Abstract
Physical properties, such as density (ρ) and speed of sound (u) of [Bmim][triflate], 1-pentanol and their binary mixtures, are measured over the whole composition range as a function of temperature between 298.15 and 328.15 K at atmospheric pressure. Experimental values are used to calculate the excess molar volumes (\(V_{\text{m}}^{\text{E}}\)), excess values of partial molar volumes (\(\overline{V}_{\text{m}}^{\text{E}}\)), partial molar volumes at infinite dilution (\(\overline{V}_{\text{m}}^{{\text{E}},\infty }\)), excess values of isentropic compressibility (\(\kappa_{\text{s}}^{\text{E}}\)), free length (\(L_{\text{f}}^{\text{E}}\)), speeds of sound (\(u_{{}}^{\text{E}}\)) and isobaric thermal expansion coefficient (\(\alpha_{\text{P}}^{\text{E}}\)) for the binary mixture. These excess properties are fitted to the Redlich–Kister-type equation to obtain the binary coefficients and the standard deviations. A qualitative analysis of these parameters indicates strong intermolecular interactions in both the systems and the interaction increases with the increase in temperature. An attempt has been made to predict derivatives of thermodynamic potentials through physicochemical parameters, and using empirical relations excess chemical potentials/molecular properties of the mixtures from nonlinear parameter are also computed at 308.15 K. The presence of strong interactions was further supported by IR spectroscopy. In addition, analysis of \(V_{\text{m}}^{\text{E}}\) data of the mixture was done through the Prigogine–Flory–Patterson theory.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) as ‘green’ solvents represent a class of liquid materials with unique properties and alternative to traditional volatile organic solvents. Their applications are escalating rapidly as utilized in several areas of technology and science [1,2,3]. Mixing of the ionic liquids with molecular solvents is one of the alternative steps to reduce the use of expensive ionic liquids and to save time for synthesizing new ionic liquids of desired properties. The mixtures of ionic liquids and conventional organic solvents may be gaining a remarkable amount of attention from both the researchers and industries from both economic and ecological points of view. Improvement in the physicochemical properties of the common ILs into their mixtures with molecular organic solvents is a distinctive approach to exploit their potential applications [4]. High viscosity of ILs may hamper their industrial and research applications. Fortunately, their mixtures with molecular solvents show reduced viscosity without affecting their advantages as green solvents. In particular, the addition of polar co-solvents can strongly influence the physical and chemical properties of ILs such as viscosity, reactivity and electrical conductivity as well as solubility and solvation properties [5]. Recently, several binary IL + molecular solvent systems have been shown to perform better than the pure ILs, and such systems have been used in numerous applications such as in biocatalyzed reactions, super capacitors, reaction media and medium for dissolution of biopolymers [6,7,8,9,10,11,12,13,14,15,16,17,18]. Therefore, IL + molecular solvent mixtures have received rising attention in the past years. Besides speed of sound, experimental data of density also play a crucial role in predicting molecular interactions between the liquids in a binary mixture.
The IL in the study 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([Bmim][triflate]) is extensively used as corrosion inhibitor [19], as a solvent in electrochemical reduction [20] and in chromatography, etc. On the other hand, 1-pentanol, which is a self-associative organic solvent, has been used in many chemical and industrial processes. In addition, being a good industrial solvent, 1-pentanol is also used as an intermediate in the manufacture of many pharmaceuticals, organic compounds, lubricants, lubricant additives, flavorings, special catalysts, production of liquid crystals and an extracting agent [21]. The mixtures containing 1-pentanol are very significant from theoretical point of view, not only because of its self-association, but also due to the strong intermolecular effects produced due to the presence of –OH. It is of great importance to understand the mixing behavior of [Bmim][triflate] in 1-pentanol and to provide accurate physicochemical data.
Systematic investigations of the physicochemical properties of [Bmim][triflate] with molecular organic solvents including water have been reported [22,23,24,25,26]. González et al. [22] reported the osmotic and apparent molar properties of [Bmim][triflate] binary mixtures with 1-propanol, 2-propanol, 1-butanol, 2-butanol and 1-pentanol. Vercher et al. [23] reported the density and speeds of sound data of binary mixtures of [Bmim][triflate] with water. Vercher et al. [24] reported the refractive indices of water with [Bmim][triflate], whereas García-Miaja et al. [25] reported the density and heat capacity of water with [Bmim][triflate]. Recently, Anwar [26] reported thermoacoustic and volumetric properties of [Bmim][triflate] with 1-butanol and ethyl acetate. A thorough literature survey reveals that thermoacoustic and volumetric data of [Bmim][triflate] with 1-pentanol were not reported.
Basing on our initial experiments, [Bmim][triflate] has been found to be totally miscible with 1-pentanol at all proportions. Hence, in the present study, it is proposed to measure the physical properties (densities, ρ, and speeds of sound, u) of the binary mixture of [Bmim][triflate] with 1-pentanol in the temperature range 298.15 to 328.15 K and over the whole composition range to estimate their excess/deviation properties for their potential application in industrial processes. Based on the measured values, thermodynamic, acoustical and optical parameters such as excess molar volumes (\(V_{\text{m}}^{\text{E}}\)), excess values of partial molar volumes (\(\overline{V}_{\text{m}}^{\text{E}}\)), partial molar volumes at infinite dilution (\(\overline{V}_{\text{m}}^{{\text{E}},\infty }\)), isentropic compressibility (\(\kappa_{\text{s}}^{\text{E}}\)), free length (\(L_{\text{f}}^{\text{E}}\)), excess speeds of sound (\(u_{{}}^{{\text{E}}}\)), isobaric thermal expansion coefficient (\(\alpha_{\text{P}}^{{\text{E}}}\)) and the other thermodynamic expressions like \(\left( {\frac{{\partial V_{\text{m}}^{{\text{E}}} }}{\partial T}} \right)_{\text{P}}\), \(\left( {\frac{{\partial H_{\text{m}}^{{\text{E}}} }}{\partial P}} \right)_{\text{T}}\) have been determined for the binary mixture. These excess properties are fitted to the Redlich–Kister-type equation to obtain the binary coefficients and the standard deviations. The results have been analyzed with the help of IR spectroscopy. In addition, analysis of \(V_{\text{m}}^{E}\) data of the mixture was done employing the Prigogine–Flory–Patterson (PFP) theory.
Experimental
Material
The IL used in this work is [Bmim][triflate] (CAS 174899-66-2) with mass fraction purity of 0.99 was purchased from Iolitec GmBH (Germany), while 1-pentanol (CAS 71-41-0) was supplied by Sigma-Aldrich. The suppliers and the purity for pure compounds are reported in Table 1. The water content in IL and 1-pentanol was determined using a Karl Fischer titrator (Metrohm, 890 Titrando) [31,32,33]. Each one of the samples was dried in vacuum of 0.1 Pa for about 72 h and at moderate temperatures (starting from room temperature and gradually increasing it over a 6 h period up to 333 K). The water content of all the samples was checked further and observed to be in the range of 0.015%, which is lower than the original pre-evacuation analysis, which indicated values in the range of < 0.02%. [Bmim][triflate] was used without any further purification, and 1-pentanol was further purified by distillation technique. The purities of IL and 1-pentanol were verified by comparing the measured values of density and speed of sound of the pure liquids with the literature values at atmospheric pressure which are shown in Table 2.
Apparatus and procedure
Sample preparation
All samples were prepared freshly in amber glass vials with screw caps having PFE septa, were sealed with parafilm to avoid absorption of moisture from the atmosphere and were then stirred for over 30 min to ensure total dissolution of the mixtures. Samples were taken from the vials with a syringe through the PFE septum. The mass of the dry bottle was initially determined. The less volatile component (RTIL) of the mixture was then introduced into the bottle, and the mass was recorded. The organic liquid (1-pentanol) was added, and the mass of bottle including two components was recorded. Samples were weighed using Mettler Toledo AB 135 balance with a precision of ± 0.01 mg. The uncertainty in the mole fractions of the mixtures was estimated as being ± 1 × 10−4.
Measurement of density and speed of sound
Densities and speed of sound are measured with an Anton Paar DSA-5000 M vibrating tube density and sound velocity meter. The density meter is calibrated with doubly distilled degassed water and with dry air at atmospheric pressure. The temperature of the instrument is controlled to within ± 0.01 K by a built-in Peltier device that corresponds to an uncertainty in density of ± 0.0002% as specified by the manufacturer. Measured density and speed of sound values (at a frequency approximately 3 MHz) are precise to 5 × 10−3 kg m−3 and 5 × 10−1 m s−1, respectively.
Measurement of infrared spectra
Infrared absorbance was measured by making use of Shimadzu Fourier transform infrared (FT-IR) spectrometer, equipped with attenuated total reflectance (ATR) accessories. A fixed cell about 0.1 mm thickness is used which is suitable for the measurement of volatile samples also. The spectral region is 650–4000 cm−1 with resolution 0.5 cm−1 and 100 scans. At least five repeated measurements were performed for each sample.
Results and discussion
The experimentally measured values of density (ρ) and speed of sound (u) for the binary mixture of [Bmim][triflate] and 1-pentanol over the whole composition range as a function of temperature between 298.15 and 328.15 K with an increment of 10 K under atmospheric pressure are specified in Table 3. The change in the values of ρ, u and n D with respect to temperature is linear and with respect to mole fraction is nonlinear. This nonlinear trend of ρ, u and n D with respect to concentration indicates that molecular interactions definitely exist at all temperature between liquids in study. The molecular interactions between unlike molecules of liquid mixtures depend on the composition, shape, molecular size and temperature [34, 35]. The structural contributions come up from several effects such as interstitial accommodation and geometrical fitting of smaller component into larger due to the large difference in the molar volume between component molecules. The chemical or specific interactions comprise the formation of hydrogen bonding between component molecules, dipole–dipole interactions, charge-transfer complexes, etc. The physical interactions or nonspecific interactions which are weak in nature are due to dispersion forces.
The changes observed in excess/deviation parameters indicate the nature and extent of interactions present between the component molecules of the binary mixture in study. The results interpreted from Table 3 obviously show that addition of 1-pentanol to [Bmim][triflate] can bring about remarkable changes in ρ, u, n D for the present binary system. The thermodynamic properties such as \(V_{\text{m}}^{{\text{E}}}\), \(\kappa_{\text{s}}^{{\text{E}}}\) and the other excess parameters calculated from the experimental data according to well-known thermodynamic equations (S1–S11) are given in the supplementary material.
The deviation/excess properties have been fitted to a Redlich–Kister-type polynomial equation given by
where \(Y^{{\text{E}}} = V_{\text{m}}^{{\text{E}}}\), \(\kappa_{\text{s}}^{{\text{E}}}\), \(L_{\text{f}}^{{\text{E}}}\), \(Z^{{\text{E}}}\), \(u_{{}}^{\text{E}}\), \(\alpha_{\text{P}}^{{\text{E}}}\) and \(\Delta_{\phi } n_{\text{D}}\). Here, x 1 and x 2 are the mole fractions of [Bmim][triflate] and 1-pentanol, respectively. Further, A i are the adjustable parameters of the function which are computed using the least square method. In the present study, ‘i’ values have been taken from 0 to 4. The resultant standard deviations σ (Y E) have been computed using the following equation.
where ‘m’ is the total number of experimental points and ‘n’ is the number of coefficients in Eq. (1). The calculated values of the coefficients A i along with the standard deviations \(\sigma (Y^{{\text{E}}} )\) are shown in Table 4.
There are many active sites in both the components such as aromatic hydrogen, more electronegative fluorine and oxygen atoms of [Bmim][triflate] and hydroxyl group of 1-pentanol to form hydrogen bonds as well as dipole–dipole interactions. In pure [Bmim][triflate], there exist H-bonds between aromatic hydrogen atoms of [Bmim]+ cation and fluorine/oxygen atoms of triflate anion in addition to electrostatic forces of attraction between cation and anion. 1-Pentanol is an associative liquid with intermolecular H-bonds. Hence, we can assume many possibilities for interactions between the two components under study in addition to geometrical factors.
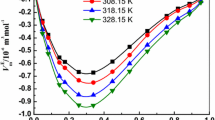
The relationship between excess molar volumes \(V_{\text{m}}^{E}\) obtained for the binary mixture [Bmim][triflate] + 1-pentanol as a function of composition in the temperature range T = 298.15–328.15 K is graphically represented in Fig. 1. The variations of excess molar volumes are found to be negative in the whole composition range except at IL-rich region at all temperatures which may be credited to the H-bonding. The hydroxyl oxygen and alkyl hydrogens of 1-pentanol are able to form H-bonds with imidazolium aromatic C–H hydrogen and fluorine/oxygen atoms of triflate anion, respectively. But H-bonding alone cannot explain the attractions between the two constituent molecules in the binary mixture. Further, favorable fitting of smaller 1-pentanol molecules (at T = 298.15 K, V m = 108.68 × 10−6 m3 mol−1) into the voids created by larger [Bmim][triflate] molecules (at T = 298.15 K, V m = 222.18 × 10−6 m3 mol−1) is definitely contributing to interactions. A similar variation in \(V_{\text{m}}^{{\text{E}}}\) values can be observed for the rest of studied temperatures. Moreover, the variation of \(V_{\text{m}}^{{\text{E}}}\) for the present system as a function of temperature becomes more negative with rise in temperature. Generally, the strength of hydrogen bond decreases with rise in temperature. As temperature increases, the self-association of 1-pentanol is disturbed which leads to the formation of free 1-pentanol molecules. Then, more favorable fitting of smaller 1-pentanol molecules into the voids created by larger IL molecules occurs, thereby, shrinkage of the volume of the mixture to a larger extent, resulting in more negative \(V_{\text{m}}^{{\text{E}}}\) values with rise in temperature. Therefore, order of strength of interaction enhances with rise in temperature. At IL-rich region, electrostatic forces of attraction dominates between oppositely charged ions which push out 1-pentanol molecules from ionic clusters resulting dispersive type of interactions between solvent and solute molecules [34].
The existing molecular interactions in the present binary system are properly reflected in the properties of partial molar volumes of the constituent molecules. Partial molar volume is the contribution that a component of a mixture makes to the total volume of the solution. The partial molar volumes \(\overline{V}_{\text{m,1}}\) of component 1 ([Bmim][triflate]) and \(\overline{V}_{\text{m,2}}\) of component 2 (1-pentanol) in the mixtures over the whole composition range have been computed using equations (S12, S13) are given in the supplementary material.
The partial molar volumes (\(\overline{V}_{\text{m,1}}^{\infty }\), \(\overline{V}_{\text{m,2}}^{\infty }\)) and excess partial molar volumes at infinite dilution (\(\overline{V}_{\text{m,1}}^{{\text{E}},\infty }\), \(\overline{V}_{\text{m,2}}^{{\text{E}},\infty }\)) were calculated from coefficients of Redlich–Kister-type polynomial equation given in Table 4 using equations (S14–S19) are given in the supplementary material.
The calculated values of \(\overline{V}_{\text{m,1}}\) and \(\overline{V}_{\text{m,2}}\) for the studied binary system are shown in Table S1 of the supplementary material. On visualizing the values of \(\overline{V}_{\text{m,1}}\) and \(\overline{V}_{\text{m,2}}^{{}}\) for the two components in the mixtures (Table S1), it is evident that the values are lower than their individual molar volumes in the pure state, which reveals that contraction of volume occurs on mixing [Bmim][triflate] with 1-pentanol at all examined temperatures. Figures 2 and 3 represent the disparity of excess partial molar volumes of \(\overline{V}_{\text{m,1}}^{{\text{E}}}\) ([Bmim][triflate]) and \(\overline{V}_{\text{m,2}}^{{\text{E}}}\) (1-pentanol), respectively, in the binary mixture at T = 298.15, 308.15, 318.15 and 328.15 K. The values of both components are negative at all temperatures. Figures 2 and 3 not only show the existence of strong forces between the unlike molecules, but also supports the inferences drawn from excess molar volume.
The pertinent data of \(\overline{V}_{\text{m,1}}^{\infty }\), \(\overline{V}_{\text{m,2}}^{\infty }\) and \(\overline{V}_{\text{m,1}}^{{\text{E}},\infty }\), \(\overline{V}_{\text{m,2}}^{{\text{E}},\infty }\) are presented in Table 5 at T = (298.15/308.15/313.15/318.15) K. The values of \(\overline{V}_{\text{m,1}}^{E,\infty }\) are found to be negative and become more negative with increase in temperature. This indicates that the association effect is greater than the dissociation effect for [Bmim][triflate] in the present system. The excess partial molar volumes at infinite dilution for 1-pentanol (\(\overline{V}_{\text{m,2}}^{{\text{E}},\infty }\)) are positive and decrease with increase in temperature. This is because for more concentrated 1-pentanol solution, most of the hydrogen bonds still exist. This argument supports the existing strong molecular interactions which are observed in the case of \(V_{\text{m}}^{{\text{E}}}\) values in the binary system and are well reproduced from the calculated properties of partial molar volumes at all examined temperatures.
Isentropic compressibility (\(\kappa_{\text{s}}\)) values are presented in Table 3, and their excess values (\(\kappa_{\text{s}}^{{\text{E}}}\)) are depicted in Fig. 4. From Table 3, it can be visualized that the isentropic compressibility decreases with increase in concentration of [Bmim][triflate] which may be due to the aggregation of solvent molecules around the ions supporting solute–solvent interaction. This suggests that when [Bmim][triflate] is added to the solvent 1-pentanol, the ions of the [Bmim][triflate] attract certain 1-pentanol molecules toward itself by wrenching the molecule species from the bulk of the solvent. Thus, less number of solvent molecules will be made available for the next incoming species which is known as compression. In Fig. 4, the \(\kappa_{\text{S}}^{{\text{E}}}\) values for the mixture [Bmim][triflate] + 1-pentanol are negative in the whole composition range at all investigated temperatures. This negative value of \(\kappa_{\text{S}}^{{\text{E}}}\) may be attributed to the strong attractive interactions between the molecules of the components [36]. In the present study, the negative \(\kappa_{\text{S}}^{{\text{E}}}\) can be attributed to a closer approach of unlike molecules and stronger interactions between components of mixtures at all temperatures. This supports the inference drawn from \(V_{\text{m}}^{{\text{E}}}\).
For the studied binary mixture, the free length (\(L_{\text{f}}\)) was calculated using equation (S2) of the supplementary material and its values are presented in Table 3. The trend of excess free length (\(L_{\text{f}}^{{\text{E}}}\)) with mole fraction of [Bmim][triflate] is shown in Fig. 5. The negative value of \(L_{\text{f}}^{{\text{E}}}\) indicates existence of specific interactions between unlike molecules in the liquid mixture which is attributed to the structural readjustments in the liquid mixture toward a less compressible phase of fluid and closer packing of molecules [36]. With rise in temperature, the values of \(L_{\text{f}}^{{\text{E}}}\) become more negative suggesting increase in the strength of interactions with temperature. The excess ultrasonic speed of sounds (\(u_{{}}^{{\text{E}}}\)) against mole fraction of [Bmim][triflate] was calculated using equation (S8) of the supplementary material and found to be positive value over the entire range of composition at all the studied temperatures (Fig. 6). Positive deviations specify the increasing strength of interaction between component molecules of binary liquid mixture, while the structure-breaking factor in the mixture dominates resulting in negative deviations in the speed of sound [37]. According to Ali et al. [38], more positive values mean much more strong interactions between the molecules (Fig. 7).
Isobaric thermal expansion coefficient (\(\alpha_{\text{P}}^{{}}\)) represents the fractional change in the volume of a system with temperature at constant pressure which is given in Table 3. The positive values of \(\alpha_{\text{P}}^{{\text{E}}}\) indicate the presence of weak interactions between the participating component molecules of the mixtures, while negative values of \(\alpha_{\text{P}}^{{\text{E}}}\) account for strong interactions between the participating component molecules [39]. Figure 8 shows negative values of \(\alpha_{\text{P}}^{{\text{E}}}\) at all investigated temperatures for the binary mixture in study. This trend supports the existence of strong interactions between the constituent molecules in binary solution. An attempt has been made to evaluate the parameters \(\left( {\frac{{\partial V_{\text{m}}^{{\text{E}}} }}{\partial T}} \right)_{\text{P}}\) and \(\left( {\frac{{\partial H_{\text{m}}^{{\text{E}}} }}{\partial P}} \right)_{\text{T}}\) at all investigated temperatures which are presented in Figs. 9 and 10, respectively. They have similar variation with the mole fraction of [Bmim][triflate] and temperature, but with opposite sign. The \(\left( {\frac{{\partial V_{\text{m}}^{{\text{E}}} }}{\partial T}} \right)_{\text{P}}\) values for the studied mixtures are thereby indicating the presence of strong interactions existing between the unlike molecules of the mixtures [40]. The isothermal pressure coefficients of excess molar enthalpy \(\left( {\frac{{\partial H_{\text{m}}^{{\text{E}}} }}{\partial P}} \right)_{\text{T}}\) are positive. This may be due to the increase in attraction forces between the components of this mixture with increasing pressure [41, 42]. The signs of these derivatives also support the presence of strong interactions between the molecules under study.
The presence of strong interactions in the system which were drawn from the above inferences of derived deviation/excess parameters is well supported by IR spectral studies. The unique properties of imidazolium cations are found in their electronic structure. The electronic structure of these cations contains delocalized 3-center-4-electron configuration across the N1–C2–N3 moiety, a double bond between C4 and C5 at the opposite side of the ring and a weak delocalization in the central region [43]. The hydrogen atom at C2 carbon is positively charged owing to the electron deficiency in the C=N bond, whereas C4 and C5 are almost neutral. The resulting acidity of the hydrogen atoms is crucial to understand the properties of these ionic liquids. The hydrogen on the C2 carbon (C2–H) has been shown to bind distinctively with solute molecules [44, 45].
Infrared transmittance was recorded from 650 to 4000 cm−1 (Fig. 10) for pure liquids as well as equimolar mixture in order to study the effects of molecular interactions. The stretching vibrations which are altered due to formation of hydrogen bonds are analyzed. For pure [Bmim][triflate], the peaks at 3115.9 and 3154.5 cm−1 are due to aromatic C2–H and C4,5–H vibrations, respectively. The peaks between 2800 and 3000 cm−1 result from aliphatic CH groups in the butyl and methyl moieties [46,47,48]. The C2–H vibrational frequency (3115.9 cm−1) is shifted to lower frequencies by about 38.6 cm−1 when compared to the C4–H and C5–H stretches (3154.5 cm−1) because of its stronger acidic character. The peaks at 1265.5 cm−1 and 1032 cm−1 are due to asymmetrical and symmetrical stretching vibrations in SO3 group of triflate anion. The peaks centered at 1226.5 and 1164.2 cm−1 belong to asymmetrical and symmetrical stretching vibrations in CF3 group of triflate anion. For pure 1-pentanol, the broad peak at 3200–3500 cm−1 belongs to O–H stretching vibrations and the peaks between 2800 and 3000 cm−1 are attributed to C–H stretching vibrations of alkyl groups. 1-Pentanol when added interacts with existing ion pairs and breaks the hydrogen bonding attractions between imidazolium aromatic C–H hydrogen and oxygen atoms of triflate anion. Consequently, oxygen atoms of 1-pentanol start forming strong hydrogen bonds with imidazolium aromatic C–H hydrogens. This leads to the weakening of the covalent character of C–H bonds in imidazolium cation, causing redshift in imidazolium C–H stretching vibrations. In the case of triflate anion, oxygen and fluorine atoms are able to form hydrogen bonds with alkyl hydrogen of 1-pentanol. Pure 1-pentanol has no peak in this range.
Upon addition of 1-pentanol, the hydroxyl oxygen and alky hydrogen of 1-pentanol start forming hydrogen bonds with imidazolium aromatic C–H hydrogen and oxygen atoms of triflate anion, respectively. Further addition of 1-pentanol significantly weakens inter-ionic interactions and eventually initiates ion pair dissociation. Once the individual ions are released, the ions are rapidly saturated with 1-pentanol. The hydrogen bonds in the system are responsible to get complete miscibility and solvation of cations/anions in the ionic liquid giving a remarkable contraction in the volume of the mixture.
The advantage of knowledge of physicochemical properties over calorimetric experiments in binary liquid mixtures has significance in theoretical and applied areas of research, and such results are often used in numerous chemical and industrial practice. Extensive number of parameters can be predicted with physicochemical properties, and it shows its bang in ultrasonics which set out from marine acoustics to medicine. These are used to compute micro- to macro-attributed characteristics significant to binary liquid system. In spite of many precautions, calorimeters lost heat to surroundings which cannot be eradicated completely. This leads to an error in measurements [49]. Hence, basing on the physicochemical properties, certain derivatives of thermodynamic potentials are computed at T = 308.15 K and are shown in Table 6.
The excess chemical potential is the vital thermodynamic quantity for phase equilibrium estimations. It is a kind of potential energy that can be absorbed or released during a chemical reaction or phase transition. Excess chemical potentials estimated [50] from Margules, Porter and Van Laar show positive trend as shown in Fig. 11 at T = 308.15 K.
The nonlinearity parameter (B/A) determines nonlinearity of the equation of state for a fluid. The (B/A) is fundamental as it decides deformation of a restricted amplitude wave propagating in the liquid. Further, it is not only linked to the molecular dynamics of the medium, but also provides information about structural properties of medium, internal pressures, clustering, intermolecular spacing, etc. Significance of B/A escalates with the growth of high-pressure technologies of food processing and preservation. In the present work, an attempt has been made to work out on molecular properties of the mixtures from empirical equations derived by Hartmann–Balizar (H&B) [51] and Ballou [52] with the Sehgal’s relations [53]. The computed molecular properties are shown in Table 7.
Prigogine–Flory–Patterson statistical theory for excess molar volume,\(V_{\text{m}}^{E}\)
The Prigogine–Flory–Patterson (PFP) theory may be used to analyze and correlate the experimental excess molar volumes of binary mixtures ILs and molecular organic solvent mixtures [48, 54,55,56,57]. We have correlated \(V_{\text{m}}^{{\text{E}}}\) of presently studied binary mixture using PFP theory over entire range of mole fractions in the temperature range T = (298.15–328.15) K. The PFP theory considered \(V_{\text{m}}^{{\text{E}}}\) in three different contributions [58]: (1) interaction contribution \(V_{\text{int}}^{{\text{E}}}\), which is associated with intermolecular specific interaction with sign of \(H_{\text{m}}^{{\text{E}}}\), (2) free volume contribution \(V_{\text{fv}}^{{\text{E}}}\), which is associated with reduced volume to reduced temperature ratio with negative sign and (3) internal pressure contribution, \(V_{\text{P}}^{{\text{E}}}\), which is associated with breaking of IL structure with introduction of molecular organic solvents and changes in reduced volume of components with positive or negative sign. The calculation of various parameters of the theory based on relevant equations is given elsewhere [59,60,61].
The pure components parameters for the PFP theory are included in Table 8. The Flory contact interaction parameter, \(\chi_{12}\), the only adjustable parameter, needed in the PFP theory was obtained by experimental \(V_{\text{m}}^{{\text{E}}}\) values due to the non-availability of experimental excess enthalpy values. The Flory contact interaction parameter, \(\chi_{12}\), was found to be positive at all the investigated temperatures and decreases with rise in temperature which suggests that the physical interactions take place when binary mixtures are formed. The values of three contributions: \(V_{\text{int}}^{{\text{E}}}\), \(V_{\text{fv}}^{{\text{E}}}\) and \(V_{\text{P}}^{{\text{E}}}\), to \(V_{\text{m}}^{{\text{E}}}\) (PFP) at equimolar composition are summarized in Table 9. The first term \(V_{\text{int}}^{{\text{E}}}\) represents the energy of interaction which is positive at all temperatures. The second term \(V_{\text{fv}}^{{\text{E}}}\) was found to be negative for the system studied as \(V_{\text{fv}}^{{\text{E}}}\) is proportional to \(- \;(\tilde{v}_{1} - \tilde{v}_{2} )^{2}\) [55]. The magnitude of negative values for \(V_{\text{fv}}^{{\text{E}}}\) depends upon the difference in Flory’s reduced volumes of involved components. Negative values of \(V_{\text{fv}}^{{\text{E}}}\) increase in magnitude as the temperature increases which shows that as the temperature increases, more free volume in the [Bmim][triflate] becomes available to accommodate the smaller 1-pentanol molecules which resulted in more negative \(V_{\text{m}}^{{\text{E}}}\). The third term, i.e., characteristic pressure \(V_{\text{P}}^{{\text{E}}}\), which depends on the relative cohesive energy of the expanded and less expanded component, is found to be negative at all investigated temperatures. It is proportional to \((\tilde{v}_{1} - \tilde{v}_{2} )^{2} - (P_{1}^{ * } - P_{2}^{ * } )\) and can have both the negative and positive signs depending upon the magnitude of \(P_{\text{i}}^{ * }\) and \(\tilde{v}_{\text{i}}\) of unlike components [55]. For the system [Bmim][triflate] + 1-pentanol, \(V_{\text{P}}^{{\text{E}}}\) is negative which is related to the structure-breaking effect of the 1-pentanol on the electrostatic interactions between the ions of [Bmim][triflate] and so the 1-pentanol molecules can be placed around the [Bmim]+ and [triflate]− ions [55, 62]. Analysis of these contributing terms \(V_{\text{int}}^{{\text{E}}}\), \(V_{\text{fv}}^{{\text{E}}}\) and \(V_{\text{P}}^{{\text{E}}}\) reveals that \(V_{\text{fv}}^{{\text{E}}}\) plays a major role along with \(V_{\text{P}}^{{\text{E}}}\) for the overall strong interactions between solute and solvent. Figure 12 indicates the composition dependence of \(V_{\text{m}}^{{\text{E}}}\) (PFP) values calculated from PFP theory for [Bmim][triflate] + 1-pentanol mixture compared with experimental \(V_{\text{m}}^{{\text{E}}}\) at 298.15 K. From the figure, the \(V_{\text{m}}^{{\text{E}}}\) values calculated from PFP theory are in good agreement with the experimental values at 1-pentanol-rich region.
Conclusions
Densities and ultrasonic speed of sounds for binary liquids of [Bmim][triflate] with 1-pentanol have been measured experimentally at atmospheric pressure over the entire composition range at temperature 298.15, 308.15, 318.15 and 328.15 K. From the experimental data, excess/deviation properties such as \(V_{\text{m}}^{{\text{E}}}\), \(\kappa_{\text{s}}^{{\text{E}}}\), \(L_{\text{f}}^{{\text{E}}}\), \(u_{{}}^{{\text{E}}}\) and \(\alpha_{\text{P}}^{{\text{E}}}\) have been evaluated. The excess and deviation parameters have been fitted to Redlich–Kister-type polynomial, and also corresponding standard deviations have been computed. In the present binary liquid systems of [Bmim][triflate] with 1-pentanol, the observed excess values clearly reflect the dominance of strong attractive forces. The positive excess molar volume, at IL-rich region, is due to the dominance of dispersion type of interactions. The order of strong interactions follows (298.15 < 308.15 < 318.15 < 328.15) K. The observed lower partial molar volumes in the liquid mixture when compared to the molar volumes of respective pure components also support the existence of strong interactions in the system. Further, an attempt has been made to evaluate derivatives of thermodynamic potentials through physicochemical parameters and excess chemical potentials/molecular properties of the mixtures from empirical equations are also estimated at 308.15 K. The IR spectral studies also supported the presence of strong interaction between molecules in study. PFP theory was able to explain the volumetric behavior of the system quite successfully.
References
Greaves TL, Drummond CJ. Protic ionic liquids: evolving structure–property relationships and expanding applications. Chem Rev. 2015;115:11379–448.
Ghandi K. A review of ionic liquids, their limits and applications. Green Sustain Chem. 2014;4:44.
Guo F, Zhang S, Wang J, Teng B, Zhang T, Fan M. Synthesis and applications of ionic liquids in clean energy and environment: a review. Curr Org Chem. 2014;19:455–68.
Shamsuri AA, Daik R. Applications of ionic liquids and their mixtures for preparation of advanced polymer blends and composites: a short review. Rev Adv Mater Sci. 2015;40:45–59.
Comminges C, Barhdadi R, Laurent M, Troupel M. Determination of viscosity, ionic conductivity, and diffusion coefficients in some binary systems: ionic liquids + molecular solvents. J Chem Eng Data. 2006;51:680–5.
Bhatt MD, Dwyer CO. Recent progress in theoretical and computational investigations of Li-ion battery materials and electrolytes. Phys Chem Chem Phys. 2015;17:4799–844.
Allen CJ, Mukerjee S, Plichta EJ, Hendrickson MA, Abraham KM. Oxygen electrode recharge ability in an ionic liquid for the Li–air battery. J Phys Chem Lett. 2011;2:2420–4.
Park S, Kazlauskas RJ. Biocatalysis in ionic liquids—advantages beyond green technology. Curr Opin Biotechnol. 2003;14:432–7.
Sureshkumar M, Lee CK. Biocatalytic reactions in hydrophobic reactions. J Mol Catal B Enzym. 2009;60:1–12.
Moniruzzaman M, Nakashima K, Kamiya N, Goto M. Recent advances of enzymatic reactions in ionic liquids. Biochem Eng J. 2010;48:295–314.
Rodrigues JV, Ruivo D, Rodriguez A, Deive FJ, Esperanca JMSS, Marrucho IM, Gomes CM, Rebelo LPN. Structural-functional evaluation of ionic liquid libraries for the design of co-solvents in lipase-catalysed reactions. Green Chem. 2014;16:4520–3.
Da Graca MN, Da Silva JMR, Da Silva JC, Alves MM. The use of organic solvents/ionic liquids mixtures in reactions catalyzed by lipase from Burkholderia cepacia immobilized in different supports. J Mol Catal B Enzym. 2015;112:1–8.
Lozano P, Diego T, Carrie D, Vaultier M, Iborra J. Continuous green biocatalytic processes using ionic liquids and supercritical carbon dioxide. Chem Comm. 2002;7:692–3.
Turner MB, Spear SK, Holbrey JD, Rogers RD. Production of bioactive cellulose films reconstituted from ionic liquids. Biomacromol. 2004;5:1379–84.
Turner MB, Spear SK, Holbrey JD, Daly DT, Rogers RD. Ionic liquid-reconstituted cellulose composites as solid support matrices for biocatalyst immobilization. Biomacromolecules. 2005;6:2497–502.
Tan SSY, MacFarlane DR, Upfal J, Edye LA, Doherty WO, Patti AF, Pringle JM, Scott JL. Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid. Green Chem. 2009;11:339–45.
Gericke M, Liebert T, Heinze T. Interaction of ionic liquids with polysaccharides, 8-synthesis of cellulose sulfates suitable for polyelectrolyte complex formation. Macromol Biosci. 2009;9:343–53.
Gericke M, Schaller J, Liebert T, Fardim P, Meister F, Heinze T. Studies on the tosylation of cellulose in mixtures of ionic liquids and a co-solvent. Carbohydr Polym. 2012;89:526–36.
Acidi A, Hasib-ur-Rahman M, Larachi F, Abbaci A. Ionic liquids [EMIM][BF4], [EMIM][Otf] and [BMIM][Otf] as corrosion inhibitors for CO2 capture applications. Korean J Chem Eng. 2014;31:1043–8.
Zhao SF, Horne M, Bond AM, Zhang J. Electrochemical reduction of aromatic ketones in 1-butyl-3-methylimidazolium-based ionic liquids in the presence of carbon dioxide: the influence of the ketone substituent and the ionic liquid anion on bulk electrolysis product distribution. Phys Chem Chem Phys. 2015;17:19247–54.
Lappe P, Hofmann T. Ullmann’s encyclopedia of industrial chemistry. Weinheim: Wiley; 2005. https://doi.org/10.1002/14356007.a19-049.
González EJ, Calvar N, Domínguez Á, Macedo EA. Osmotic and apparent molar properties of binary mixtures alcohol + 1-butyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid. J Chem Thermodyn. 2013;61:64–73.
Vercher E, Miguel PJ, Llopis FJ, Orchillés AV, Martínez-Andreu A. Volumetric and acoustic properties of aqueous solutions of trifluoromethanesulfonate-based ionic liquids at several temperatures. J Chem Eng Data. 2012;57:1953–63.
Vercher E, Llopis FJ, González-Alfaro V, Miguel PJ, Martínez-Andreu A. Refractive indices and deviations in refractive indices of trifluoromethanesulfonate-based ionic liquids in water. J Chem Eng Data. 2011;56:4499–504.
García-Miaja G, Troncoso J, Romaní L. Excess enthalpy, density, and heat capacity for binary systems of alkylimidazolium-based ionic liquids + water. J Chem Thermodyn. 2009;41:161–6.
Anwar N. Interaction of 1-butyl-3-methylimidazolium trifluoromethanesulfonate with ethyl acetate/1-butanol: thermophysical properties. J Solut Chem. 2016;45:1077–94.
Domańska U, Laskowska M. Effect of temperature and composition on the density and viscosity of binary mixtures of ionic liquid with alcohols. J Solut Chem. 2009;38:779–99.
Almasi M, Mousavi L. Excess molar volumes of binary mixtures of aliphatic alcohols (C 1–C 5) with Nitromethane over the temperature range 293.15 to 308.15 K: application of the ERAS model and cubic EOS. J Mol Liq. 2011;163:46–52.
Al-Jimaz AS, Al-Kandary JA, Abdul-Latif AH. Densities and viscosities for binary mixtures of phenetole with 1-pentanol, 1-hexanol, 1-heptanol, 1-octanol, 1-nonanol, and 1-decanol at different temperatures. Fluid Phase Equilib. 2004;218:247–60.
Kumar H, Sharma S. Acoustical studies of binary liquid mixtures of cyclopentane with 1-alkanol at different temperatures and different approaches for ideal mixing laws. J Solut Chem. 2010;39:967–86.
Kijevčanin ML, Radović IR, Djordjević BD, Tasić AŽ, Šerbanović SP. Experimental determination and modeling of densities and refractive indices of the binary systems alcohol + dicyclohexylamine at T = (288.15–323.15) K. Thermochim Acta. 2011;525:114–28.
van Miltenburg JC, van den Berg GJ. Heat capacities and derived thermodynamic functions of 1-propanol between 10 K and 350 K and of 1-pentanol between 85 K and 370 K. J Chem Eng Data. 2004;49:735–9.
Scholz E. Karl Fischer titration. Berlin: Springer; 1984.
Reddy MS, Nayeem SM, Soumini C, Raju KTSS, Babu BH. Study of molecular interactions in binary liquid mixtures of [Emim][BF4] with 2-methoxyethanol using thermo acoustic, volumetric and optical properties. Thermochim Acta. 2016;630:37–49.
Nayeem SM, Kondaiah M, Sreekanth K, Reddy MS, Rao DK. Thermoacoustic, volumetric, and viscometric investigations in the binary mixtures of 1, 4-dioxane with n-hexane or n-heptane or n-octane. J Therm Anal Calorim. 2016;123:2241–55.
Reddy MS, Nayeem SM, Raju KTSS, Babu BH. The study of solute–solvent interactions in 1-ethyl-3-methylimidazolium tetrafluoroborate + 2-ethoxyethanol from density, speed of sound, and refractive index measurements. J Therm Anal Calorim. 2016;124:959–71.
Reddy MS, Raju KTSS, Nayeem SM, Khan I, Krishna KBM, Babu BH. Excess thermodynamic properties for binary mixtures of ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate and 2-methoxyethanol from T = (298.15 to 328.15) K at atmospheric pressure. J Solut Chem. 2016;45:675–701.
Ali A, Nabi F, Tariq M. Volumetric, viscometric, ultrasonic, and refractive index properties of liquid mixtures of benzene with industrially important monomers at different temperatures. Int J Thermophys. 2009;30:464–74.
Ali A, Tariq M. Thermal expansivity and isothermal compressibility of binary liquid mixtures from ultrasonic velocity: a comparison to Flory’s theory and hard sphere models. J Pure Appl Ultrason. 2006;28:99.
Reddy MS, Nayeem SM, Raju KTSS, Rao VS, Babu BH. The study of solute–solvent interactions in 1-ethyl-3-methylimidazolium ethylsulfate + 2-ethoxyethanol from density, speed of sound and refractive index measurements. J Mol Liq. 2016;218:83–94.
Roth C, Appelhagen A, Jobst N, Ludwig R. Microheterogeneities in ionic-liquid–methanol solutions studied by FTIR spectroscopy, DFT calculations and molecular dynamics simulations. Chem Phys Chem. 2012;13:1708–17.
Reddy MS, Raju KTSS, Rao AS, Sharmila N, Babu BH. Study of thermophysical properties of the binary mixtures of ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate and 2-propoxyethanol from T = (298.15 to 328.15) K at atmospheric pressure. J Chem Thermodyn. 2016;101:139–49.
Hunt PA, Kirchner B, Welton T. Characterising the electronic structure of ionic liquids: an examination of the 1-butyl-3-methylimidazolium chloride ion pair. Chem A Eur J. 2006;12:6762–75.
Aggarwal A, Lancaster NL, Sethi AR, Welton T. The role of hydrogen bonding in controlling the selectivity of Diels–Alder reactions in room-temperature ionic liquids. Green Chem. 2002;4:517–20.
Znamenskiy V, Kobrak MN. Molecular dynamics study of polarity in room-temperature ionic liquids. J Phys Chem B. 2004;108:1072–9.
Lassegues JC, Grondin J, Cavagnat D, Johansson P. Reply to the Comment on’ New interpretation of the CH stretching vibrations in imidazolium-based ionic liquids. J Phys Chem A. 2009;114:687–8.
Grondin J, Lassegues JC, Cavagnat D, Buffeteau T, Johansson P, Holomb R. Revisited vibrational assignments of imidazolium-based ionic liquids. J Raman Spectrosc. 2011;42:733–43.
Vercher E, Llopis FJ, Gonzalez-Alfaro MV, Andreu AM. Density, speed of sound, and refractive index of 1-ethyl-3-methylimidazolium trifluoromethanesulfonate with acetone, methyl acetate, and ethyl acetate at temperatures from (278.15 to 328.15) K. J Chem Eng Data. 2010;55:1377–88.
Md Nayeem Sk. Investigation of molecular interactions & prediction of calorimetric potentials of a binary liquid system at T = 308.15 K: an insight from physicochemical parameters. Karbala Int J Modern Sci. 2017. https://doi.org/10.1016/j.kijoms.2017.05.001.
Nayeem SM, Khan I, Sampurna Rao D, Indira P, Reddy M, Kumari MS, Beebi PN, Investigation of molecular interaction in binary system through activity coefficients and application of Prigogine–Flory–Patterson theory to evaluate excess molar enthalpy at T = 308.15 K. In: Proceedings of international seminar, January; 2017, p. 108–111, ISBN 978-93 -82570-84-4.
Hartmann B. Potential energy effects on the sound speed in liquids. J Acoust Soc Am. 1979;65:1392–6.
Copens AB, Beyer RT, Ballou J. Parameter of nonlinearity in fluids. III. Values of sound velocity in liquid metals. J Acoust Soc Am. 1966;4:1443–8.
Sehgal CM. Non-linear ultrasonics to determine molecular properties of pure liquids. Ultrasonics. 1995;33(2):155–61.
Reddy MS, Raju KTSS, Nayeem SM, Krishna KBM, Babu BH. Molecular interaction studies in the binary mixture of 1-ethyl-3-methylimidazolium trifluoromethanesulphonate + 1-butanol from density, speed of sound and refractive index measurements. Phys Chem Liq. 2017;55:775–95.
Patterson D, Delmas G. Corresponding states theories and liquid models. Discuss Faraday Soc. 1970;49:98–105.
Zafarani-Moattar MT, Shekaari H. Application of Prigogine–Flory–Patterson theory to excess molar volume and speed of sound of 1-n-butyl-3-methylimidazolium hexafluorophosphate or 1-n-butyl-3-methylimidazolium tetrafluoroborate in methanol and acetonitrile. J Chem Thermodyn. 2006;38:1377–84.
Kermanpour F, Niakan HZ. Measurement and modeling the excess molar properties of binary mixtures of [C6 mim][BF4] + 3-amino-1-propanol and {[C6 mim][BF4] + isobutanol}: application of Prigogine–Flory–Patterson theory. J Chem Thermodyn. 2012;48:129–39.
Torres RB, Ortolan MI, Volpe PLO. Volumetric properties of binary mixtures of ethers and acetonitrile: experimental results and application of the Prigogine–Flory–Patterson theory. J Chem Thermodyn. 2008;40:442–59.
Flory PJ. Statistical thermodynamics of liquid mixtures. J Am Chem Soc. 1965;87:1833–8.
Abe A, Flory PJ. The thermodynamic properties of mixtures of small, nonpolar molecules. J Am Chem Soc. 1965;87:1838–46.
Vercher E, Orchilles AV, Miguel PJ, Andreu AM. Volumetric and ultrasonic studies of 1-ethyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid with methanol, ethanol, 1-propanol, and water at several temperatures. J Chem Eng Data. 2007;52:1468–82.
Vaid ZS, More UU, Oswal SB, Malek NI. Experimental and theoretical excess molar properties of imidazolium based ionic liquids with isomers of butanol. Thermochim Acta. 2016;634:38–47.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SrinivasaReddy, M., Srinivasa Rao, G., Md Nayeem, S. et al. Thermophysical investigations and prediction of calorimetric potentials in binary mixture of 1-butyl-3-methylimidazolium trifluoromethanesulfonate with 1-pentanol. J Therm Anal Calorim 132, 725–739 (2018). https://doi.org/10.1007/s10973-017-6887-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6887-y