y (Y = V or \(\kappa_{\text{S}}\) or H or C P) due to processes (i–iii) were expressed [68–70] by
The overall change in the thermodynamic properties \(Y^{\text{E}} \left( {Y = V\,{\text{or}}\,\kappa_{\text{S}} \,{\text{or}}\,H\,{\text{or}}\,C_{\text{P}} } \right)\) for the investigated (1 + 2) mixtures was then expressed by
Since ν 2/ν 2 = \(\left( {{}^{3}\xi_{\text{1}} /{}^{3}\xi_{\text{2}} } \right)\) [71], Eq. (13) was expressed as
It was, further, assumed that interaction energy parameters for the establishment of unlike contacts and formation of 1:2 molecular complex; rupture of cohesion forces in IL and dipole–dipole interactions among cycloalkanones are nearly equal, i.e., \(\chi_{{\text{12}}} \cong \chi_{12}^{*} = \chi_{{\text{12}}}^{{\prime }}\) and \(\chi_{\text{1}} \cong \chi_{2} = \chi^{*}\), respectively; Eq. (14) was then reduced to
Equation (15) contains two unknown parameters \(\chi_{{\text{ij}}}^{{\prime }}\) and χ*. These parameters were evaluated by using experimental data of V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) at two compositions. The estimated parameters were then utilized to predict V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) values at other mole fractions of x 1. The V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) values along with \(\chi_{{\text{12}}}^{{{\prime }v}}\), \(\chi^{*v}\);\(\chi_{{\text{12}}}^{{{\prime }\kappa }} ,\chi^{{{\mathbf{*}}\kappa }}\); \(\chi_{{\text{12}}}^{{{\prime }{\text{H}}}} ,\chi^{{*{\text{H}}}}\), \(\chi_{{\text{12}}}^{{{\prime }C_{\text{P}} }} ,\chi^{{*C_{\text{P}} }}\) parameters are reported in Tables 4, 5, respectively, and are compared with experimental values.
Examination of data in Table 4 has indicated that V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) values estimated by Graph theory are in agreement with experimental results. The comparison between calculated and experimental values provides additional support to the assumptions made in deriving Eq. (15). Further, V E values for mixtures changing sign with relative proportion of the constituent molecules can be estimated during Graph theory.
Conclusions
The paper reports a new experimental data of excess molar volumes, V E, excess isentropic compressibilities, \(\kappa_{\text{S}}^{\text{E}}\), excess heat capacities, \(C_{\text{P}}^{\text{E}}\) (at 293.15, 298.15, 303.15, 308.15 K), and excess molar enthalpies, H E (at 298.15 K), for [Bmim][BF4] (1) + cyclopentanone or cyclohexanone (2) mixtures. The V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) were fitted to Redlich–Kister equation to determine binary adjustable parameters and standard deviations. The analyses of V E data in terms of Graph theory suggest that while [Bmim][BF4] exist as monomer; cyclopentanone and cyclohexanone exist as mixture of cyclic and open dimer. Further, (1 + 2) mixtures are characterized by interactions between hydrogen and oxygen atoms of cyclopentanone or cyclohexanone with fluorine atom of [BF4]− anion and proton of –CH3 group attached to imidazolium ring of [Bmim]+ cation. The proposed molecular entities in pure and mixed state were utilized to estimate their connectivity parameters, \({}^{3}\xi^{{\prime }}\) which in turn were used to determine V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) data. The quantum mechanical calculations and IR spectral data of the investigated mixtures also supported the presence of proposed molecular entities in the mixtures. It has been observed that estimated V E, \(\kappa_{\text{S}}^{\text{E}}\), H E and \(C_{\text{P}}^{\text{E}}\) values by Graph theory compare well with their corresponding experimental values.
References
Hua HC, Sorianoa AN, Lerona RB, Li MH. Molar heat capacity of four aqueous ionic liquid mixtures. Thermochim Acta. 2011;519:44–9.
Sattari M, Gharagheizi F, Ilani-Kashkouli P, Mohammadi AH, Ramjugernath D. Development of a group contribution method for the estimation of heat capacities of ionic liquids. J Therm Anal Calorim. 2014;115:1863–82.
Yue D, Jing Y, Ma J, Yao Y, Jia Y. Physicochemical properties of ionic liquid analogue containing magnesium chloride as temperature and composition dependence. J Therm Anal Calorim. 2012;110:773–80.
Domanska U. Solubilities and thermophysical properties of ionic liquids. Pure Appl Chem. 2005;77:543–57.
Anouti M, Caillon-Caravanier M, Dridi Y, Jacquemin J, Hardacre C, Lemordant D. Liquid densities, heat capacities, refractive index and excess quantities for protic ionic liquids + water binary system. J Chem Thermodyn. 2009;41:799–808.
Marsh KN, Boxall JA, Lichtenthaler R. Room temperature ionic liquids and their mixtures—a review. Fluid Phase Equilib. 2004;219:93–8.
Zhao YN, Yang ZZ, Luo SH, He LN. Design of task specific ionic liquids for catalytic conversion of CO2 with aziridines under mild conditions. Catal Today. 2013;200:2–8.
Sheldon R. Catalytic reactions in ionic liquids. Chem Commun. 2001;2399–407.
Zhang W, Xu K, Zhang Q, Liu D, Wu S, Verpoort F, Song XM. Oxidative desulfurization of dibenzothiophene catalyzed by ionic liquid [BMIm] HSO4. Ind Eng Chem Res. 2010;49:11760–3.
Campos-Martin JM, Capel-Sanchez MC, Fierro JLG. Highly efficient deep desulfurization of fuels by chemical oxidation. Green Chem. 2004;6:557–62.
Te M, Fairbridge C, Ring Z. Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2O2 and formic acid/H2O2 systems. Appl Catal A. 2001;219:267–80.
Al-Shahrani F, Xiao T, Liewellyn SA, Barri S, Jiang Z, Shi H, Martine G, Green MLH. Desulfurization of diesel via the H2O2 oxidation of aromatic sulfides to sulfones using a tungstate catalyst. Appl Catal B. 2007;23:311–6.
Moosavi M, Daneshvar A, Sedghamiz E. Rheological properties of [bmim]PF6 + methanol mixtures at different temperatures, shear rates and compositions. J Mol Liq. 2015;209:693–705.
Revelli AL, Mutelet F, Jaubert JN. Extraction of benzene or thiophene from n-heptane using ionic liquids. NMR and thermodynamic study. J Phys Chem B. 2010;114:4600–8.
Ma J, Hong X. Application of ionic liquids in organic pollutants control. J Environ Manag. 2012;99:104–9.
Curras MR, Costa Gomes MF, Husson P, Padua AAH, Garcia J. Calorimetric and volumetric study on binary mixtures 2,2,2-trifluoroethanol + (1-butyl-3-methylimidazolium tetrafluoroborate or 1-ethyl-3-ethylimidazoliumtetrafluoroborate). J Chem Eng Data. 2010;55:5504–12.
Zhang X, Hu D, Zhao Z. Measurement and prediction of excess enthalpies for ternary solutions 1-ethyl-3-methylimidazolium dimethylphosphate + methanol or ethanol + water at 298.15 K and at normal atmospheric pressure. J Chem Eng Data. 2014;59:205–11.
Curras MR, Vijande J, Pineiro MM, Lugo L, Salgado J, Garcia J. Behavior of the environmentally compatible absorbent 1-butyl-3-methylimidazolium tetrafluoroborate with 2,2,2-trifluoroethanol: experimental densities at high pressures and modeling of PVT and phase equilibria behavior with PC-SAFT EoS. Ind Eng Chem Res. 2011;50:4065–76.
Fredlake CP, Crosthwaite JM, Hert DG, Aki SNVK, Brennecke JF. Thermophysical properties of imidazolium-based ionic liquids. J Chem Eng Data. 2004;49:954–64.
Balaji R, Sankar MG, Sekhar MC, Shekar MC. Thermophysical and spectroscopic properties of binary liquid systems: acetophenone/cyclopentanone/cyclohexanone with N-methylformamide. Phys Chem Liq. 2015. doi:10.1080/00319104.2015.1109996.
Dragoescu D. Refractive indices and their related properties for several binary mixtures containing cyclic ketones and chloroalkanes. J Mol Liq. 2015;209:713–22.
Kumari PG, Venkatesu P, Hofman T, Rao MVP. Excess molar enthalpies and vapour–liquid equilibrium for N-methyl-2-pyrrolidone with ketones. J Chem Eng Data. 2010;55:69–73.
Scholz E. Karl Fischer titration. Berlin: Springer; 1984.
Riddick JA, Bunger WB, Sakano TK. Organic solvents physical properties and methods of purification. 4th ed. New York: Wiley; 1986.
Dubey GP, Sharma M. Temperature and composition dependence of the densities, viscosities, and speeds of sound of binary liquid mixtures of 1-butanol with hexadecane and squalane. J Chem Eng Data. 2008;53:1032–8.
Saini N, Yadav JS, Jangra SK, Sharma D, Sharma VK. Thermodynamic studies of molecular interactions in mixtures of o-toluidine with pyridine and picolines: excess molar volumes, excess molar enthalpies and excess isentropic compressibilities. J Chem Thermodyn. 2011;43:782–95.
Sunkara GR, Tadavarthi MM, Tadekoru VK, Tadikonda SK, Bezawada SR. Density, refractive index, and speed of sound of the binary mixture of 1-butyl-3-methylimidazolium tetrafluoroborate + N-vinyl-2-pyrrolidinone from T = (298.15 to 323.15) K at atmospheric pressure. J Chem Eng Data. 2015;60:886–94.
Huo Y, Xia S, Ma P. Densities of ionic liquids, 1-butyl-3-methylimidazolium hexafluoroborate and 1-butyl-3-methylimidazolium tetrafluoroborate, with benzene, acetonitrile, and 1-propanol. J Chem Eng Data. 2007;52:2077–82.
Taib MM, Murugesan T. Density, refractive index, and excess properties of 1-butyl-3-methylimidazolium tetrafluoroborate with water and monoethanolamine. J Chem Eng Data. 2012;57:120–6.
Pal A, Kumar B. Volumetric and acoustic properties of binary mixtures of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate [bmim][BF4] with alkoxyalkanols at different temperatures. J Chem Eng Data. 2012;57:688–95.
Govinda V, Attri P, Venkatesu P, Venkateswarlu P. Evaluation of thermophysical properties of ionic liquids with polar solvent: a comparable study of two families of ionic liquids with various ions. J Phys Chem B. 2013;117:12535–48.
Sharma VK, Kataria J, Bhagour S. Thermodynamic investigations of 1-ethyl-3-methylimidazolium tetrafluoroboarate and cycloalkanone mixtures. J Therm Anal Calorim. 2014;118:431–47.
Ciocirlan O, Teodorescu M, Dragoesce D, Iulian O, Barhala A. Densities and excess volumes for binary mixtures of cyclopentanone with chloroalkanes at T = (288.15, 298.15, 308.15 and 318.15) K. J Chem Eng Data. 2010;55:3891–5.
Dragoescu D, Teodorescu M, Barhala A. Isothermal (vapour plus liquid) equilibria and excess Gibbs free energies in some binary (cyclopentanone plus chloroalkane) mixtures at temperatures from 298.15 to 318.15 K. J Chem Thermodyn. 2007;39:1452–7.
Palaiologou MM, Arianas GK, Tsierkezos NG. Thermodynamic investigation of dimethyl sulfoxide binary mixtures at 293.15 and 313.15 K. J Solut Chem. 2006;35:551–65.
Lange NA. Handbook of chemistry. 11th ed. New York: Mc Graw-Hill; 1973.
Ciocirlan O, Teodorescu M, Dragoescu D, Iulian O, Barhala A. Densities and excess molar volumes for binary mixtures of cyclohexanone with chloroalkanes at temperatures between (288.15 and 318.15) K. J Chem Eng Data. 2010;55:968–73.
Rafiee HR, Ranjbar S, Poursalman F. Densities and viscosities of binary and ternary mixtures of cyclohexanone, 1,4-dioxane and isooctane from T = (288.15–313.15) K. J Chem Thermodyn. 2012;54:266–71.
Bermudez-Salguero C, Gracia-Fadrique J, Calvo E, Amigo A. Densities, refractive indices, speeds of sound, and surface tensions for dilute aqueous solutions of 2-methyl-1-propanol, cyclopentanone, cyclohexanone, cyclohexanol, and ethyl acetoacetate at 298.15 K. J Chem Eng Data. 2011;56:3823–9.
Tsierkezos NG, Molinou IE, Filippou AC. Thermodynamic properties of binary mixtures of cyclohexanone with n-alkanols (C1–C5) at 293.15 K. J Solut Chem. 2005;34:1371–86.
Sharma VK, Rohilla A. Excess heat capacities of 1-methyl pyrrolidin-2-one and pyridine or picolines mixtures. Thermochim Acta. 2013;568:140–7.
Sharma VK, Solanki S, Bhagour S, Sharma D. Excess molar enthalpies of ternary mixtures containing 1-ethyl-3-methylimidazolium tetrafluroborate and organic solvents. Thermochim Acta. 2013;569:36–41.
Sabbah R, Xu-Wu A, Chickos JS, Leitao MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:93–204.
Rebelo LPN, Najdanovic-Visak V, Visak ZP, Nunes da Ponte M, Szydlowski J, Cerdeirina CA, Troncoso J, Romani L, Esperança JMSS, Guedesc HJR, De Sousa HC. A detailed thermodynamic analysis of [C4mim] [BF4] + water as a case study to model ionic liquid aqueous solutions. Green Chem. 2004;6:369–81.
Sanmamed YA, Navia P, Gonzalez-Salgado D, Troncoso J, Romani L. Pressure and temperature dependence of isobaric heat capacity for [Emim][BF4], [Bmim][BF4], [Hmim][BF4], and [Omim][BF4]. J Chem Eng Data. 2010;55:600–4.
Fuchs R. Heat capacities of liquid ketones and aldehydes at 298 K. Can J Chem. 1980;58:2305–6.
Nishikawa K, Ohomura K, Tamura K, Murakami S. Excess thermodynamic properties of mixtures of cyclohexanone and benzene at 298.15 and 308.15 K and the effect of excess expansion factor. Thermochim Acta. 1995;267:323–32.
Benson GC, Kiyohara O. Evaluation of excess isentropic compressibilities and isochoric heat capacities. J Chem Thermodyn. 1979;11:1061–4.
Brocos P, Amigo A, Points M, Calvo E, Bravo R. Application of the Prigogine–Flory–Patterson model to excess volumes of mixtures of tetrahydrofuran or tetrahydropyran with cyclohexane or toluene. Thermochim Acta. 1996;286:297–306.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Sharma VK, Kumar S. Topological investigations of molecular interactions in mixtures in mixtures containing 1,4 dioxane and alkanols. Thermochim Acta. 2005;428:83–90.
Sharma VK, Siwach RK, Sharma D. Excess molar volumes, excess molar enthalpies and excess isentropic compressibilities of tetrahydropyran with aromatic hydrocarbons. J Chem Thermodyn. 2011;43:39–46.
Sharma VK, Romi Kumar S. Topological investigations of binary and ternary mixtures: excess isentropic compressibilities. Thermochim Acta. 2004;417:91–7.
Solanki S, Saini N, Sharma VK. Topological investigations of binary mixtures containing ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate and pyridine or isomeric picolines. J Chem Thermodyn. 2013;56:123–35.
Singh PP, Sharma VK, Sharma SP. Topological studies of the molecular species that characterize lower alkanols + methylene bromide mixtures: molar excess volumes and molar excess enthalpies. Thermochim Acta. 1986;106:293–307.
Singh PP. Topological aspects of the effect of temperature and pressure on the thermodynamics of binary mixtures of nonelectrolytes. Thermochim Acta. 1983;66:37–73.
Kier LB, Yalkowasky SH, Sinkula AA, Valvani SC. Physicochemical properties of drugs, chapter 9. New York: Mercel Dekker; 1980. p. 282–95.
Rao CNR. Chemical application of infrared spectroscopy. London: Academic Press; 1963.
Swapnil AD, Kailas LW, Mahesh NV, Diwakar ZS, ChangKyoo Y. Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arab J Chem. 2013. doi:10.1016/j.arabjc.2013.09.034.
Silverstein RM, Bassler GC, Morrik TC. Spectroscopic identification of organic compounds. 5th ed. Singapore: Wiley; 1991.
Samadi Z, Mirzaei M, Hadipour NL, Khorami SA. Density functional calculations of oxygen, nitrogen and hydrogen electric field gradient and chemical shielding tensors to study hydrogen bonding properties of peptide group (O=C–NH) in crystalline acetamide. J Mol Graph model. 2008;26:977–81.
Yogeswari B, Kanakaraju R, Boopathi S, Kolandaivel P. Microsolvation and hydrogen bond interactions in glycine dipeptide: molecular dynamics and density functional theory studies. J Mol Graph Model. 2012;35:11–20.
Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98:5648–52.
Sekhar MC, Venkatesulu A, Mohan TM, Gowrisankar M. Density functional theory, natural bond orbital and atoms in molecule analyses on the hydrogen bonding interactions in 2-chloroaniline-carboxylic acids. Orient J Chem. 2015;31:897–906.
Housaindokht MR, Hosseini HE, Googheri MSS, Monhemi H, Najafabadi RI, Ashraf N, Gholizadeh M. Hydrogen bonding investigation in 1-ethyl-3-methylimidazolium based ionic liquids from density functional theory and atoms-in-molecules methods. J Mol Liq. 2013;177:94–101.
Huggins ML. The thermodynamic properties of liquids included solutions. Part 1. Intermolecular energies in mono atomic liquids and their mixtures. J Phys Chem. 1970;74:371–8.
Huggins ML. The thermodynamic properties of liquids included solutions. Part 2. Polymer solutions considered as diatomic system. Polymer. 1971;12:389–99.
Sharma VK, Solanki S. Topological investigations of binary mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate and anilines. J Mol Liq. 2013;177:133–44.
Kumar S, Yadav JS, Saini N, Sharma D, Sharma VK. Thermodynamic properties of liquid mixtures containing 1,3-dioxolane and anilines: excess molar volumes, excess molar enthalpies, excess Gibb’s free energy and isentropic compressibilities changes of mixing. Thermochim Acta. 2010;511:74–81.
Yadav JS, Sharma D, Sharma VK. Topological investigations of thermodynamic properties of binary mixtures containing 2-pyrrolidinone. Thermochim Acta. 2009;489:45–52.
Singh PP, Nigam RK, Singh KC, Sharma VK. Topological aspects of thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1981;46:175–91.
Acknowledgements
The authors are thankful to Mr. K. Chandrasekhar Reddy, SSBN College, Anantapur, for providing Gaussian-09 facility and C-DAC, Pune, India, for providing the computational work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, H., Solanki, S. & Sharma, V.K. Topological analysis of thermodynamic properties of binary mixtures containing 1-butyl-3-methylimidazolium tetrafluoroborate and cycloalkanones. J Therm Anal Calorim 127, 2459–2472 (2017). https://doi.org/10.1007/s10973-016-5820-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5820-0